- Volume 61 , Number 3
- Page: 462–4
lnterleukin-1β production by peripheral blood mononuclear cells f rom leprosy patients
To the Editor:
Many hypotheses have attempted to explain the cell-mediated immunity (CMI) defect in lepromatous leprosy. In the present study we have investigated the CMI defect in lepromatous leprosy by quantitating the IL-β produced by adherent cells of peripheral blood mononuclear leukocytes (PBML) in response to Mycobacterium leprae.
Sixteen lepromatous leprosy/borderline lepromatous leprosy (LL/BL) patients from Gremaltes Referral Hospital, Madras, India, diagnosed and classified by the Ridley and Jopling classification, were studied (9). Fourteen of them were untreated and two had been treated earlier with dapsone but were free from any treatment for at least 6 months before inclusion into the present study. The age range was 18-50 years, and two were females. Blood samples were taken before commencement of multidrug therapy (100 mg of dapsone, plus 50 mg of clofazimine daily, unsupervised, with 600 mg of rifampin and 300 mg of clofazimine given once monthly under supervision) and at the end of 6 months of therapy. Age- and sex-matched healthy controls were from the same endemic area.
PBML were obtained by Ficoll-Hypaque density centrifugation (1) of heparinized venous blood and washed three times with RPMI 1640 medium (GIBCO Laboratories, Grand Island, New York, U.S.A.). Two million viable PBML were added to a 35-mm diameter, tissue culture petri dish. The final volume was made up to 2 ml with RPMI 1640 culture medium supplemented with 5% heat-inactivated fetal bovine serum (Sigma Chemical Co., St. Louis, Missouri, U.S.A.), 2 mM glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10 mM HEPES, 5 × 10-5 M 2-mercaptoethanol, penicillin 200 units and streptomycin 200 µg. The plate was incubated for 1 hr at 37ºC under 5% carbon dioxide. Then the nonadherent cells were removed by vigorous washing. Two million M. leprae, obtained from armadillos and killed by gamma irradiation (kindly supplied by Dr.
R. J. W. Rees, Medical Research Council, London) were added to the plate. Culture medium to the final volume of 2 ml was added, and the plate was incubated at 37ºC in a humidified atmosphere with 5% carbon dioxide for 44 hr. The supernatant then was filtered through 0.22-µm membrane filter and stored at -70ºC until assayed. IL-β in the fluid was quantitated using a commercial ELISA kit (Cistron Biothechnology, Pine Brook, New Jersey, U.S.A.). Student's / test was used for statistical analysis.
The IL-β produced by LL/BL patients (before therapy) and healthy controls was 44.31 ± 16.06 pg/ml and 162.20 ± 55.55 pg/ml, respectively (The Figure). The quantity of IL-β produced by LL/BL patients (before therapy) is significantly less when compared to healthy controls (p < 0.05). The correlation coefficient between the bacterial index (BI) and IL-β (before therapy) was calculated in a computer-based NCSSplus program and no correlation was seen between them (r = -0.0621; p > 0.05). Six out of 16 patients (before therapy) produced IL-β which was below the lower limit of the IL-β range of healthy controls, while 5 out of 16 patients did not produce any detectable quantity of IL-β at all.
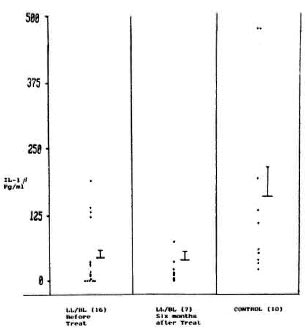
The figure. IL-1β produced by adherent cells of106 PBML/ml from BL/LL patients when stimulated with M. leprae = mean ± S.E.M.; figures in parentheses = number of subjects).
The quantity of IL-β produced by LL/ BL patients after 6 months of multidrug therapy was 40.28 ± 17.24 pg/ml, showing that after 6 months of multidrug therapy the IL-β level did not change from its pretreatment level (p > 0.05) (The Figure).
IL-1 is produced in two distinct forms- IL-lα and IL-β-and the IL-β protein represents the predominant IL-1 produced by stimulated mononuclear phagocytes (7). Apart from producing IL-1, macrophages also can inhibit immune responses through the release of prostaglandins, such as PGE2, and other poorly characterized inhibitors (6). These can inhibit the proliferative responses of mouse thymocytes to IL-1 in bioassays (11). On the other hand, thymocyte assay also may respond to additional cofactors, such as IL-4 and IL-6 (2). Thus, the inhibitor or enhancer problem makes the quantitative data generated by functional bioassays difficult to interpret; ELISA for IL-β is not affected by these enhancers or inhibitors that may be present in the culture supernatants (11) and provides a much needed specificity to the studies of IL-1 release by mononuclear phagocytes (3). Moreover, natural human IL-β from macrophage culture supernatants and recombinant human IL-β purified by the same procedures seem to have identical biological and immunological properties (5).
