- Volume 61 , Number 1
- Page: 8–15
Clinical features and outcome of reversal (type 1) reactions in Hyderabad, India
ABSTRACT
A retrospective survey of the notes on all patients attending Dhoolpet Leprosy Research Center, India, during 1985 was done to establish the frequency, timing, and clinical features of reversal (type 1) reactions; 494 case notes were examined and clinical evidence of a reversal reaction was found in 44 cases (10.9%). Reactions were most common in borderline patients, with 11.4% and 14.8% of borderline tuberculoid (BT) and borderline lepromatous (BL) patients developing reactions, respectively. Presentation in reaction was frequent with 47.5% of reactional patients having signs of a reversal reaction at the time of their first visit to the Dhoolpet clinic; 50% of skin reactions developing in patients on antileprosy treatment occur within the first month of treatment. Neurological reactions occur later and over a longer time course. Late reactions may occur up to 6 ½ years after the start of treatment. Further reactional episodes occurred in 31.8% of the patients, and may be repeated. Steroid treatment produced improvement of both skin lesions and neuritis, but improvement in clinical signs and symptoms occurred in only 50% of the neuritic episodes.RÉSUMÉ
Nous avons réalisé une étude rétrospective des dossiers de tous les patients fréquentant le "Dhoolpet Leprosy Research Center" en Inde en 1985 pour établir la fréquence, le moment et les caractéristiques clinques des réaction reverses (type 1); 494 dossiers ont été examinés et on a trouvé une évidence clinque de réaction réverse dans 44 cas (10,9%). Les réactions étaient les plus fréquentes chez les patients borderlines, avec respectivement 11,4% et 14,8% des patients borderline tuberculoides (BT) et borderline lépromateux (BL) développant des réactions. Parmi les patients qui ont présenté une réaction, 47,5% avaient des signes de réaction reverse au moment de leur première visite à la clinque de Dhoolpet; 50% des réactions cutanées se développant chez les patients en traitement anti-Icpreux surviennent au cours du premier mois de traitement. Les réactions neurologiques surviennent plus tardivement, et s'étalent sur une plus longue période. Des réactions tardives peuvent survenir jusqu'à 6 ½ ans après le début du traitement. Des épisodes réactionncls ultérieurs sont survenus chez 31,8% des patients, et peuvent se répéter. Un traitement stéroide produit une amélioration des lésions aussi bien cutanées que nerveuses, mais l'amélioration des signes clinques et des symptômes est survenue dans seulement 50% des épisodes névritiques.RESUMEN
Se hizo un estudio retrospectivo de todos los pacientes atendidos durante 1985 en el Dhoolpet Leprosy Research Center de la India para establecer la frecuencia, el tiempo de aparición, y las características clínicas de las reacciones reversas del tipo 1. Se examinaron 494 casos, encontrándose evidencias de reacción reversa en 44 de los mismos (10.9%). Las reacciones fueron más comunes en los pacientes con lepra intermedia (11.4% en los casos BT y 14.8% en los BL). El 47.5% de los pacientes tuvieron signos de reacción reversa en el tiempo de su primera visita a la clínica Dhoolpet; el 50% de las reacciones en piel ocurrieron durante el primer mes de tratamiento antileproso. Las reacciones neurológicas ocurrieron después y a lo largo de un periodo más extenso de tiempo. Las reacciones tardías pueden ocurrir hasta 6.5 años después de comenzar el tratamiento. El 31.8% de los pacientes presentaron episodios reaccionales adicionales, algunos de repetición. El tratamiento con esteroides produjo mejoría tanto de las lesiones en piel como de las neuritis, pero la mejoría en los síntomas y signos clínicos sólo ocurrió en el 50% de los episodios neuríticos.Reversal reactions (type 1 reactions), which are an important complication ofleprosy, are episodes of acute inflammation affecting skin and nerves occurring when a patient develops increased cell-mediated immunity toward Mycobacterium leprae (1,3) and moves toward the tuberculoid end of the leprosy spectrum. Ridley and Radia (13) have described the histological features of reversal reactions which include edema, lymphocytic infiltration and giant cell formation. The outcome of this cellular activation is the elimination of leprosy bacilli with a fall in the bacterial index (BI) (13). Since this acute immune response is directed at M. leprae antigens, the inflammatory response is seen at the sites of localization of M. leprae antigens, skin and peripheral nerve, and is manifest clinically as inflammatory skin lesions and acute neuritis. It is this neuritic component of reversal reaction pathology which is particularly important because unless neuritis is promptly diagnosed and treated, rapid and severe nerve damage may ensue. Despite the practical importance of reversal reactions there have been few studies of their epidemiology or natural history. We have focused solely on reversal reactions, and are not considering here the other major reactional state in leprosy, erythema nodosum leprosum (ENL; type 2 reactions).
