- Volume 61 , Number 3
- Page: 455–8
Cutaneous sarcoidosis masquerading as relapsed borderline tuberculoid leprosy?
ABSTRACT
A patient with cutaneous sarcoidosis is presented. The patient was diagnosed initially as borderline tuberculoid leprosy downgrading to borderline lepromatous leprosy and received a full 2 years of World Health Organization multibacillary drug treatment (MDT). Bilateral hilar lymphadenopathy on chest X-ray and cutaneous ancrgy manifested by negative Mantoux, candidin and trichophytin skin tests sug gested the diagnosis of sarcoidosis. Histopathology confirmed this diagnosis. The subcutaneous nodule seen in this patient is a rare type of cutaneous sarcoidosis.In an effort to increase the utility of the JOURNAL in continuing medical education, in this section we welcome contributions dealing with practical problems in leprosy work. Submissions to this section will undergo minimal editorial changes and may well contain controversial points. Letters to the Editor pointing out other viewpoints are welcome.
Cutaneous sarcoidosis and leprosy maintain so close a relationship that they often produce a diagnostic dilemma for the clinician.1, 2 Thus, in a country where leprosy is endemic it is likely that sarcoidosis of the skin occasionally can be misdiagnosed as leprosy. The correct diagnosis is suspected either when the patient fails to respond to antileprosy treatment or during clinical and/ or histopathological follow up.2,3
We describe here a patient who initially was diagnosed as borderline tuberculoid (BT) leprosy and accordingly received the World Health Organization (WHO) multibacillary antileprosy treatment for 2 years. Later, during follow up, he developed lesions which on investigation turned out to be those of sarcoidosis.
CASE REPORT
A 58-year-old male was seen in January 1990 with three erythematous infiltrated plaques of 1-year duration, one each on the dorsum of the hands and the right knee, with 60% loss of sensation. The lesion over the knee showed central clearing with a welldefined inner border and an ill-defined outer border. He also had radiating pain along the right forearm, tenderness over the skin lesions, and a moderately thickened, tender, right ulnar nerve of 20 days' duration. He had been receiving WHO MDT paucibaciliary treatment for 7 months from another institution.
A diagnosis of BT leprosy downgrading to borderline lepromatous (BL) leprosy with a type 1 reaction was made clinically. Slitskin smears from the lesions and earlobes were negative for acid-fast bacilli; a skin biopsy showed an epithelioid cell granuloma suggestive of BT leprosy and a lepromin test was 20 mm at 3 weeks. He was started on WHO MDT multibacillary treatment along with 30 mg prednisolone daily. The prednisolone was tapered and stopped after 6 months. Antileprosy treatment was stopped in January 1992, when all the lesions had healed.
During follow up after 7 months in August 1992, two erythematous, asymptomatic, shiny succulent noduloplaques (1.5 cm x 1 cm and 1 cm x 1 cm) were noted over the face (Fig. 1). In addition, skin-colored subcutaneous nodules were observed over the right sole and the medial aspect of the left great toe. He was admitted with clinical possibilities of sarcoidosis and BT leprosy in relapse.

Fig. 1. A single, erythematous, shiny, succulent, noduloplaque lesion over the right nasolabial fold and area below right nostril.
A chest X-ray revealed bilateral hilar lymphadenopathy (Fig. 2). His erythrocyte sedimentation rate was 32 mm/1st hr; serum calcium, 9.4 mg%; total serum proteins, 8.3% (albumin 4.3, globulin 4 A:G = 1). Mantoux, candidin and trichophytin skin tests were negative. Pulmonary function tests showed mild obstructive features.
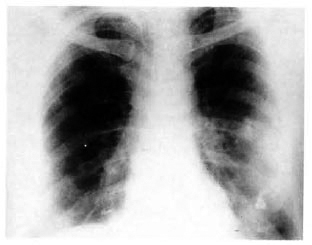
Fig. 2. Chest X-ray showing bilateral hilar lymphadenopathy.
A histopathological examination of the biopsies obtained from one of the lesions over the face and one from the right sole showed closely packed clusters of noncaseating epithelioid cell granulomas situated deeper in the dermis. There was sparse lymnoduloplaque lesion over the right nasolabial fold and area below right nostril. phocytic infiltrate around the granuloma (Fig. 3A). Typical asteroid inclusions were noticed also (Fig. 3B). Staining for reticulin revealed a fine reticulin network encircling the granulomas and penetrating them at places (Fig. 3C).
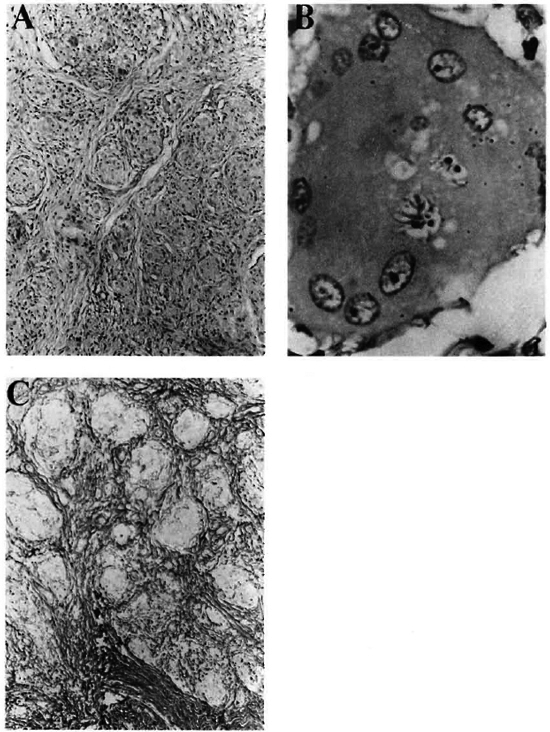
Fig. 3. A = Multiple circumscribed, compact, noncaseating granulomas with Langhans' type of giant cells (H&E x240); B = A large multinucleated giant cell with asteroid bodies (H&E x 1375); C = Reticulin fibers surround and permeate epithelioid cell granulomas (reticulin stain, x 240).
