- Volume 61 , Number 1
- Page: 35–43
Serological monitoring of the response to chemotherapy in leprosy patients
ABSTRACT
Sixty-five patients initially seropositive for IgM anti-phenolic glycolipid-I (PGL-I) antibodies were tested for antibody levels to PGL-I, lipoarabinomannan (LAM), and the 35-kDa protein of Mycobacterium leprae at regular intervals for up to 30 months following the commencement of multidrug therapy (MDT). There was a steady decline in IgM anti-PGL-I and anti-35-kDa antibody levels to a mean of 17% and 14%, respectively, of the starting level at 24 months. The development of type 1 and type 2 reactions or the presence of drugresistant organisms in a small number of patients had no significant influence on the changes in antibody level. The rate of decline was similar in different disease categories, but a higher proportion of lepromatous patients remained seropositive at the end of 2 years of treatment than borderline tuberculoid patients. By contrast, the mean IgG anti-LAM antibody levels remained stable or increased. Again the occurrence of type 1 or type 2 reactions had no significant effect on antibody level over 2 years. Falls in the IgM anti-PGL-I antibody levels mirrored the falls in the bacterial index in individual patients and provide an additional parameter for monitoring the response to chemotherapy.RÉSUMÉ
On a dosé à intervalles réguliers jusqu'à 30 mois après le début de la polychimiothérapie (PCT), chez soixante-cinq patients originellement séropositifs pour les IgM anti-glycolipide phénoliquc-I (PGL-I), les taux d'anticorps vis-à-vis du PGL-I, lipoarabinomannan (LAM) et la protéine de 35-kDa de Mycobacteriuym leprae. Il y a eu une diminution régulière des taux d'IgM anti-PGL-I et anti-35-kDa jusqu'à atteindre à 24 mois des taux moyens respectifs de 17% et 14% des taux de départ. Le développement de réactions de type 1 et type 2 ou la présence d'organismes résistants chez un petit nombre de patients n'a pas eu d'influence significative sur les modifications des taux d'anticorps. Le taux de diminution des anticorps était semblable pour les différents types de la maladie, mais une proportion plus grande de patients lépromatcux que de patients borderline tuberculoides sont restés séropositifs à la fin des deux ans de traitement. Par contraste, la moyenne des taux d'anticorps IgG anti-LAM est restée stable ou a augmenté. A nouveau, la survenue de réactions de type 1 ou 2 n'a pas eu d'effet significatif sur le taux d'anticorps après deux ans. Les chutes des taux d'anticorps IgM anti-PGL-I reflétaient la chute de l'indice bactérien chez les patients individuels et fournit un paramètre supplémentaire pour la surveillance de la réponse à la chimiothérapie.RESUMEN
Se midieron los niveles de anticuerpos anti-GLF-I, anticuerpos anti-lipoarabinomanana, y anticuerpos contra la proteína de 35 kDa del Mycobacterium leprae, en sesenta y cinco pacientes inicialmente seropositivos para anticuerpos IgM anti-glicolípido fenólico-I (GLF1). El estudio se hizo a intervalos regulares durante los 30 meses siguientes del inicio del tratamiento con poliquimioterapia. A los 24 meses, se observó una disminución sostenida en los niveles de IgM anti-GLF-I y anti-35-kDa, hasta un nivel medio de 17% y 14%, respectivamente, en relación a los niveles iniciales. Ni el desarrollo de reacciones tipo 1 o tipo 2, ni la presencia de organismos resistentes a la droga, tuvieron una inlluencia significativa sobre los cambios en los niveles de anticuerpos en un número pequeño de pacientes. La velocidad de decaimiento fue similar en las diferentes categorias de la endermedad pero la proporción de pacientes que permanecieron seropositivos al final del segundo año de tratamiento fue mayor en el grupo lepromatoso que en el tuberculide subpolar. En contraste, los niveles medios de anticuerpos IgG anti-LAM permanecieron estables o incrementados. Otra vez, las reacciones tipo 1 o 2 no modificaron significativamente los niveles de anticuerpos a lo largo de 2 años. La caída en los niveles de anticuerpo IgM anti-GLF-I correlacionó con la caída en el índice bacteriano en los pacientes individuales constituyendo así un parámetro adicional para evaluar la respuesta al tratamiento.Monitoring the response of leprosy patients to drug therapy has depended upon clinical observation and measurement of the fall in the numbers of acid-fast bacilli (AFI3) in slit-skin smears. The fall in the bacterial index (BI) in lepromatous patients is estimated to be 0.5 to 1.0 log units per year and, therefore, patients with high bacterial loads may remain smear positive for years (30).
