- Volume 61 , Number 1
- Page: 29–34
Effects of chemotherapy on antibody levels directed against PGL-I and 85A and 85B protein antigens in lepromatous patients
ABSTRACT
IgG antibodies against antigens 85A and 85B f rom Mycobacterium bovis BCG, IgM antibodies against phenolic glycolipid-I (PGL-I) and circulating PGL-I antigen were measured in the serum of 11 patients with lepromatous leprosy receiving multidrug therapy (MDT). Before treatment, 6 patients were reactive to antigen 85A, 10 patients to antigen 85B, and 11 patients to PGL-I; circulating PGL-I was detected in the sera of all of them. After 2 years of MDT PGL-I antigen could no longer be detected in all of the patients, except for two who were not compliant with treatment. IgG antibodies directed against the 85A and 85B antigens and IgM antibodies against the PGL-I antigen also decreased significantly during treatment but more slowly. The determination of circulating PGL-I antigen remains the most appropriate tool for monitoring lepromatous leprosy under MDT.RÉSUMÉ
Les anticorps IgG vis-à-vis des antigènes 85A et 85B du BCG à base de Mycobacterium bovis, les anticorps IgM vis-à-vis du glycolipide phenolique-I (PGL-I) et l'antigène PGL-I circulant ont été mesurés dans le serum de 11 patients atteints de lèpre lépromatcuse recevant une polychimiotherapic (PCT). Avant traitement, 6 patients réagissaient à l'antigène 85A, 10 patients à l'antigène 85B, et 11 patients au PGL-I; du PGL-I circulant a été détecté dans le scrum de chacun d'eux. Après deux ans de PCT, l'antigène PGL-I ne pouvait plus être détecté chez les patients, à l'exception de deux, qui ne suivaient pas leur traitement régulièrement. Les anticorps IgG dirigés contre les antigènes 85A et 85B et les anticorps IgM dirigés contre l'antigène PGL-I ont aussi diminué progressivement en cours de traitement, mais plus lentement. La détermination de l'antigène PGL-I circulant reste l'outil le plus approprié pour la surveillance de la lèpre lépromatcuse traitée par PCT.RESUMEN
Se midieron los niveles de anticuerpos IgG contra los antígenos 85A y 85B del Mycobacterium bovis BCG, los niveles de anticuerpos IgM contra el glicolípido fenólico (GLF-I), y los niveles de GLF-I circulante, en el suero de 11 pacientes con lepra lepromatosa tratados con poliquimioterapia (PQT). Antes del tratamiento, 6 pacientes fueron reactivos con el antígeno 8 5A, 10 pacientes con el antígeno 85B, y 11 pacientes con el GLF-I. En todos los pacientes se encontró GLF-I circulante. Después de 2 años de tratamiento con PQT ya no se encontró GLF-I en el suero de ninguno de los pacientes, excepto en dos casos que no fueron constantes en su tratamiento. Los anticuerpos IgG dirigidos contra los antígenos 85A y 85B y los anticuerpos IgM contra el GLF-I, también disminuyeron significativamente durante el tratamiento pero lo hicieron más lentamente. La determinación del GLF-I circulante es la forma más apropiada para seguir la evolución de la enfermedad en los pacientes lepromatosos en tratamiento con PQT.The lepromatous forms of leprosy are characterized by an important mycobacterial load, by a low specific cellular immune response, and by a high production of specific antibodies which do not seem to have any protective role (2,18).
Serology has been used for disease diagnosis as well as for monitoring response to treatment of lepromatous leprosy. Most of the serological assays have involved the use of phenolic glycolipid-I (PGL-I) or its synthetic glycoconjugatcs, whole Mycobacterium M. leprae sonicates, the 35-kDa or the 36-kDa protein antigens of M. leprae (3-7,10,15,16,19,21,22).
An alternative approach has been based on the utilization of M. bovis or M. tuberculosis antigens since M. leprae has many antigens in common with these two species. These antigens include the secreted and fibroncctin-binding antigen 85 complex (1,8) consisting of three proteins: 85A (32 kDa), 85B (30 kDa) and 85C (33 kDa) (25). Recently, high IgG antibody levels against component 85A and especially against component 85B of this complex were described in lepromatous leprosy (12,13,17,23).