The data presented here were collected during a retrospective study of case notes at Dhoolpet Leprosy Research Centre, Hyderabad, India. The study was designed to document a) the frequency of reactions; b) the timing of reactions, particularly in relation to the onset of leprosy symptoms and treatment; c) the symptoms and signs of reaction, particularly the signs of neurological involvement; d) the response to treatment and e) the frequency of late reactions.
METHODS
The Dhoolpet clinic is situated on the edge of Hyderabad old city. It does not have a leprosy control program so most patients are self-referred, with a few being referred from local practitioners. The clinic is funded as a research center by the British Medical Research Council, and has developed a strong interest in nerve damage under the direction of Dr. J. M. H. Pearson (who was the clinician in charge in 1985). All notes for patients registering as new cases with the clinic from 1 January until 31 December 1985 were reviewed in March 1991 by one person (DNJL). These dates were chosen to allow a lengthy follow up for the detection of late reactions. Patients who did not have leprosy or did not attend again were excluded from the analysis. All patients had been seen by either one of the two clinicians running the clinic. A reversal reaction was deemed to have occurred if any of the following criteria were met: a) there was new erythema of existing skin lesions without evidence of downgrading, b) there were new erythematous skin lesions without the features of ENL, c) new acute neuritis, or d) the histopathological report was of a reversal reaction. Data were collected on age, sex, time at which symptoms of leprosy were first noted, commencement of treatment, and appearance of reactional lesions. Particular attention was paid to the symptoms and signs of reversal reactions, the description of skin lesions and neuritis. Records were specifically examined for data on the ulnar, median, radial, radial cutaneous, lateral popliteal (common peroneal), posterior tibial and sural nerves. For those nerves, the following symptoms and signs were specifically recorded: nerve tenderness; motor symptoms (symptomatic weakness); signs of paresis (a score of less than 5/5 using the MRC testing scale); sensory symptoms (paresthesia, anesthesia) and sensory signs (diminished sensation to pin prick, light touch or temperature). Treatment details for the reversal reactions were noted together with the time it took for improvement to occur. Any further reactional episodes were noted.
RESULTS
A total of 810 new cases were registered at the clinic between 1 January and 31 December 1985, of these cases 144 were not leprosy and 167 were seen only once and were not included in the study. The notes on live patients were unavailable. The study population of 494 was clinically classified as tuberculoid (TT) 77 (15.6%); borderline tuberculoid (BT) 218 (44.1%); midborderline (BB) 3 (0.6%); borderline lepromatous (BL) 67 (13.5%); lepromatous (LL) 123 (24.8%) and 6 "others" (5 indeterminate, 1 polyneuritic case).
