- Volume 61 , Number 3
- Page: 464–6
Higher specificity in the serodiagnosis of leprosy by combined titration of antiphenolic glycolipid-l and antiphospholipid antibodies
To the Editor:
The early diagnosis of infectious cases of leprosy would significantly improve the prospects for successful leprosy control. It has long been hoped that this could be achieved with a specific serological test using an IgM phenolic glycolipid-I (PGL-I) enzyme-linked immunosorbent test (ELISA)(3). Unlike the initial encouraging results, studies report that the IgM anti-PGL-I ELISA fails to represent a reliable test for the serodiagnosis of leprosy (2).
Antiphospholipid antibodies have been reported recently in leprosy patients (4,8).
In this study a PGL-I-ELISA serological test and an anti-phospholipid ELISA have been performed in leprosy patients and "healthy controls" to determine the performance values of the two immunological tests as well as to determine if the combination of the two independent tests can be superior to either assay alone.
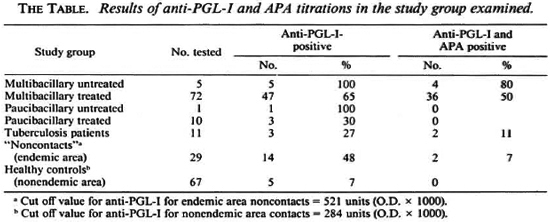
The study group included: a) 88 leprosy patients, 31 from Eritrea, 36 from Italy, and 21 from other areas (Latin America, Asia, North Africa) classified as multibacillary untreated (N = 5), multibacillary treated (N = 72), paucibacillary untreated (N = 1), paucibacillary treated (N = 10); b) 11 patients affected with active pulmonary tuberculosis; c) 29 healthy people from an endemic area ("noncontacts"); d) 65 healthy controls from a nonendemic area (Genoa, Italy).
Sera from all subjects were assayed for antiphospholipid antibodies (APA) and anti-PGL-I using ELISA techniques previously described (5, 9).
The positivity threshold for the APA was established referring to standard sera provided by Dr. Harris, Department of Medicine, University of Louisville, Kentucky, U.S.A.
The definition of a positive anti-PGL-I test was established using one negative control group from an endemic area (Ethiopia) and one negative control group from a nonendemic area (Italy). The correspondent threshold values were 521 units (O.D. × 1000) for the endemic area and 284 units for the nonendemic area (5).
Of 88 leprosy patients, 56 (64%) had significant anti-PGL-I antibodies and 40 (45%) were seropositive to both PGL-I and APA. Ofthe 11 tuberculosis patients, 3 (27%) were PGL-I positive and 2 (11%) were positive to both anti-PGL-I and APA.
Among the 29 "noncontacts" from the endemic area, 14 (48%) were PGL-I-positive but only 2 (7%) were positive to both antigens.
Among the 67 healthy controls from the nonedemic area, 62 (93%) were PGL-I-negative and none of them was positive to both antigens (The Table).
The PGL-I positivity rate among the leprosy patients studied was consistent with previous reports (1) but lower than others(7), more than likely because the majority of our patients had been treated for significant periods of time, leading to a reduction of the anti-PGL-I titers.
The percentage of the APA response among leprosy patients was higher than those previously reported (8).
For patients positive for PGL-I and negative for APA, different factors such as genetic background, clinical form, reactional phase, disease duration and treatment status should be taken into account in justifying the different immunological responses. However the number of patients studied was too small, and the clinical information was insufficient to control fully the relevance of each of these factors in influencing the immunological behavior.
The positivity rate among the tuberculosis patients was significantly high and was comparable with the "false-positive" rate among the noncontact population from the endemic area, confirming the variable degree of specificity of the anti-PGL-I titration.
Finding anti-PGL-I-positive sera in healthy people from a nonendemic area was undoubtedly intriguing since all of the subjects included in our "negative control" group were reportedly healthy and without clinical evidence of leprosy, tuberculosis or any other mycobacterial disease. Only further studies will help to throw light on the aspect of "false-positive" PGL-I.