The quality of slit-skin smears in many leprosy control programs is poor, and the value of regular smears in the assessment of the response to therapy has been questioned (10). In recent studies we have found that 42% of smear-negative patients have antibodies to one of two Mycobacterium leprae -specific antigens: phenolic glycolipid-I (PGL-I) and the ML-04 monoclonal antibody defined epitope on a 35kDa protein. All new primary neuritic (PN), tuberculoid (TT), and 95% of the borderline tuberculoid (BT) patients assessed prospectively were smear negative; however, 41% of the PN (23) and TT patients and 44% of the BT patients (22) were seropositive for antibodies to either PGL-I or the 35-kDa protein. When IgG anti-lipoarabinomannan (LAM) antibodies were measured in addition, 47% of PN and 48% of paucibacillary (PB) patients had antibodies to one of the three leprosy antigens. Of new multibacillary (MB) patients 93% were seropositive to one of these three antigens (24). Therefore, many PB patients have antibodies in the absence of a positive skin smear, and MB patients have similar rates of seropositivity and skin-smear positivity (24).
Cross-sectional studies have documented that IgM anti-PGL-I antibodies are lower in treated than untreated patients (2), as are anti-35-kDa antibodies and IgG anti-LAM (18,20). Fewer studies have examined the rate of fall of antibody levels in individual patients with therapy, although reports confirm a decline in both anti-PGL-I and anti-protein antibodies during a chemotherapeutic trial (6,15). In addition, fluctuations in anti-PGL-I and anti-LAM antibody levels occur during type 2 and type 1 reactions, respectively (1,18).
Since a large proportion of untreated patients are seropositive to a panel of antigens, we have examined whether serial measurements of antibody levels are of value in assessing the response to multidrug therapy (MDT) in a field situation. In particular, we have compared the rate of change in antibody levels with the decline in the BI and examined the effect of dapsone resistance and of reactional episodes on the change in antibody levels.
MATERIALS AND METHODS
Patients The subjects consisted of 65 previously untreated patients or patients who had defaulted from regular monotherapy for more than 2 years. Patients were enrolled over a 2-year period and were initially positive for IgM anti-PGL-I (although not necessarily other) antibodies. The diagnosis of leprosy was made on clinical and bacteriological grounds according to the Ridley-Jopling classification (21), and 49 patients were males and 16 females with an age range of 10 to 73 years. The clinical classifications of the patients were borderline tuberculoid (BT, N = 11), borderline (BB, N = 4), borderline lepromatous (BL, N = 30) and lepromatous (LL, N = 20). This diagnosis was confirmed in 18 patients by histopathological examination of a skin biopsy. Forty-nine patients had no history of previous treatment and 16 had irregular monotherapy with dapsone (DDS) for 6 to 34 years more than 2 years prior to commencing MDT. Sixty-two patients were assigned to the multibacillary or MB-MDT treatment regimen; two the paucibacillary (PB-MDT) treatment regimen, and one patient received dapsone (DDS) and clofazimine (Lamprene®) alone due to developing a sensitivity to rifampin after five doses of MB-MDT.
All patients were examined regularly at clinics for evidence of type 1 (reversal) or type 2 (erythema nodosum leprosum, ENL) reactions, and compliance to therapy was recorded. Scrum samples were collected before therapy commenced and at approximately 3-month intervals thereafter into 0.01% sodium azidc and stored at - 20ºC.