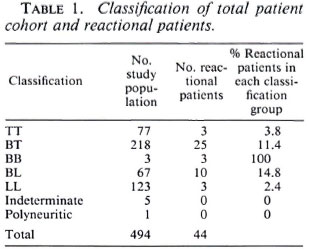
A total of 45 patients met our definition of reversal reaction during the 6-year study period; 1 patient was subsequently excluded because his histology report showed evidence of relapse, leaving a reversal reaction group of 44 patients -52.3% of reversal reactions were in males, 47.7% in females. The age range of the patients at the time of reaction varied from 12-89 years (mean 35 years). Diagnostic skin biopsies were done in 27 patients. The clinical and histological classifications were concordant in only 15 cases, of the remaining 12 patients 9 were closer to the tuberculoid end of the spectrum and 2 were more lepromatous than anticipated on clinical examination. These latter two patients were clinically classified as BL but on histology the classification was of subpolar lepromatous (LLS) in reaction; they also both responded to steroids which helps confirm their reactional state. For our analysis a patient's classification is his/her histological one when histology has been done, otherwise it is his/her clinical one. Table 1 gives the classification of the 44 patients studied. Reversal reactions are most likely to occur in patients from the borderline groups (BT, BB and BL) with 86.3% of our patients being in these borderline groups.
Data on patient Bis were available for 12 patients before, during and after reaction; on 38 patients during reaction and on 18 during and after reaction. All Bis fell or remained at 0 before, during and after reaction with the exception of one patient whose BI fell from 3.7 at presentation to 0 during reaction and then rose a year later to 2.3 when he relapsed. His reversal reaction had been confirmed histologically.
In 1985 the World Health Organization (WHO) multidrug therapy (MDT) had not been introduced for all patients in Hyderabad, and so 37% of the patients had been treated with dapsone (DDS) alone, 56.5% with DDS and rifampin and 6.5% with DDS, rifampin and clofazimine.
Clinical features of reversal reactions. Of the 44 patients who had a reversal reaction, 19 (43.1%) had skin lesions alone, 10 (22.7%) had both skin lesions and neuritis, and 14 (31.8%) had only neuritis (Fig. 1). One patient was found to have histological evidence of reaction on a skin biopsy from a nonreactional-looking lesion; 26 patients had erythematous skin lesions and in only 9 cases was edema present also. No ulceration of skin lesions was noted. New skin lesions were reported in 5 patients (2 BT, 3 BL).
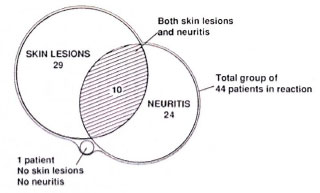
Fig. 1. Distribution of skin lesions and neuritis in 44 Indian reactional patients.
There was evidence of neuritis in 24 (54.5%) patients. (Table 2 shows for each peripheral nerve the percentages of patient nerves that were found to have evidence of neuritis.) There was no evidence for lateralization of the neuritis. The ulnar nerve is the most commonly affected nerve by any parameter of neuritis, with nerve tenderness being found in 25% and motor signs in 33.3% of the nerves examined. Nerve tenderness was rarely elicited in the lateral popliteal (common peroneal) and posterior tibial nerves, although evidence of motor dysfunction was found in 14.5% of lateral popliteal nerves. No evidence of radial nerve involvement was recorded, although four radial cutaneous nerves were reported to be tender. Nine patients had neuritis in one nerve only, five patients were affected in two nerves and 10 patients had multiple nerve involvement. A score was constructed to determine the severity of neuritis: for each nerve 1 point was allocated for each possible sign of neuritis (tenderness, sensory symptoms, sensory signs, motor symptoms, motor signs). For 10 nerves the maximum possible score was 50. (Table 3 gives the distribution of scores.) Patients who had neuritis usually had more than one sign of neuritis. Paucibacillary and multibacillary patients did not differ in their clinical presentation of reactions with respect to the presence of skin lesions or the numbers and types of nerves involved.
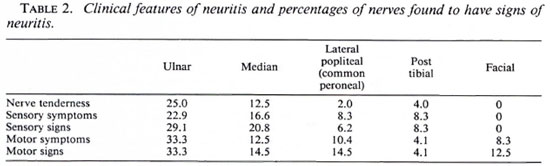
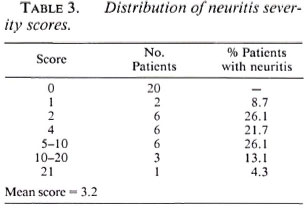
Figure 2 shows the numbers of patients developing symptoms of skin and nerve reactions during defined time intervals from their first symptoms of leprosy. The period of 12-24 months after first symptoms is the peak time for the development of reactional symptoms, although reactional symptoms occurred as long as 12 years after the initial symptoms of leprosy. Skin reactional symptoms developed earlier than neurological symptoms, with a median first symptomto-reaction time for skin lesions of 13.0 months (S.D. 37.8 months) and 27.5 months (S.D. 33.1 months) for neuritis. There were a substantial number of patients in whom the diagnosis of leprosy was only considered when they developed a reaction.
