- Volume 61 , Number 1
- Page: 25–8
Fixed duration MDT in paucibacillary leprosy (classical and modified)
ABSTRACT
We analyzed the records of 1022 patients of paucibacillary leprosy who had received either 6 doses of WHO-MDT alone ("classical" MDT, 668 patients) or had post-MDT dapsone for at least 6 months ("modified" MDT, 354 patients). The duration of posttherapy surveillance ranged f rom 6 months to 7 years (mean 20.4 months). We found that the incidence of unfavorable events was significantly higher with the classical regimen when patients were graded as active at the end of the fixed duration regimen, especially when patients with > 2 lesions were considered. In the patients who were graded as inactive at the end of 6 doses, there was a slight excess of unfavorable events in the modified regimen, although not statistically significant. No correlation was found between unfavorable events and the regularity of treatment or the lepromin status. Overall, the incidence of adverse events was higher in patients with multiple lesions, and more than 90% of the adverse events occurred during the first 2 years of follow up. It is felt that 6 doses of MDT is adequate in the majority of patients who have few lesions or who have become inactive at the end of the treatment period. However, caution should be exercised in those with multiple lesions or in those considered active at the end of 6 doses.RÉSUMÉ
Nous avons analysé les dossiers de 1022 patients atteints de lèpre paucibacillaire qui avaient reçu soit 6 doses de PCT-OMS seule (PCT "classique." 668 patients), soit de la dapsone après la PCT pour au moins 6 mois (PCT "modifiée," 354 patients). La durée de la surveillance post-thérapeutique s'est étalée de 6 mois à 7 ans (moyenne 20,4 mois). Nous avons trouvé que l'incidence d'événements défavorables était SIGNIFICAtivement plus élevée avec le régime classique lorsque les patients étaient notés comme "actifs" à la lin du traitement de durée fixe, particulièrement en ce qui concerne les patients qui présentaient plus de deux lésions. Chez les patients notés comme "inactifs" après les 6 doses, il y avait un léger excès, bien que non statistiquement significatif, d'événements défavorables pour le régime modifié. Aucune correlation n'a été trouvée entre les événements défavorables et la régularité du traitement ou le statut à la lépromine. Au total, l'incidence d'événements défavorables était plus élevée chez les patients présentant des lésions multiples, et plus de 90% des événements défavorables sont survenus au cours des deux premières années de suivi. Il nous semble que 6 mois de PCT sont suffisants pour la majorité des patients qui présentent peu de lésions ou qui sont devenus inactifs à la fin de la période de traitement. Cependant, il faudrait être prudent pour les patients qui présentent des lésions multiples ou ceux considérés actifs à la fin des 6 doses.RESUMEN
Se analizaron los registros de 1022 pacientes con lepra paucibacilar que habían recibido 6 dosis de la poliquimioterapia recomendada por la OMS (PQT clásica, 668 pacientes), o la PQT seguida por al menos 6 meses de tratamiento con dapsona (PQT modificada, 345 pacientes). La duración de la vigilancia posterior a la terapia osciló de 6 meses a 7 años (media 20.4 meses). Encontramos que la incidencia de eventos desfavorables fue significativamente más elevada con la PQT clásica cuando los pacientes se clasificaron como activos al final del tiempo establecido de tratamiento, especialmente cuando se consideraron aquellos pacientes con más de 2 lesiones. En los pacientes clasificados como inactivos al final de las 6 dosis, hubo un ligero exceso de eventos desfavorables en el grupo con el tratamiento modificado, aunque éste no fue estadísticamente significativo. No se encontró correlación alguna entre eventos desfavorables y la regularidad del tratamiento o la reactividad a la lepromina. La incidencia global de efectos adversos fue más alta en los pacientes con lesiones múltiples, y más del 90% de los efectos adversos ocurrieron durante los primeros 2 años de seguimiento. Pensamos que 6 dosis de PQT es adecuada en la mayoría de los pacientes que tienen pocas lesiones o que han llegado a ser inactivos al final del periodo de tratamiento. Sin embargo, debe tenerse cuidado en aqucllos casos con lesioncs multiples o en aquellos considerados activos al final de las 6 dosis.Almost 10 years have elapsed since the introduction of the World Health Organization (WHO) recommended multidrug therapy (MDT) regimen for the paucibacillary (PB) form of leprosy (16). The WHO recommendation of an arbitrary 6-month period of treatment for all PB cases, although generally accepted (8,14), has drawn adverse comments from several workers (3,6,12), some of whom have suggested continuation of dapsone (DDS) with or without rifampin for either a limited period (6) or until the subsidence of all signs of activity (2).
In order to evaluate the efficacy of the "classical" MDT (as envisaged by the WHO) vis-a-vis a "modified" version of the same (6 doses of MDT followed by 6 months of dapsone alone), we compared the clinical outcome in patients given either of these regimens over a period of 8 years. Previously, we had reported a study of relapses in PB leprosy after MDT which was given for varying lengths of time with or without post-MDT dapsone, until clinical inactivity was reached (5).