The mean BI was calculated from slitskin smears taken from four sites, including one clinical lesion, at an average interval of 4 months. The same sites were sampled on each occasion.
Serological assays
IgM anti-PGL-I antibodies were measured by enzyme-linked immunosorbant assay (ELISA) using the glycoconjugate disaccharide-bovine serum albumin (dBSA; provided by the IMMLEP program of the World Health Organization) as described previously (22). The ELISA was performed on all samples within a week of collection and before the sample was frozen. Results were expressed as absorbances obtained under standard conditions at 492 nm.
IgG anti-LAM antibodies were measured by ELISA using M. tuberculosis LAM (kindly provided by Dr. P. J. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A.) using assay conditions as described previously (22). Results were expressed as absorbances at 492 nm.
Antibodies to the M. leprae-sptcific 35kDa protein (28) were measured by a monoclonal inhibition ELISA using ML-04 antibody (kindly provided by Dr. J. Ivanyi, MRC TB and Related Infections Unit, London) under conditions described previously (22). Results were expressed as the serum titer causing 50% inhibition of ML-04 binding to M. leprae sonicate (ID50).
Dapsone (DDS) compliance
Compliance to daily DDS therapy was assessed by the presence of DDS metabolites in the urine using the "spot test" (11).
Drug sensitivity
The sensitivity of M. leprae from untreated patients to DDS and rifampin was tested in the mouse foot pad (27). An inoculum containing 1 x 104 AFB from a fresh skin biopsy was injected in a 30-µl volume into the hindfoot pads of Swiss albino mice. The mice were fed dapsone at one of three concentrations (0.01%, 0.001%, or 0.0001% w/w) mixed with the feed; or rifampin (300 µl of a 0.05% solution) by gastric lavage once weekly. Foot pads were harvested and counted when the growth of M. leprae in mice not receiving any drugs had reached 10h AFB/footpad.
Statistics
Differences in the proportions seropositive between groups were tested with the chi-squared test. Differences in the range of antibody levels at different time points and in different patient groups were tested by means of the Mann-Whitney U test. The relationship between the change in antibody levels and the change in BI in individual patients over 24 months of chemotherapy was tested by measurement of the correlation coefficient.
RESULTS
Clinical findings
Patients were monitored for an average of 17 months (range 6 to 30 months) during which time an average of five samples were taken from each patient (range 3 to 10) at an average interval of 3.4 months. During the study period, 19 (29%) of the patients (5 BT, 2 BB, 12 BL) developed type 1 reactions and type 2 reactions occurred in 11 (17%) patients (7 BL, 4 LL). Despite fulfilling the WHO MDT guidelines for regular therapy (31), 13 patients were found to be negative on one occasion by means of the spot test for DDS. Subsequent urine tests in these patients were positive.
Six patients were considered likely to be infected with drug-resistant M. leprae by mouse foot pad assays. In four, isolates of M. leprae showed secondary dapsone resistance [three at high level (0.01%) and one at mid-level (0.001%)]. In two patients, growth of M. leprae was demonstrated in rifampin-fed mice. However, this putative rifampin resistance was not confirmed on later passage of the organisms.
For the assessment of the effect of type 1 and type 2 reactions and drug resistance on the change in antibody levels, the antibody levels in these patients were compared with 34 patients who were drug sensitive and without a history of reaction; these included 6 I3T, 2 BB, 13 BL and 13 LL patients. Five patients, three with both type 1 and 2 reactions and two patients with drug-resistant leprosy who developed ENL, were excluded from these comparisons.
Changes in antibody levels
The patterns of change in the three antibody levels were similar in BT, BB/BL, and LL patient groups, whether expressed as antibody values (Table 1) or as a percentage of initial antibody level. The mean changes in antibody levels and Bis expressed as a percentage of initial levels for the whole patient group are illustrated in The Figure. There were distinct patterns of change in the three antibodies measured.