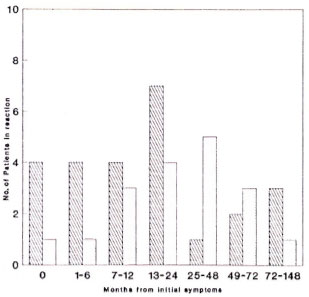
Fig. 2. Time intervals from initial symptoms of leprosy to development of reactional skin lesions and neuritis. Skin =  ; nerve =
; nerve =  . Median symptom-reaction time: skin = 13.0 mos. ± 37.8 mos; nerve = 27.5 mos. ± 33.1 mos.
. Median symptom-reaction time: skin = 13.0 mos. ± 37.8 mos; nerve = 27.5 mos. ± 33.1 mos.
Reactional symptoms developed in 45.4% of our patients before they sought treatment for leprosy, 38.6% were on treatment at the time of reaction, and 9% developed reaction after treatment (treatment details were incomplete for three patients). Only one patient admitted to previous irregular treatment. Of the 17 (1 TT, 10 BT, 1 BB, 4 BL, 1 LL) patients who were on treatment at the time of reaction, 6 were receiving DDS monotherapy, 2 of whom were irregular in taking their medication. Ten patients were on DDS and rifampin; 1 was irregular. One patient was on multibacillary MDT.
We have analyzed the temporal relationship between starting treatment and a reaction separately for skin lesions and neuritis (Fig. 3). The median treatment-to-skinlesion interval is 1.0 month (S.D. 18.4 months); for neuritis, 3.0 months (S.D. 17.8 months). Of patients developing skin reactions 25% had done so more than 1 month before seeking treatment, while 32% sought treatment at the time of new, acute symptoms. Of the 12 patients developing a skin reaction after starting treatment six did so within a month of starting treatment. A further five patients developed reactions more than 6 months after starting treatment, with one patient having a skin reaction 74 months after starting treatment.
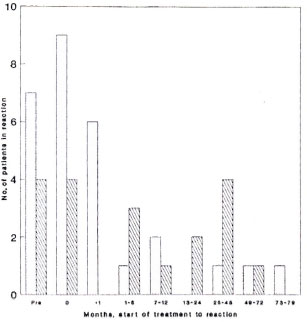
Fig. 3. Time intervals from start of treatment to development of reactional skin lesions and neuritis. Skin =  ; nerve =
; nerve =  . Median treatment-reaction time: skin = 1 mo. ± 18.4 mos; nerve = 3 mos. ± 17.8 mos; 0 = at presentation.
. Median treatment-reaction time: skin = 1 mo. ± 18.4 mos; nerve = 3 mos. ± 17.8 mos; 0 = at presentation.
The timing of neurological reactions in relation to treatment is shown in Figure 2; 21.1% of patients reported neurological signs in the 6 months before treatment was started, a further 21.1 % at the start of treatment, and 36.3% of patients developing neuritis on treatment did so in the first 7 months. The remaining patients developed neurological signs at a steady rate up to 50 months after the start of treatment. We found no difference in the timing of either skin or neurological reactions from the start of treatment between paucibacillary and multibacillary patients.
Seven patients (5 BT, 2 BL) developed reactions more than 24 months after starting treatment. Four patients had signs in both skin and nerve, three in skin alone. The BT patients had received either DDS monotherapy (N = 2, one irregularly) or DDS and rifampin. The BL patients had taken DDS and rifampin, one irregularly.