The aim of the present study was to compare in lepromatous leprosy, during a 2-year course of multidrug therapy, the evolution of IgM antibody reactivity against PGL-I (ELISA) and of IgG humoral response against the 85A and 85B components of the BCG 85 complex separated by isoelectric focusing. PGL-I antigen was also quantified in the serum during this treatment.
MATERIALS AND METHODS
Patients. Nine patients with polar lepromatous leprosy (B to J) and two with borderline lepromatous leprosy (A and K) were diagnosed in French Polynesia and classified according to the Ridley-Jopling (18) classification following histopathological analysis of biopsy specimens. The number of acid-fast bacilli (AFB) per mg of skin tissue was determined by homogenization of the samples (Prof. J. Grosset, Hôpital Pitié-Salpêtrière, Paris, France).
Sera from the patients were collected on admission and sequentially during the 2-year period of therapy and were stored at -20ºC until use.
Since 1982, a multidrug therapy has been implemented in French Polynesia. For multibacillary patients, it consists of a daily administration for 24 months of dapsone (DDS; 100 mg) and rifampin (10 mg/kg) with a daily supplement of 100 mg of clofazimine during the first 12 months and 5 mg/kg of prothionamide also during the first 2 months. In this regimen, rifampin was given daily instead of monthly as recommended by the World Health Organization (WHO).
Eight patients adequately followed their treatment; three patients did not. Patient G stopped his treatment after 17 months; patient H, after 12 months, interrupted his therapy for 10 months and was again treated for 6 months; patient J stopped chemotherapy after 6 months because of developmcnt of hepatitis 13. After 2 months of interruption, rifampin was given monthly. The occurrence of erythema nodosum lcprosum (ENL) was noted in five patients (A, C, D, F and I) during multidrug therapy.
Isoelectric focusing and Western blot analysis. To separate the three components of antigen 85, vertical nondenaturing isoelectric focusing (IEF) was performed in a 2.5 to 6.5 pH gradient as previously described (12,23,24). The proteins were next electrophoretically transferred onto nitrocellulose sheets (0.2 µm; Bio-Rad Laboratories, Richmond, California, U.S.A.). The nitrocellulose strips were incubated overnight with serial twofold dilutions of sera diluted in TBS-0.05% Tween (TBS-T), washed in TBS-T and incubated for another 4 hr with antihuman IgG, IgA or IgM peroxidase-conjugatcd rabbit immunoglobulins (Dakopatts, Glostrup, Denmark) diluted 1/500. After rinsing in TBS-T, staining was performed by the addition of peroxidase substrate (Bio-Rad) containing alphachloronaphthol in the presence of hydrogen peroxide.
The reactivity of the sera against the antigen 85 complex was tested twice. Positive and negative control sera were included in each run.
The degree of staining of each of the antigen 85 components was quantified using a video densitometer (Model 620; Bio-Rad) and integrated reflectance values, expressed in color yield units, were determined for each peak (12). The titer of each tested serum was defined as the reciprocal of the highest dilution giving a reflectance value higher than 0.1.
Taking as the cut-off level a titer of 100 when measuring the reactivity against the 85B component, the specificity calculated in a control group of 153 healthy subjects was 99.3%. For antigen 85A, a titer of 200 led to a 98% specificity. These healthy subjects were from leprosy-endemic areas and included contacts of leprosy patients.
Anti-PGL-I IgM by ELISA. IgM anti-PGL-I antibodies were quantitated by ELISA as previously described (3). Briefly, the antigen used was the semi-synthetic natural trisaccharide 3-p-hydroxy-phenylpropionate (NTP) coupled to bovine serum albumin (Lot XI-66-860717; Fuji, Nara, Japan) (14). Nonspecific binding of the sera was measured on control wells not coated with antigen, and all of the sera were tested in duplicate. After washing, specific IgM was detected by goat antihuman IgM peroxidase conjugate (BIOSYS, Compiegne, France). The reaction was developed when the chromogenic substrate orthophenylcthylene-diamine (OPD; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) was added with H2O2 ; solution at 0.013% final concentration. Automatic optical density (OD) readings and calculations were performed by a micro-ELISA reader (Titertek; Flow Laboratories, Helsinki, Finland). The results are expressed as the difference in OD between the antigen-coated and control wells. The cut-off level of the test, determined on 396 healthy Polynesian blood donors, was OD = 0.200 (mean OD + 2 S.D.) at a 1/250 dilution of the sera. The serum titer was calculated from the reciprocal of the highest dilution giving an OD higher than 0.200.