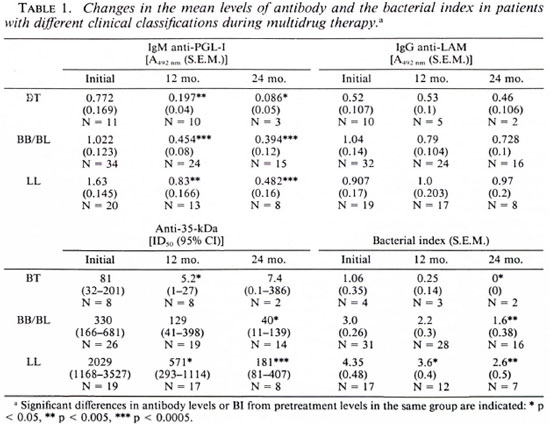
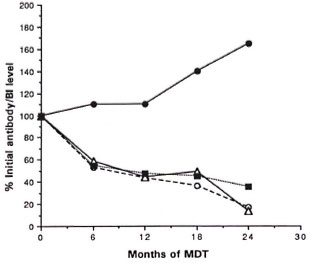
The figure. Mean changes in the three anti-ALleprae antibody levels (PGL-I, 35-kDa, and LAM) andin the Ills of 65 leprosy patients over 2 years of mul-tidrug therapy expressed as percentages of pretreat-ment values: Ο = IgM anti-PGL-I;  = IgG anti-LAM; Δ = anti-35-kDa protein;
= IgG anti-LAM; Δ = anti-35-kDa protein;  = bacterial index.
= bacterial index.
IgM anti-PGL-I antibodies. These antibodies fell rapidly from the commencement of therapy in all patients, the average decline being 83% over the 2-year period of study (Table 1). Since lepromatous leprosy patients had higher initial IgM anti-PGL-I antibody levels, 78% remained seropositive at the end of 2 years in contrast with none in the BT group. Once seronegativity was achieved, no patient became seropositive again. Although antibody levels generally did not rise above the initial levels, this did occur in 2 of 9 BT patients during the first 6 months of therapy. These levels subsequently fell. There were no significant differences in the change in antibody levels over 2 years in patients with reactions or in the small number with drug-resistant bacilli compared with other patients (Table 2).
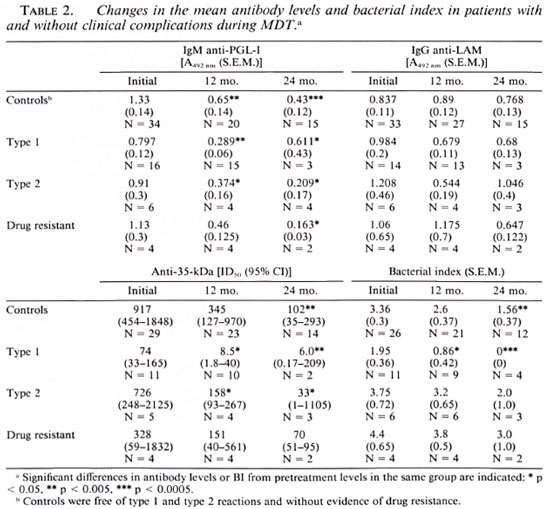
IgG anti-LAM antibodies. The mean level of anti-LAM antibodies showed little change after 1 or 2 years or a modest increase. When results were expressed as the percent of initial levels, there was a modest increase in IgG anti-LAM levels in contrast with the fall seen in IgM anti-PGL-I and anti-35-kDa antibodies (The Figure). Moreover, 69% (9 of 13) of patients who were initially seronegative for anti-LAM antibodies became seropositive during treatment. This was seen in each leprosy patient group (3 of 5 BT, 2 of 3 BL, 4 of 5 LL). Rcactional episodes did not affect IgG anti-LAM levels over the 2-year study period (Table 2).