Treatment. Prednisolone was used to treat 63% of the patients, and all patients with any evidence of neuritis received steroids. The dosages used ranged from 10-30 mg daily (mean 15 mg daily). The examining clinician's notes were used to determine whether a lesion had improved or resolved. Skin lesions were more likely than neuritis to improve on treatment with improvement being noted in 93.1% of skin lesions and only 50% of neuritic episodes. The response of skin lesions also started quickly, with 38.1% improving at 1 month and 81.0% improving by 6 months. Complete resolution of skin lesions occurred in only 52.9% of the patients and had occurred within 6 months for 35.7% of this group; for 85.7%, by 12 months.
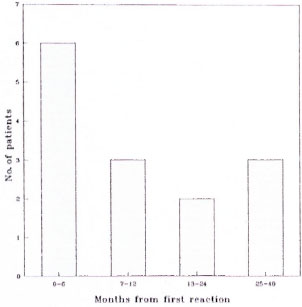
Fig. 4. Time intervals from initial reaction to next reactional episode. Median time to first recurrence = 9.0 mos. (S.D. ± 11.0 mos.).
Neurological improvement was deemed when there was either a recorded subjective improvement of symptoms or a discernable improvement on motor or sensory testing; 50% (N = 12) of the patients with neuritis had some neurological improvement, 5 patients were lost to follow up, and 7 did not improve. Comparing those who improved with those who did not showed no' differences in the type of disease, pattern of nerve involvement (single nerves versus multiple nerves), or dose of steroids used. The neuritis severity score was higher for improvers than for nonimprovers (7.25 vs 5.6). Reversal reactions had been confirmed histologically for three of the nonimprovers, and there was no deterioration in the Bis or clinical evidence of relapse in the other four. Thus, failure to improve was not due to undiagnosed relapsing disease. Neurological improvement occurred more slowly than skin improvement, with a mean time to improvement of 8.5 months. Complete resolution of neurological signs was rare, occurring in only two patients.
Recurrent reactions. Fourteen patients (6 paucibacillary, 8 multibacillary) had a further reaction, either worsening or new skin lesions, or worsening or new neuritis. Skin biopsies were done on six patients, and all biopsies confirmed the presence of either a reversal reaction or active disease; no evidence of relapsing disease was found. Seven patients had just one recurrence, and of the remaining 7 patients, 1 patient had two recurrences, 2 had three recurrences, 3 had four recurrences, and 1 had five recurrences. Recurrences occurred up to 40 months after the initial diagnosis of reversal reaction with the peak incidence during the first 6 months after the first reactional episode. Recurrences seemed equally likely to affect skin or nerve, with 50% of the patients having episodes of neuritis. There was some response to further courses of steroids; of 8 patients treated with steroids, 4 improved, 2 deteriorated and 2 remained static.
DISCUSSION
Although reversal reactions are a wellrecognized complication of leprosy, there are few published reports of their frequency and natural history (10,14). Our data should be interpreted with some caution since the study is retrospective and derived from a self-referred population rather than a population in a leprosy control area. This might tend to produce high figures for the incidence of reversal reactions, since patients in reaction may be prepared to travel to a clinic known to have an interest in nerve damage. The retrospective nature of the study also precluded us from studying possible etiological factors that may precipitate reactions.
Our data confirm previous observations (6,12) that borderline patients are most likely to develop reversal reactions, with 11.4% and 14.8% of BT and BL patients developing reactions compared with only 3.8% and 2.4% of TT and LL patients, respectively. However, it is important to note that TT and LL patients may develop reactions; the three LL patients presenting with reactional lesions were all histologically subpolar lepromatous (LLS).