The novelty of our study consists in performing the ELISA PGL-I test in parallel with the titration of antiphospholipid antibodies. By considering as "positive" only those subjects with both anti-PGL-I and APA, the percentage of positivity among the leprosy patients decreased from 64% (when anti-PGL-I was considered alone) to 45%, when PGL-I and APA were both considered.
On the other hand, improvement of test specificity was remarkable since the percentage of negative subjects among all nonpatients was raised from an unsatisfactory 52% to 93% in the healthy group from the endemic region and up to 100% in the subjects from the nonendemic area. If such findings are not so important in endemic countries, where even PGL-I fails to be useful for leprosy detection in large-scale studies, they are extremely important when applied in countries which are nonendemic for leprosy.
The performance values of an immunological method determine the potential use of the test. Sensitivity of a method does not significantly influence the predictive value, neither in areas of low endemicity nor in people at high risk for the disease (e.g. household contacts).
On the other hand, the specificity of a test, used both as a diagnostic screening tool and in epidemiological studies, has to be very high when the prevalence rate is low because even minor changes in the specificity give dramatic changes in the predictive value for positive results (6). The predictive value for a positive result (PV-POS) is calculated as percent positive patients among all positive individuals. If we consider such a value in our study, we can see that the PV-POS was 67% when anti-PGL-I positivity was considered alone, but was 91% when both titrations were considered.
Therefore, the serological method herein proposed seems able to improve significantly the predictive value for serodetection of leprosy, even though clinical examination always will be the primary method for diagnosing leprosy.
If other studies confirm the encouraging results of this preliminary report, it would be conceivable to consider anti-PGL-I titration as the preliminary test for screening surveys and APA titration as the "second step" procedure to be done on anti-PGL-Ipositive subjects in order to improve the degree of specificity and the rate of predictive values.
- Paolo Fiallo, M.D.
Paolo P. Cardo, B.Sc.
Enrico Nunzi, M.D.
C.I.R.LEP
University of Genoa
Viale Benedetto XV, 7
1-16132 Genoa, Italy
REFERENCES
1. AGIS, F., SCHLICH, P., CARTEL, J.-L., GUIDI, C. and BACH, M.-A. Use of anti-A/. leprae phenolic glycolipid-I antibody detection for early diagnosis and prognosis of leprosy. Int. J. Lcpr. 56(1988)527-536.
2. BAUMGART, K., BRITTON, W., BASTEN, A. and BAGSHAVE, A. Use of phenolic glycolipid-I for scrodiagnosis of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81(1987)1030-1032.
3. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
4. ESCOBAR-GUTIERREZ, A., AMEZCUA-CHAVARRIA, M. E., PASTEN, S., CASTRO, E., FLORES O. and RODRIGUEZ, O. Anti-cardiolipin antibodies in Mexican lepromatous leprosy patients. Int. J. Lepr. 58(1990)723-724.
5. FIALLO, P., TRAVAGLINO, C, CARDO, P. P., PERSI, A. and NUNZI, E. Dosaggio di anticorpi antiglicolipide fenolico I (Mycobacterium leprae) in una popolazione della Eritrea. G. Ital. Dermatol. Venereol. 126(1991)273-278.
6. KRONVALL, G. The potential of immunological tests as tools in the epidemiology of leprosy. Lepr. Rev. 52 Suppl. 1(1981)207-219.
7. LEFFORD, M. J., HUNEGNAW, M. and SIWIK, E. The value of IgM antibodies to PGL-I in the diagnosis of leprosy. Int. J. Lcpr. 59(1991)433-440.
8. QUISMORO, F. P. and REA, T. H. Serum antiphospholipid antibodies in leprosy. (Abstract) Int. J. Lcpr. 56(1988)682.
9. ROBERT, E., PARODI, A., CARDO, P. P. and NUNZI, E. Gli anticorpi anti-cardiolipina nel lupus eritematoso. G. Ital. Dermatol. Venereol. 124(1989)5-7.
Reprints requests to Dr. Nunzi.