- Volume 61 , Number 1
- Page: 44–50
Circulating immune complexes in leprosy sera: demonstration of antibodies against mycobacterial glycolipidic antigens in isolated immune complexes
ABSTRACT
Circulating immune complexes (CIC) were assayed in sera of leprosy patients. Using an immunoassay for two mycobacterial antigens-phenolic glycolipid-I (PGL-I) and glycolipid IV (SL-IV)-sera f rom 65 patients with leprosy (38 lepromatous, 18 borderline, and 9 tuberculoid) were studied. The CIC were isolated by polyethylene glycol (PEG) precipitation, washed, treated with an acid buffer, neutralized, and tested using an enzyme-linked immunosorbent assay (ELISA). We demonstrated that CIC could contain IgG and IgM antibodies reacting against PGL-I and SL-IV. The high levels of antibodies in the precipitable CIC showed concordance with high levels in the original sera, although some patients presented high levels of precipitable CIC in the absence of high titers of antibodies in their sera. It was concluded that some of the CIC observed in patients with leprosy were composed of IgG and IgM immunoglobulins against specific mycobacterial antigens.RÉSUMÉ
Les complexes immuns circulant (CIC) ont été dosés dans le serum de malades de la lèpre. On a étudié le scrum de 65 malades de la lèpre (38 lépromatcux, 18 borderline et 9 tubereuloides) à l'aide d'un test immunologique pour deux antigènes mycobactériens, le glycolipide phénolique-I (PGL-I) et le glycolipide IV (SL-IV). Les CIC ont été isolés par précipitation au polyethylene glycol (PEG), lavés, traités avec un tampon acide, neutralisés, et testés à l'aide d'un test enzymatique (ELISA). Nous avons montré que les CIC pouvaient contenir des anticorps IgG et IgM réagissant vis-à-vis de PGL-I et SL-IV. Les taux élevés d'anticorps dans les CIC précipitables montraient une concordance avec des taux élevés dans le scrum original, bien que certains patients présentaient des taux élevés de CIC précipitables en l'absence de taux élevés d'anticorps dans leur serum. On en conclut que certains des CIC observés chez des malades de la lèpre se composent d'immunoglobulines IgG et IgM dirigées contre des antigènes mycobactériens spécifiques.RESUMEN
Se buscaron complejos inmunes circulantes (CIC) en los sueros de los pacientes con lepra. Utilizando un inmunoensayo para dos antígenos micobacterianos (glicolipido fenólico I [GLF-I] y glicolipido IV [SL-IV]), se analizaron los sueros de 65 pacientes con lepra (38 lepromatosos, 18 intermedios, y 9 tubereuloides). Los complejos inmunes circulantes se aislaron por precipitación con polietilen glicol (PEG), se lavaron, se trataron con regulador ácido, se neutralizaron, y se probaron usando un inmunoensayo enzimático (ELISA). Se demostró que los CIC pueden contener anticuerpos IgG e IgM reaccionando con GLF-I y con SL-IV. Los elevados niveles de anticuerpos en los CIC precipitables, mostraron concordancia con sus niveles elevados en los sueros originales, aunque algunos pacientes presentaron altos niveles de CIC precipitables aún en ausencia de títulos elevados de anticuerpos en sus sueros. Se concluyó que algunos de los CIC observados en los pacientes con lepra estuvieron compuestos de inmunoglobulinas IgG e IgM contra antígenos micobacterianos específicos.Circulating immune complexes (CIC) have been demonstrated in a wide variety of diseases including: autoimmune diseases (systemic lupus erythematosus, Wegener's granulomatosis, Hashimoto's thyroiditis); infectious diseases (lepromatous leprosy, bacterial subacute endocarditis, sepsis) as well as neoplastic disorders (8,10).
Methods for CIC detection have recently been introduced by many investigators: binding of exogenous Clq to complexes using gel diffusion (1); platelet aggregation test (8); anticomplementary activity (12); precipitation with polyethylene glycol (5); inhibition of phagocytosis using labeled aggregated IgG and macrophages (14); quantitation of immune complexes by binding to radiolabeled Clq and complex precipitation (13); and detection of CIC by their inhibitory effect on the agglutination of IgGcoatcd particles by rheumatoid factor or Clq (11). These methods have been largely employed in the study of diseases in which CIC could play an important role.