A transbronchial lung biopsy showed interstitial fibrosis. Serum transaminase, alkaline phosphatase, blood urea, creatinine, and electrocardiograph and echocardiograph, and X-rays of the hands and feet were within normal limits. Serum angiotensin converting enzyme and Kveim tests were not done because of nonavailability.
He was administered interlesional triamcinolone acetonide with moderate improvement, and is presently on follow up with us.
DISCUSSION
Apart from lupus pernio, all other types of skin lesions of sarcoidosis can be mimicked closely by leprosy. However, a subcutaneous nodule in a relapsed BT leprosy patient is practically unknown.4,5 Moreover, relapses occur 1 year after stopping treatment and lesions reappear at the same site.4
According to Scadding and Mitchell, cutaneous anergy manifesting as negative Mantoux, candidin, and trichophytin skin tests and the lack of sensitization to DNCB is quite suggestive of sarcoidosis.6 In our patient the Mantoux, trichophytin and candidin skin tests were negative. Also, asymptomatic bilateral hilar lymphadenopathy (classically known as clinico-radiological dissociation) is highly suggestive of sarcoidosis.7
Histopathologically, at times it may be difficult to differentiate between BT leprosy and sarcoidosis.8 However, granulomatous infiltration of dermal nerves is probably the most consistent differentiating feature between these two.9 But the deeper situation of a compact, naked, epithelioid cell granuloma permeated by a fine reticulin network and containing plentiful asteroid bodies certainly points toward a diagnosis of sarcoidosis,9 as in our case.
The incidence of subcutaneous nodules in sarcoidosis is 2%-6%.10,11In view of their asymptomatic nature, they are frequently overlooked by both patients and physicians.13 In the present case, the coexistence of both of these rare happenings was observed. None of the 40 patients with sarcoidosis reported from our institute had such a type of skin lesion.14
The patient was treated with intralesional corticosteroid since there was no indication for systemic therapy.15
We postulate that leprosy as a mycobacterial disease may have played some triggering role in the development of sarcoidosis. Careful and meticulous follow up of all leprosy patients is recommended.
- Surrinder Kaur, M.D., F.A.M.S.
Sandipan Dhar, M.D., M.A.M.A.
Department of Dermatology, Venereology and Leprology
- Pradeep Bambery, M.D.
Department of Internal Medicine
- Amrinder J. Kanwar, M.D.
Department of Dermatology, Venereology and Leprology
- Arvind Khajuria, M.D.
Department of Pathology
Postgraduate Institute of Medical Education and Research
Chandigarh 160012, India
1. James, D. G. and Jopling, W. H. Sarcoidosis and leprosy. Trop. Med. Hyg. 1(1961)42-46.
2. Ramanujam, K. Tuberculoid leprosy or sarcoidosis? A diagnostic dilemma. Lepr. India 54(1982)318-323.
3. Morrison, J. S. Sarcoidosis in Bantu; necrotizing and mutilating forms of the disease. Br. J. Dermatol. 90(1974)649-655.
4. World Health Organization. A guide to leprosy control. 2nd edn. Geneva: World Health Organization, 1988, pp. 40-42.
5. Jacobson, R. R. Treatment. In: Leprosy. Hastings, R. C, ed. London: Churchill Livingstone, 1985, pp. 209-211.
6. Scadding, J. G. and Mitchell, D. N. The immunology of sarcoidosis. In: Sarcoidosis. 2nd edn. London: Chapman and Hall, 1985, pp. 414-418.
7. Winterbauer, R. H., Behe, N. and Mootes, K. K. A clinical interpretation of bilateral hilar adenopathy. Ann. Intern. Med. 78(1973)65-71.
8. Ridley, D. S. Pathogenesis of Leprosy and Other Related Diseases. London: Wright, 1988, pp. 145-154.
9. Zumla, A. and James, D. G. Sarcoidosis and leprosy-an epidemiological, clinical, pathological and immunological comparison. Sarcoidosis 6(1989)88-96.
10. Vainsencher, D. and Winklemann, R. K. Subcutaneous sarcoidosis. Arch. Dermatol. 120(1984)1028-1031.
11. Kalb, R. E., Epstein, W. and Grossmann, M. E. Sarcoidosis with subcutaneous nodules. Am. J. Med. 85(1988)731-736.
12. Erenchun, F. R. D., Vazquez-Doval, F. J., Idate, M., Leache, A. and Quintanilla, E. Sarcoidosis nodules as the first clinical manifestation of sarcoidosis. Clin. Exp. Dermatol. 17(1992)192-194.
13. Savin, J. A. Sarcoidosis. In: Textbook of Dermatology, Vol. 4, 5th edn. Champion, R. H., Burton, J. L. and Ebling, F. J. G., cds. Oxford: Blackwell Scientific Publications, pp. 2383-2406.
14. Bambery, P., Behera, D., Gupta, A. K., Kaur, U., Jindal, S. K., Deodhar, S. D. and Malik, S. K. Sarcoidosis in north India: the clinical profile of 40 patients. Sarcoidosis 4(1987)155-158.
15. Johns, C. J., Scott, P. P. and Schonfeld, S. A. Sarcoidosis. Ann. Rev. Med 40(1989)353-371.
Reprint requests to Dr. (Mrs.) S. Kaur, House No. 2352, Phase XI, S.A.S. Nagar (Mohali), Punjab 160059, India.