Anti-35-kDa protein antibodies. A significant decline in the serum titer causing a 50% inhibition of ML-04 binding occurred in all patients (The Figure). None of the LL group with high initial titers (mean 982) were seronegative at the end of the study period; however one third of the BL and one half of the BT groups had become negative by the last quarter of therapy (Table 1). There were no differences in the rates of change in antibody levels in patients with reactions over 2 years (Table 2).
Changes in the BI. There were marked declines in the BI in all patients over the 2-year study period. The annual rate of fall in the BI was 0.81 log units in BT patients, 0.8 log units in BB/BL patients, and 0.75 log units in LL patients. At the end of 2 years of MDT, 50% (2 of 4) of BT, 17% (4 of 23) BB/BL, and 6% (1 of 18) of LL patients had become smear negative.
Patients with drug-resistant leprosy or who developed type 2 reactions during therapy had higher initial Bis than did the disease controls (Table 2). The initial Bis of patients who developed type 1 reactions were significantly lower than those of reaction-free patients (p < 0.01) because of the higher proportion of BT patients in this group. All patients with type 1 reactions were smear negative by the end of therapy compared with 33% of those reaction free and with type 2 reactions and none of those with drug-resistant leprosy.
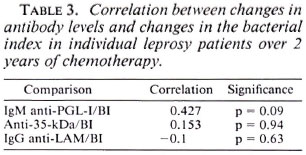
Correlation between changes in antibody levels and changes in BI. The correlations between change in antibody levels and the BI in individual patients with 2 years of treatment are shown in Table 3. The fall in IgM anti-PGL-I antibodies, expressed as a percentage of the pretreatment levels, correlated moderately with the fall in BI. Despite the fall in anti-35-kDa antibody levels (The Figure), these did not correlate with the fall in BI in individual patients (Table 3). There was no correlation between the change in IgG anti-LAM antibodies and the BI.
DISCUSSION
Antibody responses to glycolipid antigens in leprosy patients arc typical of T-cell-independent antibody responses, being composed of predominantly IgM isotype and changing as mycobacterial antigen levels vary during chemotherapy.
Phenolic glycolipid I (PGL-I) is a major secretory product of M. leprae (12) and is found in high levels in the sera and tissues of untreated MB patients (25,29). The viability of M. leprae in axenic culture can be assessed by the incorporation of radiolabeled palmitic acid into PGL-I (7). Rapid fall in the serum level of PGL-I antigen in previously untreated MB patients is evident following a single dose of rifampin (25). Antibodies to PGL-I are predominantly of the IgM isotype (32) and the anti-PGL-I antibody levels reflect the bacterial load in untreated patients (22,24). As M. leprae are killed with chemotherapy, PGL-I synthesis ceases, the antigen is cleared from the sera and tissues and, subsequently, the antibody levels fall.
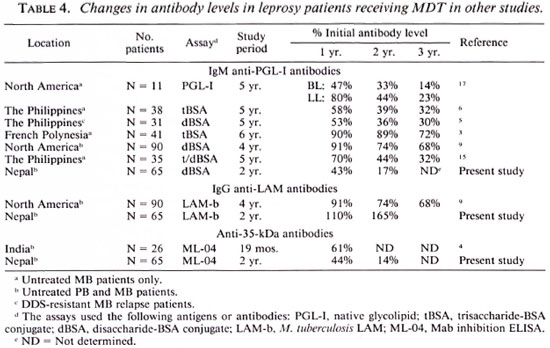
Comparisons of the results of the present study with results obtained by other authors are shown in Table 4. Changes in IgM anti-PGL-I antibody levels during the first year of MDT in this study are consistent with studies in Philippine MB leprosy patients in drug trials and a study in North America (5,6,15,17), but larger falls were evident in our patients in the second year. Smaller falls were seen in patients in French Polynesia and in a second study in North America (3,9). In these patients, the falls in the BI were also less than seen in the present study.