Some of these methods have been performed for detecting the presence of CIC in leprosy sera: demonstrating the whole Mycobacterium leprae and its molecular-components (4,17); detecting high levels of CIC (19,22); and also demonstrating tissue deposition of immune complexes (21,24,26). The purpose of the present work was to apply an enzyme immunoassay for the titration of the antibodies isolated by polyethylene glycol (PEG) precipitation of the CIC in leprosy sera. We analyzed these isolated antibodies using two different antigens: the phenolic glycolipid-I (PGL-I) of M. leprae (2) and the glycolipid-IV (SL-IV) of M. tuberculosis (7). This procedure was previously described by Louzir, et al. (10) and involved the CIC precipitation with PEG, treatment of the isolated CIC with an acid buffer, neutralization and the analysis of the antibodies by enzyme immunoassay.
MATERIALS AND METHODS
Sera. Sixty-five sera from patients with leprosy [38 lepromatous, 18 dimorphous (borderline) and 9 tuberculoid] were obtained from the Department of Dermatology and Health Center, Escola Paulista de Medicina, São Paulo, Brazil. Their ages ranged from 17 to 68 years. The diagnosis was made by clinical, bacteriological, histopathological and immunological features, and the cases were classified according to the Madrid classification (9). Paucibacillary patients were treated with dapsone 100 mg/ day and multibacillary patients received dapsone 100 mg/day and rifampin 600 mg/ day; some of them were under corticosteroid treatment. Of these 65 patients, some developed type 2 reactions (erythema nodosum leprosum) and others, type 1 reactions. In addition, sera from 10 tuberculosis patients from São Paulo, and sera from 26 normal controls from Paris, France, were included as controls.
Antigens. We used two lipidic antigens: the PGL-I of M. leprae (2) that was kindly supplied by Dr. R. J. W. Rees (WHO-IMMLEP) and the SL-IV (diacyl-trehalose IV) of M. tuberculosis (7). The methods for isolation, purification and characterization of the SL-IV antigens were reported previously (15,16).
PEG precipitation. The PEG precipitation test was performed according to the method described previously by Louzir, et al. (10), developed from the original technique by Digeon, et al. (8). The sera were diluted 1:1 with phosphate buffered saline (PBS) without Tween, pH 7.4. To 200 µl of diluted scrum 200 µl of 5% PEG 6000 (Prolabo, France) in 150 mM NAC1, 20 mM potassium phosphate buffer, pH 7.4, was added and the mixtures were incubated overnight at 4ºC. The precipitates were collected by centrifugation (1500 x g x 20 min, 4ºC) (Sigma-Bioblock, France), were washed twice with 2.5% PEG, and then were dissolved in 100 ,d of 150 mM NaCl, 20 mM potassium phosphate buffer, pH 7.5, 10 mM EDTA (Sigma, France).
To 50 µl of the obtained solution, 50 µl of cold 0.2 M HCl-glycine, pH 2.8, was added and incubated at 4ºC for 15 min. This solution was neutralized with 25 µl of 1 M K2HP04 , pH 9.0, was diluted 1:20 by adding 1825 µl of 1% bovine serum albumin (BSA) without Tween 80, and was then tested within 30 min.