Semiquantitative PGL-I detection by DOT-ELISA. The PGL-I was extracted from 500 µl of lyophilizcd serum and quantitated by DOT-ELISA according to a protocol already described (3). Each scries of extractions included a control made of normal scrum spiked with a known amount of PGL-I (the reference PGL-I and anti-PGL-I hyperimmune rabbit serum were kindly supplied by Prof. P. J. Brennan, Colorado State University, Fort Collins, Colorado, U. S.A.). 4-Chloronaphthol 0.05% containing H2O2 0.009% was used as substrate. A positive result appears as a blue-purple dot after 20 min at 37ºC. The last concentration or dilution giving a positive result was noted, and serial dilutions of purified PGL-I were used as standards. The results were expressed in nanograms of PGL-I per ml of serum. As little as 6 ng of PGL-I/ml could be detected in this assay (3), but sera were considered positive at a cut-off of 12 ng/ml, resulting in 100% specificity as determined on sera from 85 Polynesian blood donors.
Statistical analysis. Since the sample size was small, nonparametric statistical methods were used to analyze the results (20).
RESULTS
IgG antibodies directed against antigens 85A and 85B and IgM antibodies against PGL-I are shown in The Table. Taking as the cut-off value a titer of 200 for the anti85A IgG response, 6 of 11 patients had a positive value before treatment. For anti853 IgG antibodies, a cut-off value for positivity was set at 100 and 10 of 11 patients were found to be positive. When testing the IgM anti-PGL-I response (cut-off antibody titer of 250), 11 of 11 patients were positive.
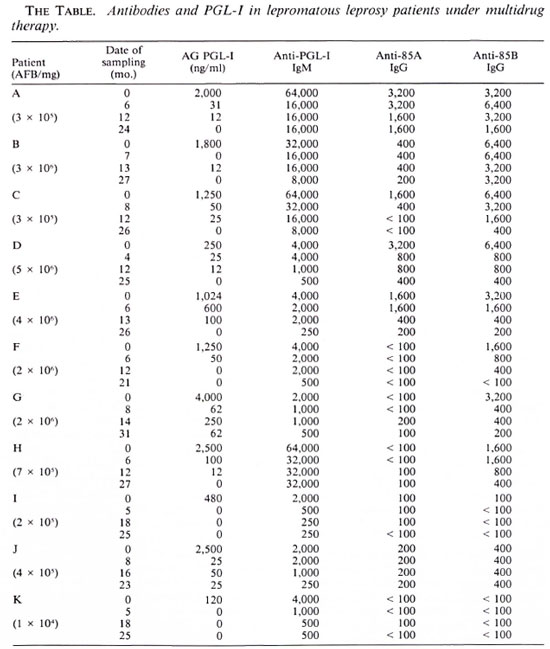
Antibody levels decreased during treatment (mean fourfold decrease for anti-85B IgG and anti-PGL-I IgM, mean twofold decrease for the anti-85A IgG) but remained higher in most of the patients than the cutoff values at the end of the treatment: respectively, in 4 of the 6 patients positive for anti-85A IgG at onset of chemotherapy, in 8 of the 10 for anti-85B IgG, and in 11 of the 11 for anti-PGL-I IgM. IgA antibodies directed against antigen 85A or 85B were too low to be quantified. High IgM responses directed against the 85A and the 85B components were demonstrated in only four patients, and these responses were not affected by the treatment. Since a high IgGspccific response was measured in the same four patients, we conclude that IgM studies did not augment the sensitivity of our test.
Circulating PGL-I antigen was found in all of the patients at the beginning of treatment. PGL-I levels decreased sharply during treatment and in 9 of 11 patients circulating PGL-I had become undetectable at the end of 2 years of treatment. After 2 years, persistent circulating PGL-I (> 12 ng/ml) was still detected in sera from two patients who were not compliant with their therapy (patients G and J).