In our study, LL/BL patients (before therapy) produced significantly smaller quantities of IL-β when compared to healthy controls. Makonkawkeyoon, et al. (4) had shown that on stimulation with lipopolysaccharide significantly lower levels of IL-1 were produced by monocytes of LL, BL, BB and BT patients than by normal controls. Watson, et al. (10) also showed that adherent cells of 5 of 13 BL/LL patients did not produce detectable IL-1 in response to lipopolysaccharide or phorbal myristate acetate. Ridel, et al. (8) reported that IL-1 produced by LL patients is comparable to that of healthy controls, but the mouse thymocyte assay for IL-1 used by them could have been influenced by prostaglandin E2 (as reported by themselves), and many other substances in the culture fluid. Moreover, after 6 months of multidrug therapy the quantity of IL-β produced by the adherent cells from PBML of LL/BL patients did not change significantly from its pretreatment level.
- Venugopal Jayapal, M.D.
Gopal Selvi Bai, M.Sc.
Department of Microbiology
Dr. ALM Post Graduate Institute of Basic Medical Sciences
University of Madras
Taramani, Madras 600113, India
- Dere k Lobo, M.B.B.S.
Gremaltes Referral Hospital
Shenoy Nagar
Madras, India
- Sandras Panchatcharam Thyagarajan, Ph.D.
Department of Microbiology
Dr. ALM Post Graduate Institute of Basic Medical Sciences
University of Madras
Taramani, Madras 600113, India
Acknowledgment. This investigation received support from the Indian Council for Medical Research, New Delhi. We wish to thank Mr. R. Annadurai for technical assistance and Dr. Vilvanathan Devanbu, Dr. Mani Mathews, and the field and hospital workers of Gremaltes Referral Hospital, Madras.
REFERENCES
1. Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21 Suppl. 97(1968)77-89.
2. Hodgkin, P. D., Bond, M. W., O'Garra, A., Frank, G., Lee, F., Coffman, R. L., Zlotnik, A. and Howard, M. Identification of IL-6 as a T cell derived factor that enhances the proliferative response of thymocytes to IL-4 and phorbol myristate acetate. J. Immunol. 141(1988)151-157.
3. Kenney, J. S., Masada, M. P., Eugui, E. M., Delustro, B. M., Mulkins, M. A. and Allison, A. C. Monoclonal antibodies to human recombinant interleukin 1 (IL 1)β quantitation of IL 1β and inhibition of biological activity. J. Immunol. 138(1987)4236-4242.
4. Makonkawkeyoon, S., Kasinrerk, W., Supajatura, V., Hirunpetcharat, C. and Vithayasi, V. Immunologic defects in leprosy patients. II. Interleukin 1, interleukin 2, and interferon production in leprosy patients. Int. J. Lepr. 58(1990)311-318.
5. Molvig, J., Sehested Hansen, B., Worsaae, H., Hejnaes, K. R., Helle, M., Dalboge, H. and Nerup, J. Comparison of biological and immunological activities of human monocyte-derived IL-1β and human recombinant IL-1 β. Scand. J. Immunol. 31(1990)225-235.
6. Monick, M., Glazier, J. and Hunninghake, G. W. Human alveolar macrophages suppress interleukin-I (IL-I) activity via the secretion of prostaglandin E2. Am. Rev. Respir. Dis. 135(1987)72-77.
7. Oppenheim, J. J., Kovacs, E. J., Matsushima, K.. and Durum, S. K. There is more than one interleukin 1. Immunol. Today. 7(1986)45-56.
8. Ridel, P. R., Jamet, P., Robin, Y. and Bach, M.-A. Interleukin-1 released by blood-monocyte derived macrophages from patients with leprosy. Infect. Immun. 52(1986)303-308.
9. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
10. Watson, S., Bullock, W., Nelson, K., Schauf, V., Gelber, R. and Jacobson, R. Interleukin 1 production by peripheral blood mononuclear cells from leprosy patients. Infect. Immun. 45(1984)787-789.
11. Wewers, M. D. and Herzyk, D. J. Alveolar macrophages differ from blood monocytes in human IL-1 β release; quantitation by enzyme-linked immunoassay. J. Immunol. 143(1989)1635-1641.