ELISA. We used the enzyme-linked immunosorbent assay (ELISA) for the isolated CIC, using the same method for lipidic mycobacterial antigens, as described by Cruaud, et al. (6). Polystyrene microtiter plates purchased from CML-Nemours (France) were coated with PGL-I (250 ng/well) and SL-IV (100 ng/well) antigens. For coating, the indicated amounts of antigen in 25 µl of hexane were placed in the wells, and the solutions were let to dry overnight at 37ºC. For verification of nonspecific adsorption, one well treated with 25 µl of hexane without antigen was included for each test. We also tested the sera without PEG precipitation. The main difference between the CIC precipitation test and the current ELISA serology was the dilution factor for the CIC, 1/20, and for the sera, 1/250. The tested sera were diluted in PBS containing 0.5% of BSA and each was tested in duplicate, as the isolated CIC. After saturation by PBS containing 5% of BSA (overnight at 4ºC), the plates were washed with PBS without Tween (Titertek Microplate Washer; Flow Laboratories, Puteaux, France) and 100 µl of diluted sera/isolated CIC was incubated at 37ºC for 1 hr 30 min for IgG and for 2 hr for IgM determinations. After washings, the conjugates with appropriate dilution were allowed to react for 2 hr at 37ºC; the conjugates were goat antihuman IgG (H + L) and IgM/β-galactosidase (Biosys, Compicgne, France). After washing, the appropriate substrate was added, and the plates were incubated at 37ºC for 1 hr. To correlate the data, three titrated sera used as standards having low, medium, and high levels of IgG or IgM antibodies were included in each plate, and PBS with 0.5% of BSA was used as the control (zero point activity). Aliquots of the standard sera were stored at -80ºC until ready for use.
After reading, for each plate, a curve was drawn using the zero and standard values after calculation of the slope and correlation coefficient. If these data were not satisfactory (slope too low, fit below 98%), the plate was rejected and the assays were repeated. The values of tested sera were corrected as follows: First, the difference between absorbance of serum and the nonspecific absorption was taken and a mean value was calculated. Then, the normalized data were calculated to establish the corrected 414 values by using the curve of the standards. These calculations were made using the Multiscan apparatus (Flow) connected to an IBM PS-60 computer using the Flow Laboratories Titersoft program revision 2.OA serial 0.197. It should be noted that all calculations can be easily performed using a statistical calculator.
Statistical analysis. Because of the nonnormal distribution of the obtained values, the data were analyzed by the nonparametric Kruskal-Wallis test to confirm the analysis of variance results (25). The percentile 5% statistics was used to determine the normal limit of the control group. Because of the concentration of the sera, it was not possible to compare the results obtained by the CIC precipitation test followed by an ELISA with the classical serologic test and, consequently, we used the McNemar test (20) to analyze the discordances between these two tests.
RESULTS
After the CIC were dissociated the liberated immunoglobulins (IgG and IgM) were assayed by ELISA using the PGL-I and SL-IV antigens. As shown in The Figure, CIC were manifestedly predominant in lepromatous and borderline leprosy, and high IgG and IgM anti-PGL-I titers were detected (The Figure, A and B). The same pattern was observed using SL-IV as the antigen (The Figure, C and D). CIC were also demonstrated in tuberculoid leprosy. However, anti-PGL-I titers were low: surprisingly, anti-SL-IV antibody titers were high in CIC from tuberculoid patients' sera. Analyzing the tuberculosis group, we observed that anti-PGL-I and anti-SL-IV titers were demonstrated, although the Kruskal-Wallis test showed a statistically significant difference just for IgG anti-PGL-I compared with the control group. The Figure shows the antibody levels' distribution in the different groups, and we can also observe that the antibody levels were higher in PEG precipitates of patients with lepromatous and borderline leprosy.
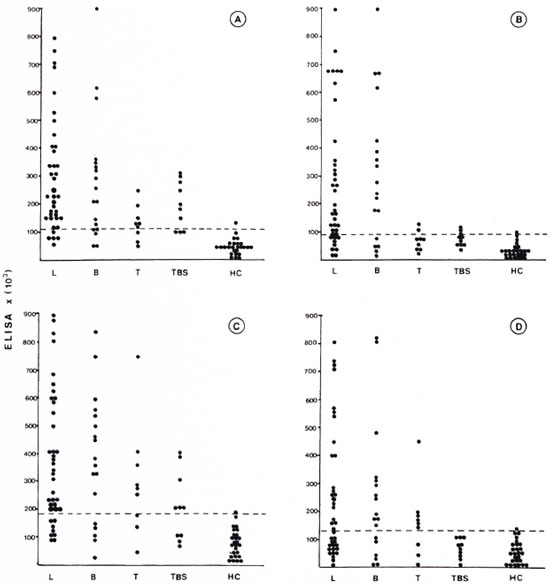
The figure. ELISA activity in precipitable CIC from patients with lepromatous leprosy (L), borderline leprosy (B), tuberculoid leprosy (T), tuberculosis (TBS), and healthy controls (HC). (---) = cut-offlevel for this test. A = anti-PGL-I IgG; B = anti-PGL-I IgM; C = anti-SL-IV IgG; D = anti-SL-IV IgM.