Our findings emphasize the immunological instability of borderline patients, many of whom had developed reactions spontaneously. This instability is reflected in the comparison of clinical and histological findings where we found that 33% of the patients had a skin biopsy more tuberculoid than the clinical picture, suggesting that the patient was upgrading rapidly with the skin signs lagging behind the histological picture. It is striking that although 41.3% of our patients had presented in reaction yet, on questioning, they admitted that they had leprosy symptoms for several months. Probably many patients are prompted to seek treatment only when they develop reactional skin lesions, particularly facial lesions or neuritis. All patients were asked about previous treatment, and none of the patients presenting with leprosy for the first time and in reaction admitted to having taken prior treatment. In any future study it would be useful to check that no dapsone (DDS) had been taken recently by screening the urine for dapsone.
Like Naafs and Wheate (10) we feel that spontaneous reversal reactions are a feature of borderline disease, and are not necessarily a result of antileprosy treatment as has also been suggested (5). However, there is a clear association between the initiation of treatment and the development of reactions, with 50% of our patients developing reactional skin lesions within 1 month of starting treatment and a further 25% going into reaction within the first 12 months of treatment. Neurological reactions develop more slowly after starting treatment, with 36.3% of neuritic episodes developing within 7 months of treatment.
Boerrigter, et al. (2) found in Malawi that the risk of reversal reaction was much higher in self-reporting patients than in those patients found by active case finding. The self-reporting group probably has more advanced disease and a higher M. leprae antigen load.
It has been shown that reversal reactions are associated with an increase in lymphocyte responsiveness to M. leprae antigens (1), and it could be argued that spontaneous reactions result from macrophages destroying bacilli with liberation of M. leprae antigens. When treatment is started many more bacilli are destroyed with the resultant liberation of large antigen loads that can then provoke a cell-mediated immune (delaycdtype hypersensitivity) response. The destruction of leprosy bacilli will be greatest at the start of treatment, and so this is when reactions are most likely to occur.
Our data suggest that skin reactions occur earlier than nerve reactions. This might be because people notice skin damage sooner than nerve damage. Alternatively, it might be that Schwann cells are in relatively protected sites with slower killing of bacilli and later release of antigen, resulting in a later onset of reactions in nerves.
Although the majority of reactions occur around the time of initiation of treatment, wc found two skin and one nerve reactions developing more than 4 years after treatment was started (one BT, one BL patient). This reinforces the principle that when releasing patients from treatment it should be ensured that they recognize the symptoms of reactions and understand the need to return for assessment and treatment. Clinicians should certainly be aware that very late reactions may occur, and our longest treatment-reaction interval was 6 ½ yrs. Reversal reactions rarely are reported as being potentially recurrent events (4) yet 33% of our patients had repeated reactions. We would emphasize that reactions frequently recur and patients should be warned of possible recurrences.
It can be difficult to differentiate clinically between relapse and reaction (11,16). Ideally, a skin biopsy will be available to distinguish between the two conditions. In this study we have adopted a strategy of stringent clinical criteria coupled with independent scrutiny of the case notes and confirmation by appropriate Bis and histology to identify reactional patients. We do not think that those patients who did not respond to steroids were in relapse; there was no evidence from histology or serial, deteriorating Bis that they were relapsing. Furthermore, other studies have reported similar improvement rates on steroid therapy (7,9,17).
Only 20% of the patients in this study had both reactional skin lesions and neuritis. This is an important practical observation, for it means that one must look specifically for evidence of neuritis and not rely on the presence of reactional skin lesions as an indicator of potential neuritis. The vulnerability of the ulnar nerve to leprous neuritis in reversal reactions is well illustrated by our data. While this might be due to the exposed site of the ulnar nerve, it should be remembered that the ulnar and median nerves are the easiest nerves to examine. Perhaps a prospective study would show a higher incidence of involvement of other nerves (5).
Steroids have been used with moderate success to treat and ameliorate the nerve damage associated with reversal reactions (8,9). While the 1985 regimens used perhaps lower and shorter doses of steroids than would now be recommended, it is striking that while steroids aid resolution of skin lesions they are less effective in producing improvement and resolution of neurological damage. This serves to emphasize the need for new drugs and approaches to controlling reversal reactions.
Acknowledgment. DNJL is a Wellcome Trust research fellow. Dhoolpet Leprosy Research Center is managed by Victoria Hospital, Dichpalli, India, together with the Medical Research Council of Great Britain.
