- Volume 61 , Number 3
- Page: 468–71
Electrocardiographic alterations in lepromatous leprosy patients with concomitant Trypanosoma cruzi infection
To the Editor:
Leprosy is endemic in Argentina and prevails mostly in the Littoral area, a region extending from the border with Paraguay in the north to the city of Buenos Aires in the south, where more than 30,000 persons are estimated to be infected with the disease (11). Coexisting with the leprosy problem, many people living in this area also are at risk of infection with Trypanosoma cruzi (Tc), the causative agent of American trypanosomiasis or Chagas' disease. Chagas' disease, whose acute infection is often asymptomatic and self-resolving, may result in chronic lesions with the heart as the main organ involved. Clinical manifestations of chronic chagasic myocardiopathy (CCM) appear years or decades after initial infection, and consist of cardiac enlargement with disturbances of cardiac rhythm and/or conduction (9).
Despite much effort, the pathogenesis of the chronic heart lesions remains incompletely elucidated, and several mechanisms have been put forward to reflect perhaps its multifactorial basis. Some authors state that lesions of intracardiac autonomic ganglia and neurons constitute an important factor in the generation of the heart disease (7). Others, instead, invoke autoimmune reactions as mainly responsible for the occurrence of chronic myocardial injury (13). More recently, microvascular abnormalities, resulting in local ischemia and focal pathological changes, also have been implicated in the genesis of the heart damage (15,17).
Since lepromatous leprosy (LL) is associated with autoantibody formation (1, 10), and may result in autonomic nerve dysfunction along with degenerative changes of striated muscle fibers (4, 5, 8), a study was undertaken to investigate whether Tc-infected LL patients show a different pattern of heart involvement.
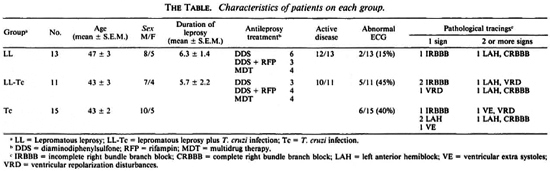
A sample of 39 individuals was studied. They were distributed as follows: 13 LL patients with no serologic evidence of Tc infection; 10 LL patients with positive Tc serology (LL-Tc); and 15 persons who yielded positive responses for the detection of specific anti-Tc antibodies (Tc). The subjects were matched for age and sex. Patients from both leprosy groups showed no major differences as to the duration of illness, antileprosy treatment, the extent of skin involvement, variety of lesions, and occurrence of type 2 reactional episodes (The Table). Except for two cases (one in group 1 and the other in group 2), all LL patients had active disease. The presence or absence of Tc infection relied on the firm evidence of positive or negative specific serology, respectively. Serology was performed by the direct agglutination, indirect hemagglutination, and indirect immunofluorescence techniques.
The subjects were carefully examined to rule out the existence of additional pathlogical disorders, particularly concomitant cardiomyopathies, i.e., congenital, rheumatic, and hypertensive. Upon them, a 12lead resting electrocardiogram (ECG) was taken. The data were recorded and interpreted by the same cardiologist blinded to the study groups.
The subjects did not have any cardiac complaint, and no significant differences in blood pressure levels were registered among the groups (data not shown). While an abnormal ECG was recorded more frequently in people with Tc infection, such a trend did not reach statistical significance when compared with LL patients, since two of them also had an altered ECG (The Table). Although electrocardiographic abnormalities were minimal in one of these cases, the other patient (a man with widespread neural involvement) had disturbances highly compatible with those recorded in CCM.
The prevalence and pattern of pathological ECG tracings in groups 2 and 3 were quite similar. No major differences as to the PR interval, heart rate and corrected QT interval were detected among the groups.
Whether Icpromatous leprosy is likely to produce some degree of heart involvement appears worth exploring given the potential implications it may carry for patients living in Latin American countries where Chagas' disease is endemic.
Lepromatous leprosy has long been recognized to be associated with many autoimmune-like serological aberrations, suggestive of faulty immunoregulation, and some clinical manifestations of anti-self reactions, such as those seen during erythemanodosum leprosum episodes(6, 10). In this regard, and as observed in chagasic individuals (2), a pattern of EVI antibody (an antibody reacting against myocardial structures, endothelium, vessels and interstitium) was recently detected in some lepromatous sera (unpublished observations).
From a different standpoint, several reports of altered autonomic nerve function have been described in lepromatous leprosy, i.e., impaired responses to Valsalva's maneuver among others (3). Such abnormality is also present among chagasic persons, and is strongly indicative of a disturbance of the autonomic control of the heart which may contribute to the generation of arrhythmias and myocardial contractile dysfunction (7, 12, 6).