MATERIALS AND METHODS
Our organization is a field-based, voluntary project functioning in the L and M municipal wards of Greater Bombay, India, with an estimated population of 1.4 million. We have been giving MDT according to the WHO guidelines since 1983. The duration of therapy is left to the discretion of the individual doctor with the proviso that at least 6 doses of MDT have to be completed. Monthly clinical assessments, random tablet counting, as well as urine tile testing for dapsone are done routinely to ensure compliance.
However, in a number of patients when there exists a doubt in the mind of the attending doctor about the activity status, we have a tendency to extend MDT. Even in cases where only 6 doses were administered, many subsequently have been given at least 6 months of dapsone alone, especially when potentially unfavorable factors existed (large or numerous lesions, nerve involvement, etc.).
After releasing the patients from treatment, every effort was made to assess the patients clinically at least every 6 months (with an annual bacteriological assessment) for a minimum period of 2 years.
At the end of 1990, the records of all patients put on fixed-duration (6 dose) MDT with or without post-MDT dapsone were analyzed. A record was made of the number of lesions (skin and nerves) at the beginning of the therapy. Regularity of treatment, lepromin status (if given), and status at the time of stopping treatment (active/inactive) also were recorded. The total number of patients with at least one 6-month assessment was 1022.
The mean duration of surveillance was 20.4 months (range 6 months to 7 years). Patients with only one 6-month assessment were 17.8% of the total; 36.8% of the patients were on surveillance for 1 year, 23.5% for 2 years, 12.6% for 3 years, 7.1% for 4 years, and the rest for more than 4 years.
The current status of each patient (active/ inactive, reversal reactions/relapse, stable/ deteriorated) was also noted. We did not attempt to differentiate between delayed reversal reaction and relapse since this is difficult under field conditions (11).
The criteria of activity (5,16) were: a) thickening or erythema of skin lesions, b) change in size or number of skin lesions, c) thickening and tenderness of nerves. Inactivity was defined as the absence of the above signs.
The criteria for relapse were predominantly clinical (7,9): a) extension, thickening, erythema or infiltration of the existing lesions or the appearance of new lesions suggestive of leprosy; b) thickened and tender nerves and new paralysis of muscles and c) bacteriological positivity. The last was not seen by us in any patient. Thus, we have compared retrospectively the outcome of the patients on the classical versus the modified therapy using these parameters.
RESULTS
Although we had fewer cases in the classical regimen, of the active patients, there was a significantly higher deterioration rate in this regimen (especially in those with more than two lesions) compared to patients treated with the modified regimen (Table 1).
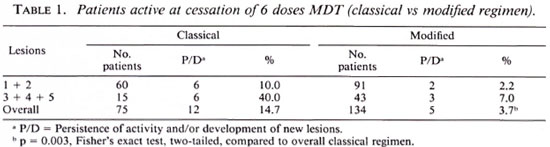
In the inactive group, we had a higher number of patients on the classical regimen, obviously due to the fact that in an "inactive" patient we felt justified in stopping all treatment even though intensive follow up was not always possible. Overall, we actually found a higher relapse rate in the modified group compared to the classical group, although this was not statistically significant (Table 2).
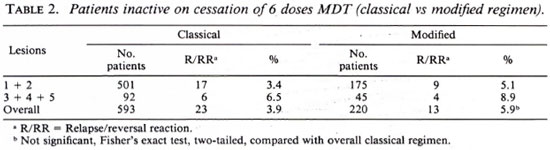
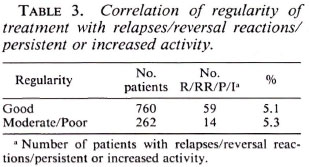
There was no correlation between regularity of treatment and unfavorable events such as relapses, reversal reactions, and persistent activity (Table 3). A similar lack of correlation was also found with lepromin status.
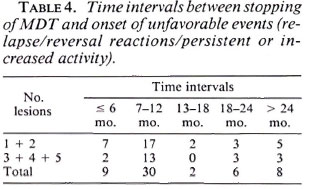
It can be seen that a majority of the unfavorable events occurred about 7-12 months after stopping MDT and more than 90% of these occurred in the first 2 years of follow up (Table 4).
DISCUSSION
At present, most of the controversies in the management of PB patients center on a) the duration of therapy and b) the role of post-MDT dapsone. In the present study, we compared the classical (6 dose MDT) with the modified regimen (post-MDT dapsone of at least an additional 6 months' duration). The assessment of improvement in PB cases is very subjective. Therefore, the criterion employed was the occurrence of unfavorable events such as persistence of activity and development of new lesions. We found that these were more with the classical regimen, especially when patients with multiple lesions were considered. This was true, however, in only those patients who were graded "active" at the end of 6 doses of MDT. In the case of inactive patients, we actually found a higher rate of reversal reactions/relapses in the modified group. It is interesting to speculate whether post-MDT dapsone actually precipitates (delayed) reversal reactions.