Lipoarabinomannan (LAM) is a complex glycolipid associated in large amounts (15 mg per g of bacilli) with the cell walls of M. leprae and M. tuberculosis (8,13). LAM provokes both IgM and IgG antibody responses (16) and, in contrast to the decline seen in IgM anti-PGL-I antibodies, we observed that IgG anti-LAM antibody levels persisted following treatment and, in some cases, rose. Although this may be due to subclass differences in the rate of antibody decline, it may also be due to differences in the rate of clearance of LAM and PGL-I. While levels of actively synthesized antigens such as PGL-I fall with bactericidal chemotherapy (25), dead leprosy bacilli remain in the tissues of patients for prolonged periods (30). Therefore the immune system continues to be exposed to LAM as M. leprae is slowly degraded, and this may be responsible for the observed increase in anti-LAM antibody levels. Increased anti-LAM antibody levels may also result from an anamestic antibody response on exposure to M. tuberculosis or environmental mycobacteria containing LAM.
Two other studies have documented variable falls in IgG anti-LAM antibodies during therapy. One cross-sectional study showed lower antibody levels to the arabinomannan from M. smegmatis in treated compared with untreated leprosy patients (17). A 5-year follow up of patients in North America, measuring antibodies to M. tuberculosis LAM, showed a variable decline in antibody levels with therapy (9) (Table 4). A substantial proportion (42%) of the patients in the latter study were still seropositive for anti-LAM antibodies after 10 years of chemotherapy. The increase in mean antibody levels observed in Nepali patients is largely due to the inclusion of initially seronegative patients who developed anti-LAM antibodies during the course of chemotherapy. Increases in an- titubercule antibodies, including anti-arabinomannan antibodies have been noted to rise in tuberculosis patients during therapy (14).
The M. leprae 35-kDa protein containing the ML-04 defined epitope is associated with the cell membrane (19). The immunoglobulin isotype involved in the response to this protein antigen has not been determined but may include IgM as well as IgG isotypes. In a previous cross-sectional study anti-35-kDa antibodies were lower in treated than in untreated patients and correlated with the length of therapy (20). Recent studies of sequential samples from Indian lepromatous patients show a similar fall in anti-35-kDa antibody levels as shown in the present study (4) (Table 4). The 35-kDa protein is presumably a rapidly degraded antigen, and the decline in antigen levels causes a corresponding decrease in antibody levels. By contrast, antibody levels to other M. leprae proteins, such as the 36-kDa (15) and the 18-kDa (26), show little difference between treated and untreated patients. Serological monitoring may also be of value in the one third of PB patients who have anti-35-kDa antibodies because the antibody levels fall rapidly with therapy (Table 1).
Although there is a consistent fall in the BI with therapy (Tables 1 and 2) the practical difficulties in assessing Bis in field programs have recently been highlighted (10). We were interested, therefore, in examining whether changes in antibody levels were a surrogate marker of the response to therapy. Changes in anti-PGL-I but not anti-35-kDa or anti-LAM antibodies correlate with the change in BI in patients over the first 2 years of MDT (Table 3). Hence, serological monitoring provides a parameter of similar sensitivity to the BI in assessing the response to chemotherapy in MB patients. Further, in the subgroup of seropositive PB patients who are smear negative, the change in antibody levels provides a new parameter to assess the impact of MDT.
Acknowledgment. We are grateful to the patients and staff of the Anandaban Leprosy Hospital, Kath mandu, Nepal, for their kind cooperation without which this study would not have been possible. The Mycobacterial Research Laboratory is fully funded by The Leprosy Mission International. We thank the IMMLEP Program of WHO for provision of the disaccharide BSA antigen; Dr. Patrick Brennan of the Colorado State University, Fort Collins, Colorado, U.S.A., for provision of LAM (prepared under NI H contract Al52582) and Prof. J. Ivanyi of the MRC Tuberculosis & Related Infections Unit, Hammersmith Hospital, London, for provision of the ML-04 monoclonal antibody.