Circulating PGL-I and antibody titers were significantly different before and at the end of therapy as determined using the Wilcoxon test: circulating PGL-I, p < 0.01 ; IgM anti-PGL-I, p < 0.01; IgG anti-85A, p < 0.05; IgG anti-85B, p < 0.01.
There was no significant correlation between circulating PGL-I and IgG anti-85A or -85B (Ag PGL-I/IgG anti-85A, r' = 0.17, p = 0.6; Ag PGL-I/IgG, r' = 0.14, p = 0.7) or between IgM anti-PGL-I and IgG anti85 A or -85B (IgM anti-PGL-I/IgG anti-85A, r' = 0.16, p = 0.6; IgM anti-PGL-I/IgG anti85B, r = 0.47, p = 0.14) as determined by Spearman's correlation coefficient.
DISCUSSION
Many authors, including us, have previously investigated the serologic monitoring of lepromatous leprosy patients during chemotherapy (3,5,6,10,15,16,19,21,22). IgM antibodies against PGL-I have generally been found to be the most reliable parameter.
In this study, we have compared the humoral response against the 85A and 85B antigens of M. bovis BCG and a synthetic analog of PGL-I (NTP) during a 2-year multidrug therapy of 11 lepromatous leprosy patients. The treatment comprised daily administration of rifampin. Circulating PGL-I antigen was also monitored in these patients. At the beginning of therapy, significant antibody levels to PGL-I, 85A and 85B could be detected in 11 of 11, 6 of 11 and 10 of 11 patients, respectively. Antibody titers decreased significantly after 2 years of treatment for the three antigens used.
Although diminished after 2 years, anti-PGL-I titers remained higher in this study than those observed in the control group of 396 healthy blood donors.
A longer follow up of our patients would probably have shown further reduction of their antibody titers down to negativity (5,6) but, on the other hand, it must be noted that persistently elevated titers of IgM anti-PGL-I were previously described after 5 years of chemotherapy (6). Confirming previous findings (5,15), no differences were observed in the pattern of decrease in antibody in patients with ENL as compared to the other lepromatous patients' reactions.
IgG antibodies directed against the 85B component of the BCG antigen 85 complex have recently been shown to be associated with the active forms of tuberculosis (17,23,24) and with the lepromatous forms of leprosy (12,13,17,23). Antigen 85B seems to be secreted more abundantly by the virulent M. bovis and M. tuberculosis strains (11) than the attenuated M. bovis BCG strain, and probably shares B-cell epitopes with the 85B antigen of M. leprae. An 84.5% homology between the M. leprae and M. bovis BCG antigen 85B mature proteins was recently reported (9).
In this study, 10 of 11 patients showed significantly elevated IgG antibody titers directed against the 85B component at the onset of treatment. The only patient [patient K (BL)] who had no IgG antibodies against the 85B component was found to have the lowest number of AFB/mg in his biopsy material. In tuberculosis patients anti-85B antibody levels evolve in parallel with disease activity and decrease at the end of therapy (23). We observed a similar phenomenon in the present study when following lepromatous leprosy patients. The mean fourfold decrease of anti-85B titer was comparable to the one observed for anti-PGL-I IgM antibodies.
Circulating PGL-I antigen was present in all sera at the beginning of therapy, and decreased to detection limits or completely disappeared in 7 of 11 patients after 1 year of treatment. After 2 years of therapy, only two patients demonstrated a substantial level of free PGL-I (patients G and J). Interestingly, these two patients had interrupted their medication. High PGL-I antigen concentration at the beginning of treatment was usually associated with slower antigen decrease.
In conclusion, the follow-up study of these 11 patients who evolved favorably under multidrug therapy showed a significant decrease of IgG antibody levels directed against the antigen 85B from M. bovis BCG similar to the decrease in IgM anti-PGL-I antibodies. Although antibody detection tests are easier to perform, circulating PGL-I antigen detection remains the most appropriate tool for monitoring lepromatous leprosy under multidrug therapy.