The evidence recorded in this preliminary sample does not seem to support the possibility of lepromatous leprosy as an aggravating element in the outcome of chronic Chagas' disease. However, when considering that the lepromatous leprosy patient with serious neural compromise showed pathological ECG tracings quite similar to the ones registered in Chagas' disease (14), the question arises whether lepromatous leprosy in some instances might itself affect the heart tissue.
- Oscar A. Bottasso, M.D., Ph.D.
Julio C. Morini, M.D., Ph.D.
Division Ininunologia
Facultad de Ciencias Medicas
Universidad Nacional de Rosario
Santa Fe 3100
Rosario 2999, Argentina
- Guillermo Salerni, M.D.
Ricardo Arce, M.D.
Policlinico Intendente Carrasco de Rosario
Rosario, Argentina
Acknowledgment. The authors wish to acknowledge financial grant No. 3088900/88 from CONICET for the partial support of this work.
REFERENCES
1. CABRINI, J. M., BOTTASSO, O. A., MAROASIN, S., SCHUJMAN, L., MANGIATERRA, L. and MORINI, J. C. Biological false positive test for syphilis in lepromatous leprosy patients with concomitant hepatitis B virus infection. J. Invest. Allergol. Clin. Immunol. 1(1991)45-48.
2. Cossio, P. M., LAGUENS, R. P., DIEZ, C, SZARF-MAN, A., SEGAL, A. and ARANA, R. M. Chagasic cardiopathy: antibodies reacting with plasma membrane of striated muscle and endothelial cells. Circulation 50(1974)1252-1259.
3. DABHOLKAR, V. R. and GAITONDE, B. B. A study of autonomic functions in leprosy. Lepr. India 54(1982)303-317.
4. DASTUR, D. K. and DAVER, S. M. Striated muscle in four categories of leprosy. II. Fine structural changes. Int. J. Lepr. 48(1980)149-158.
5. DAVER, S. M., DASTUR, D. K., REVANKAR, C. R. and SHAH, J. S. Striated muscle in four categories of leprosy. I. Histology and histochemistry. Int. J. Lepr. 48(1980)140-148.
6. ESPITIA, C, SCIUTTO, E., BOTTASSO, O. A., GONZALEZ-AMARO, R., HERNANDEZ-PANDO, R. and MANCILLA, R. High antibody levels to the mycobacterial fibronectin-binding antigen of 3-31 kD in tuberculosis and lepromatous leprosy. Clin. Exp. Immunol. 87(1992)362-367.
7. KOBERLE, F. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv. Parasitol. 6(1968)63-116.
8. Kyriakidis, M. K., Noutsis, C. G., Robinson-Kyriakidis, C. A., Venetsianos, P. J., Vyssoulis, G. P., Toutouzas, P. C, Parissis, N. G. and AVGOUSTAK-IS, D. G. Autonomic neuropathy in leprosy. Int. J. Lepr. 51(1983)331-335.
9. LARANJA, F. S., DÍAS, E., NOBREGA, G. and MIRANDA, A. Chagas' disease; a clinical, epidemiological and pathologic study. Circulation 14(1956)1035-1060.
10. MÁSALA, C, AMENDOLEA, M. A., NUTI, M., RI-CARDUCCI, R., TARABINI, C. G. L. and TARABINI, C. G. Autoantibodies in leprosy. Int. J. Lepr. 47(1979)171-175.
11. MOTTA, C. P. The epidemiological situation in the Americas. Lepr. Rev. 52 Suppl. 1(1981)61-68.
12. OLIVEIRA, J. S. M. A natural human model of intrinsic heart nervous system denervation: Chagas' cardiopathy. Am. Heart. J. 110(1985)1092-1098.
13. PETRY, K. and EISEN, H. Chagas' disease: a model for the study of autoimmune diseases. Parasitol. Today 5(1989)111-116.
14. ROSENBAUM, M. Chagasic cardiomyopathy. Prog. Cardiovasc. Dis. 7(1964)199-225.
15. Rossi, M. A., GONÇALVES, S. and RIBEIRO-DOS-SANTOS, R. Experimental Trypanoso macruzi cardiomyopathy in BALB/c mice: the potential role of intravascular platelet aggregation in its genesis. Am. J. Pathol. 114(1984)209-216.
16. SOARES, J. D. and JUNQUEIRA, L. F., JR. Incidencia de arritmias associadas a manobra de Valsalva nas diversas formas clinicas da doenca de Chagas. Rev. Soc. Bras. Med. Trop. 20 Suppl. 11(1987)58.
17. TANOMITZ, H. B., BURNS, E. R., KUMAR SINHA, A., KAHN, N. N., MORRIS, S. A., FACTOR, S. M., HATCHER, V. B., BILEZIKIAN, J. P., BAUM, S. G. and WITTNER, M. Enhanced platelet adherence and aggregation in Chagas' disease: a potential pathogenic mechanism for cardiomyopathy. Am. J. Trop. Med. Hyg. 43(1990)274-281.