REFERENCES
1. ANDREOLI, A., BRETT, S. J., DRAPER, P., PAYNE, S. N. and ROOK, G. A. W. Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int. J. Lepr. 53(1985)211-217.
2. BACH, M.-A., WALLACH, D., FLAGUEL, B., HOFFENBACH, A. and COTTENTOT, A. Antibodies to phenolic glycolipid-! in leprosy patients-evolution under chemotherapy. Int. J. Lepr. 54( 1986)256-267.
3. CHANTEAU, S., CARTEL, J.-L., CELERIER, P., PLI-CHART, R., DESFORGES, S. and Roux, J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int. J. Lepr. 57(1989)735-743.
4. CHATURVEDI, V., SINHA, S., GIRDHAR, B. K. and SENGUPTA, U. On the value of sequential serology with a Mycobacterium leprae-spccific antibody competition ELISA in monitoring leprosy chemotherapy. Int. J. Lepr. 59(1991)32-40.
5. DOUGLAS, J. T., HIRSCH, D. S., FAJARDO, T. T., CELLONA, R. V., ABALOS, R. M., DE LA CRUZ, E. C, MADARANG, M. G., DE WIT, M. Y. and KLAT-SER, P. R. Evaluation of Mycobacterium leprae antigens in the serological monitoring of a chlofazamine-based chemotherapcutic study of dapsonc resistant lepromatous leprosy patients in Cebu, Philippines. Lepr. Rev. 60(1989)8-18.
6. DOUGLAS, J. T., STEVEN, L. M., FAJARDO, T., CELLONA, R. V., MADARANG, M. G., ABALOS, R. M. and STEENBERGEN, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
7. FRANZBLAU, S. G., HARRIS, E. B. and HASTINGS, R. C. Axenic incorporation of [U-MC] palmitic acid into the phenolic glycolipid-I of Mycobacterium leprae. FEMS Microbiol. Lett. 48(1987)407-411.
8. GAYLORD, H. and BRENNAN. P. J. Leprosy and the leprosy bacillus: recent developments in characterization of antigens and immunology of the disease. Ann. Rev. Microbiol. 41(1987)645-675.
9. GELBER, R. H., LEE, F., CHO, S-N., BYRD, S., RA-JAGOPALAN, K. and BRENNAN, P. J. Serum antibodies to defined carbohydrate antigens during the course of treated leprosy. Int. J. Lepr. 57(1989)744-751.
10. GEORGIEV, G. D. and MCDOUGALL, A. C. A reappraisal of clinical and bacteriological criteria in the implementation of MDT for leprosy control programs and proposals for their better use. Lepr. Rev. 64(1990)64-72.
11. HUIKESHOVEN, H. and MADARANG, M. G. Spot test for the detection of dapsonc in urine: an assessment of its validity and interpretation in monitoring DDS self administration. Int. J. Lepr. 54(1986)21-24.
12. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid of Mycobacterium leprae}. Biol. Chem. 257(1982)15072-15078.
13. HUNTER, S. W., GAYLORD, H. and BRENNAN, P. J. Structure and antigenicity of the phosphorylatcd lipopolysaccharidcs of the tubcrcule and leprosy bacilli. J. Biol. Chem. 261(1986)12345-12351.
14. KAPLAN, M. H . and CHASE, M. W . Antibodies to mycobacteria in human tuberculosis. I. Development of antibodies before and after anti-microbial therapy. J. Infect. Dis. 142(1980)825-834.
15. KLATSER, P. R., DE WIT, M. Y. L., FAJARDO, T. T., CELLONA, R. V., ABALOS, R. M., DE LA CRUZ, E. C, MADARANG, M. G., HIRSCH, D. S. and DOUGLAS, J. T. Evaluation of M. leprae antigen in the monitoring of a dapsonc based chemotherapy of previously untreated lepromatous patients in Cebu, Philippines. Lepr. Rev. 60(1989)178-186.