Acknowledgment. This work was supported by the National Fund for Scientific Research (Belgium), Damiaanaktic Brussels, the Fondation Erasme, and the Fondation Raoul Follcrcau. Wc thank Prof. P. J. Brcnnan for providing PGL-I antigen and antiserum through funds from NIAID (contract no. I-AI-52582) and Dr. T. Fujiwara for providing NTP antigen.
REFERNCES
1. ABOU-ZEID., C, RATLIFF, T. L., WIKER, H. G., HARBOE. M., BENNEDSEN, J. and ROOK, G. A. W. Characterisation of fibronectin binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect. Immun. 56(1988)3046-3051.
2. BOTHAMLEY, G., BECK, J. S., BRITTON, W., EL-SAGHIER, A. and I VANYI, J. Antibodies to Mycobacterium tuberculosis-specific epitopes in lepromatous leprosy. Clin. Exp. Immunol. 86(1991)426-432.
3. CHANTEAU, S., CARTEL, J. L., CELERIER, P., PLI-CHART, R., DESFORGES, S. and Roux, J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int. J. Lepr. 57(1989)735-743.
4. CHANTEAU, S., CARTEL, J.-L., ROUX, J., PLICHART, R. and BACH, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid 1 in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-776.
5. CHATURVEDI, V., SINHA, S., GHIRDHAR, B. K. and SENGUPTA, U. On the value of sequential serology with a Mycobacterium leprae-speciñe antibody competition ELISA in monitoring leprosy chemotherapy. Int. J. Lepr. 59(1991)32-40.
6. CHO, S.-N., CELLONA, R. V., FAJARDO, T. T., AIIALOS, R. M., DELA CRUZ, E. C, WALSH, G. P., KIM, J. D. and HRENNAN, P. J. Detection of phenolic glycolipid-I antigen and antibody in sera from new and relapsed lepromatous patients treated with various drug regimens. Int. J. Lepr. 59(1991)25-31.
7. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
8. CLOSS, O., HARBOE, M., AXELSEN-CHRISTENSEN, N. H. and MAGNUSSEN, J. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophorcsis: a reference system. Scand. J. Immunol. 12(1980)249-263.
9. DE MENDONCA LIMA, L., CONTENT, J., VAN HEUVERSWYN, H. and DEGRAVE, W . Nucleotide sequence of the gene coding for the 85-B antigen of Mycobacterium leprae. Nucl. Acids Res. 19(1991)789.
10. DOUGLAS, J. T., STEVEN, L. M., FAJARDO, T., CELLONA, R. V., MADARANG, M. G., ABALOS, R. M. and STEENBERGEN, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
11. DROWART, A., DE BRUYN, J., HUYGEN, K., DAMIANI, G., GODFREY, H., STELANDRE, M., YERNAULT, J. C. and VAN VOOREN, J. P. Isoelcctrophorctic characterization of protein antigens present in mycobacterial culture filtrates and recognized by monoclonal antibodies directed against the Mycobacterium bovis BCG antigen 85 complex. Scand. J. Immunol. 36(1992)697-703.
12. DROWART, A., LAUNOIS, P., DE COCK, M., HUYGEN, K., DE BRUYN, J., YERNAULT, J. C. and VAN VOOREN, J. P. An isoelectric focusing method for the study of the humoral response against the antigen 85 complex of Mycobacterium bovis BCG in the different forms of leprosy. J. Immunol. Methods 145(1991)223-228.
13. ESPITIA, C, SCIUTTO, E., BOTTASSO, O., GONZALEZ-AMARO, R., HERNANDEZ-PANDO, R. and MANCILLA, R. High antibody levels to the mycobacterial fibroncctin-binding antigen of 30-31 kD in tuberculosis and lepromatous leprosy. Clin. Exp. Immunol. 87(1992)362-367.
14. FUJIWARA, T. and IZUMI, S. Synthesis is of the ncoglycoconjugate of phenolic glycolipid related trisaccharides for the serodiagnosis of leprosy. Agricul. Biol. Chem. 51(1987)2539-2545.
15. GELBER, R. H., LI, F., CHO, S.-N., BYRD, S., RA -JAGOPALAN, K. and BRENNAN, P. J. Serum antibodies to defined carbohydrate antigens during the course of treated leprosy. Int. J. Lepr. 57(1989)744-751.