We found that most of the unfavorable events occurred in the first 2 years of follow up, which only shows the importance of the mandatory surveillance period (1,4,10).
Interestingly, we did not find any correlation between regularity of treatment and adverse events (9,13); a similar negative correlation was found with lepromin also (7).
In conclusion, we would recommend that the fixed-duration chemotherapy be followed in those patients with few lesions and especially when patients are inactive at the end of 6 doses. In cases with multiple lesions and in patients with active disease at the end of 6 doses of MDT, it is better to be cautious and to give either a reasonable course of post-MDT dapsone or continue MDT until the point of clinical inactivity. We are conscious, however, that the final word on this issue has not yet been said and further research, especially under field conditions, is certainly needed.
REFERENCES
1. BOURLAND, J., JANSSENS, L., GROENEN, G., PATTYN, S. R. and THE COLLABORATIVE STUDY GROUP ON THE TREATMENT OF LEPROSY. Incubation time of relapses after treatment of paucibacillary leprosy. (Abstract) Int. J. Lepr. 52(1984)686.
2. DHARMENDRA. Editorial comment in correspondence. Indian J. Lepr. 57(1985) 227.
3. DHIR, R., GUHA, P. K. and SINGH, G. Short term chemotherapy of paucibacillary leprosy. Indian J. Lepr. 58(1986)549-554.
4. EKAMBARAM, V. Duration of treatment for disease arrest of non-Iepromatous cases and relapse rates in these patients. Lepr. Rev. 50(1979)297-302.
5. GRUGNI, A., NADKARNI, N. J., KINI, M. S. and MEIITA, V. R. Relapses in paucibacillary leprosy after MDT-a clinical study. Int. J. Lepr. 58(1990)19-24.
6. KATOCII, K., RAMANATHAN, U., NATARAJAN, M., BAGOA. A. I., BHATIA, A. S., SAXENA, R. K. and RAMU, G. Relapses in paucibacillary patients after treatment with three short term regimens containing rifampin. Int. J. Leper. 57(1989)458-464.
7. KATOCH, K., RAMU, G. and RAMANATHAN, U. Short term WHO advised multiple drug treatment of paucibacillary patients (Letter). Indian J. Lepr. 58(1986)351-353.
8. KUMAR, B. and KAUR, I. Short term combination therapy for paucibacillary leprosy -histological evaluation and follow-up study. Indian J. Lepr. 59(1987)54-62.
9. PANDYAN, T. D., SEETHAMHARAM, M., BHARATHI, R. and RAMU, G. A study of relapse in non-Iepromatous and intermediate group of leprosy. Indian J. Lepr. 57(1985)49-58.
10. PATTYN, S. R. Incubation time for relapses after treatment for paucibacillary leprosy. Lepr. Rev. 55(1984)115-120.
11. PATTYN, S. R., GROENEN, G., BOURLAND, J., GRILLONE, S. and JANSSENS, J. L. and THE COLLABORATIVE STUDY GROUP FOR THE TREATMENT OF LEPROSY IN ZAIRE AND RWANDA. A controlled therapeutic trial in paucibacillary leprosy comparing a single dose of rifampicin followed by one year of daily dapsone with 10 weekly doses of rifampicin. Lepr . Rev. 58(1987)349-358.
12. RAMU, G. and GIRDHAR. B. K. WHO chemothcrapeutic regimen for paucibacillary' cases. (Letter) Indian J. Lepr. 57(1985)226-228.
13. RANGARAJ, M. and RANGARAJ, J. Experience with multidrug therapy in Sierra Leone: clinical, operational and managerial analysis. Lepr. Rev. 57 Suppl. 3(1985)77-91.
14. REVANKAR, C. R., KARLIKAR, U. G., GURAV, V. J. and GANAPATI, R. Clinical assessment of paucibacillary leprosy under multidrug therapy-three years follow-up. Indian J. Lepr. 61(1989)355-357.
15. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
16. WORLD HEALTH ORGANIZATION. A guide to leprosy control. 2nd cdn. Geneva: World Health Organization, 1988.
1. M.D.; Lok Seva Sangam, D-1, Everard Nagar, Sion-Tromby Road, Bombay 400022, India.
2. M.D., Medical Superintendent; Lok Seva Sangam, D-1, Everard Nagar, Sion-Tromby Road, Bombay 400022, India.
3. M.B.B.S., Lok Seva Sangam, D-1, Everard Nagar, Sion-Tromby Road, Bombay 400022, India.
Reprint requests to Dr. Grugni.
Received for publication on 25 March 1992.
Accepted for publication in revised form on 26 October 1992.