16. LEVIS, W. R., MEEKER, H. C, SCHULLER-LEVIS, G. B., SERSEN, E., BRENNAN, P. J. and FRIED, P. Mycobacterial carbohydrate antigens for serological testing of patients with leprosy. J. Infect. Dis. 156(1987)763-769.
17. MILLER, R. A., GORDER, D. and HARNISCH, J. P. Antibodies to phenolic glycolipid-I during longterm therapy: serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
18. MILLER, R. A., HARNISCH, J. P. and BUCHANAN, T. M. Antibodies to mycobacterial arabinomannan in leprosy: correlation with reactional states and variation during treatment. Int. J. Lepr. 52(1984)133-139.
19. MOHAGHEGHPOUR, N., MUNN, M. W., GELBER, R. H. and ENGLEMAN, E. G. Identification of an immunostimulating protein from Mycobacterium leprae. Infect. Immun. 58(1990)703-710.
20. MWATHA, J., MORENO, C, SENGUPTA, U., SINHA, S. and IVANYI, J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr. Rev. 59(1988)195-199.
21. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity: a five-group system. Int. J. Lepr. 34(1966)255-273.
22. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., LUDWIG, H., THEUVENET, W. J. and ADIGA, R. B. Heterogeneity of serological responses in paucibacillary leprosy: differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58(1990)319-327.
23. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., THEUVENET, W. J., LAVENDER, M. and ADIGA, R. B. Serology in primary ncuritic leprosy. Trans. R. Soc. Trop. Med. Hyg. 85(1991)299-302.
24. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., WILLIAMS, D., PRADAN, H. M. and THEUVENET, W. J. Operational value of serological measurements in multibacillary leprosy: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58(1990)480-490.
25. ROCHE, P. W., BRITTON, W. J., NEUPANE, K. D., FAILBUS, S. S., CHO, S.-N. and THEUVENET, W. J. The response to chemotherapy of serum Mycobacterium leprae specific antigen in multibacillary leprosy patients. Am. J. Trop. Med. Hyg. 44(1991)702-706.
26. ROCHE, P. W., PRESTIDGE, R., WATSON, J. D. and BRITTON, W. J. Antibody responses to the 18kDa protein of Mycobacterium leprae in leprosy and tuberculosis patients. Int. J. Leepr. 60(1992)201-207.
27. SHEPARD, C. C. and CHANG, Y. T. Effect of several anti-leprosy drugs on the multiplication of the human leprosy bacillus in the foot pads of mice. Proc. Soc. Exp. Biol. Med. 109(1963)636-638.
28. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by a monoclonal antibody based immunoassay. Int. J. Lepr. 53(1985)33-38.
29. VENKATESAN, K., SINGH, H. S., BHARDWAJ, V. P. and RAMU, G. Isolation, purification and quantification of phenolic glycolipid I from human leprosy tissues. Trans. R. Soc. Trop. Med. Hyg. 82(1985)321-323.
30. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988 . Tech. Rep. Ser. 768.
31. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
32. YOUNG, D. B., DISSANAYAKE, S., MILLER, R. A., KHANOLKAR, S. R. and BUCHANAN, T. M. Humans respond predominately with IgM immunoglobulin to the species specific glycolipid of Mycobacterium leprae. 1. Infect. Dis. 149(1984)870-873.
1. B.App.Sc; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P. O. Box 151, Kathmandu, Nepal.
2. M.Sc; Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P. O. Box 151, Kathmandu, Nepal.
3. Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P. O. Box 151, Kathmandu, Nepal.
4. M.D., Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P. O. Box 151, Kathmandu, Nepal.
5. Ph.D., Department of Medicine and Centenary Institute for Cancer Medicine & Cell Biology, University of Sydney, Sydney NSW 2006, Australia.
Reprint requests to Dr. Britton.
Received for publication on 11 May 1992.
Accepted for publication in revised form on 5 January 1993.