16. KLATSER, P. R., DE WIT, M. Y. L., FAJARDO, T. T., CELLONA, R. V., ABALOS. R. M., DELA CRUZ, E. C, MADARANG, M. G., HIRSCH, D. S. and DOUGLAS, J. T. Evaluation of Mycobacterium leprae antigens in the monitoring of a dapsonc-bascd chemotherapy of previously untreated lepromatous patients in Ccbu, Philippines. Lepr. Rev. 60(1989)178-186.
17. PESSOLANI, M. C, PERALTA, J. M., RUMJANEK, F. D., GOMES, H. G., DE MELO MARQUES, M. A., ALMEIDA, E. C. C, SAAD, M. H. F. and SARNO, H. N. Serology leprosy: immunoassays comparing immunoglobulin G antibody responses to 28 and 30 kilodalton proteins purified from Mycobacterium bovis BCG. J. Clin. Microbiol. 29(1991)2285-2290.
18. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
19. ROCHE, P. W., BRITTON, W. J., NEUPANE, K., FAILBUS, S. S., CHO, S.-N. and THEUVENET, W. J. The response to chemotherapy of serum Mycobacterium leprae -specific antigen in multibacillary leprosy patients. Am. J. Trop. Hyg. 44(1991)702-708.
20. SIEGEL, S. Nonparametric Statistics for Behavioral Sciences. New York: McGraw Hill Inc., 1956.
21. SINHA, S., MCENTEGART, A., GIRDHAR, B. K., BHATIA, A. S. and SENGUPTA, U. Appraisal of two Mycobacterium leprac-spcc'xfxc serological assays for monitoring chemotherapy in lepromatous (LL/ BL) leprosy patients. Int. J. Lepr. 57(1989)24-32.
22. VAISHNAVI, C, GANGULY, N. K., SHARMA, V. K., KAUR, H. and KAUR, S. Effect of multidrug therapy on the levels of antibodies to Mycobacterium leprae GLYCOLIPID-1 in the leprosy spectrum. Indian J. Lepr. 60(1988)530-534.
23. VAN VOOREN, J. P., DROWART, A., DE BRUYN, J., LAUNOIS, P., MILLAN, J., DELAPORTE, E., DEVELOUX, M., YERNAULT, J. C. and HUYGEN, K. Humoral immune response against the 85A and 85B antigens of Mycobacterium bovis BCG in patients with leprosy and tuberculosis. J. Clin. Microbiol. 30(1992)1608-1610.
24. VAN VOOREN, J. P., DROWART, A., DE COCK, M., VAN ONCKELEN, A., D'HOOP, M. H., YERNAULT, J. C, VALCKE, C. and HUYGEN, K. Humoral immune response of tuberculosis patients against the three components of the Mycobacterium bovis BCG complex separated by isoelectric focusing. J. Clin. Microbiol. 29(1991)2348-2350.
25. WIKER, H. G., HARBOE, M., NAGAI, S. and BENNEDSEN, J. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am. Rev. Respir. Dis. 141(1990)830-838.
1. M.D.; Chest Department, Hôpital Erasme, 808 Rte de Lennik, 1070 Brussels, Belgium.
2. Tech. Asst.; Chest Department, Hôpital Erasme, 808 Rte de Lennik, 1070 Brussels, Belgium.
3. Ph.D., Chest Department, Hôpital Erasme, 808 Rte de Lennik, 1070 Brussels, Belgium.
4. Ph.D., Chest Department, Hôpital Erasme, 808 Rte de Lennik, 1070 Brussels, Belgium.
5. Ph.D.; Institut de echerches Médicales Louis Malardé, Tahiti, French Polynesia.
6. M.D., Institut de echerches Médicales Louis Malardé, Tahiti, French Polynesia.
7. Ph.D.; Institut Pasteur van Brabant, Brussels, Belgium.
8. M.Sc., Institut Pasteur van Brabant, Brussels, Belgium.
9. Ph.D., Institut Pasteur de Dakar, Senegal.
Reprint requests to Dr. Van Vooren.
Received for publication on 4 August 1992.
Accepted for publication in revised form on 17 November 1992.