We would like to thank Dr. J. Keystone and Dr. M. F. R. Waters for helpful criticism of this manuscript.
REFERENCES
1. BARNETSON, R. ST.C, BJUNE, G. and PEARSON, J. M. H. Antigenic heterogeneity in patients with reactions in borderline leprosy. Br. Med. J. 4(1975)435-437.
2. BOERRIGTER, G., PONNIGHAUS, J. M. and FINE, P. E. M. Preliminary appraisal of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
3. GODAL, T., MYRVANG, T., SAMUEL, D. R., ROSS, W. F. and LOFGREN, M. Mechanism of "reactions" in borderline tuberculoid (BT) leprosy. Acta Pathol. Microbiol. Scand. [A] 236Suppl.(1973)45-53.
4. HOGEWEG, M., KIRAN, K. U. and SUNEETHA, S. The significance of facial patches and type 1 reaction for the development of facial nerve damage in leprosy; a retrospective study among 1226 paucibacillary leprosy patients. Lepr. Rev. 62(1991)143-149.
5. JOPLING, W. H. Any questions: dapsone in leprosy. Trop. Doc. 4(1974)140.
6. JOPLING, W. H. and MCDOUGALL, A. C. A Handbook of Leprosy. 3rd cdn. London: Heincmann, 1990.
7. KIRAN, K. U., STANLEY, J. N. A. and PEARSON, J. M. H. The outpatient treatment of nerve damage in patients with borderline leprosy using a semistandardized steroid regime. Lepr. Rev. 56(1985)127-134.
8. NAAFS, B., PEARSON, J. M. H. and BAAN, A. J. M. A follow up study of nerve lesions in leprosy during and after reactions using motot conduction velocity. Int. J. Lepr. 44(1976)188-197.
9. NAAFS, B., PEARSON, J. M. H. and WHEATE, H. W. Reversal reaction: the prevention of permanent nerve damage; comparison of short and long term steroid treatment. Int. J. Lepr. 47(1979)7-12.
10. NAAFS, B. and WHEATE, H. W. The time interval between the start of anti-leprosy treatment and the development of reactions in borderline patients. Lepr. Rev. 49(1978)153-157.
11. PANNIKAR, V., JESUDASAN, K., VIJAYAKUMARAN, P. and CHRISTIAN, M. Relapse or late reversal reaction? Int. J. Lepr. 57(1989)526-528.
12. RIDLEY, D. S. Reactions in leprosy. Lepr. Rev. 40(1969)77-81.
13. RIDLEY, D. S. and RADIA, K. B. The histological course of reactions in borderline leprosy and their outcome. Int. J. Lepr. 49(1981)383-391.
14. RIDLEY, D. S. and WATERS, M. F. R. Significance of variations within the lepromatous group. Lepr. Rev. 40(1969)143-152.
15. ROSE, P. and WATERS, M. F. R. Reversal reactions in leprosy and their management. Lepr. Rev. 62(1991)113-121.
16. SEHGAL, V. N. , BHATTACHARYA, S. N. and JAIN, S. Relapse or late reversal reaction? Int. J. Lepr. 58(1990)118-120.
17. TOUW-LANGENDIJK, E. M. J., BRANDSMA, J. W. and ANDERSEN, J. G. Treatment of ulnar and median nerve function loss in borderline leprosy. Lepr. Rev. 55(1984)41-46.
1. M.R.C.P.; Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E7HT, U.K.
2. F.R.C.P., Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E7HT, U.K.
3. M.B.B.S.; Dhoolpet Leprosy Research Center, Karwan, Hyderabad 500006, India.
4. M.B.B.S., Ph.D., Dhoolpet Leprosy Research Center, Karwan, Hyderabad 500006, India.
5. Ph.D., National Institute for Medical Research, The Ridgeway, Mill Hill, London NW7 1AA, U.K.
Received for publication on 7 April 1992.
Accepted for publication in revised form on 19 November 1992.