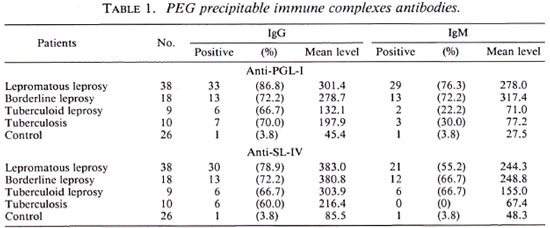
Table 1 shows the positivity and mean level of antibodies isolated from CIC in the analyzed groups. The Kruskal-Wallis test comparing the five groups (lepromatous leprosy, borderline leprosy, tuberculoid leprosy, tuberculosis, and normal control) showed that the lepromatous and borderline leprosy groups presented high levels of antibodies-a statistically significant difference compared with the control group. We observed a similar distribution in the serology for PGL-I and SL-IV.
When we analyzed all of the leprosy groups by the McNcmar test, we observed that the PEG precipitation did not increase the positivity of the serologic test, except for the antigen SL-IV -IgM in the lepromatous group (positivity for the serology 34.2% and positivity after CIC precipitation 55.3%) and the borderline leprosy group (positivity for serology 27.7% and positivity after CIC precipitation 66.7%) (data not shown).
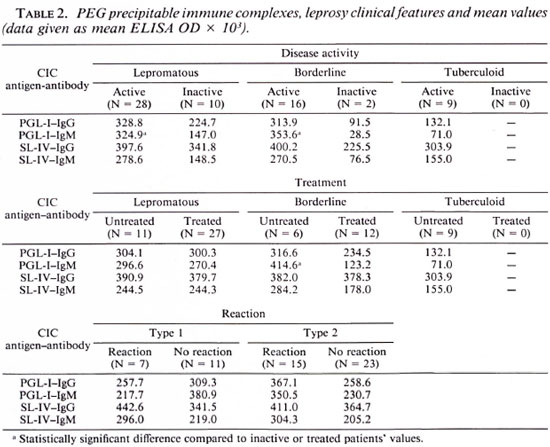
Comparing the clinical features and the results obtained, we observed high titers of IgM anti-PGL-I from isolated CIC among untreated borderline leprosy patients. Similarly, high IgM anti-PGL-I titers were observed when we analyzed the disease activity (clinical and bacteriological activity) among the lepromatous and borderline groups. We did not observe any relationship between the isolated CIC titers and type 1 or type 2 reactions. Table 2 represents the different clinical features and the main value obtained with isolated CIC. The Mann-Whitney test showed a statistical difference among the analyzed groups.
DISCUSSION
The presence of CIC in leprosy sera has been reported (4,17). However, there is little information concerning the antigen and the antibody that are responsible for the CIC formation. The present study describes a technique showing that PEG is suitable for precipitation and isolation of CIC in human sera. It was also shown that an immunoenzymatic test could be performed using the mycobacterial antigens PGL-I and SL-IV.
Analyzing the PEG precipitable immune complexes, the anti-PGL-I and anti-SL-IV antibody levels were likely to be higher in patients with the multibacillary form (lepromatous leprosy and borderline leprosy) than in the PEG precipitates from tuberculoid leprosy and controls. It was also interesting to note that the analyzed immunoglobulin classes -IgG and IgM-were present in these precipitable immune complexes. In these CIC we did not note any difference between the IgG and IgM levels.
An interesting aspect was observed when we analyzed the clinical features and the titers obtained. Among the multibacillary form (lepromatous and borderline leprosy), high IgM anti-PGL-I titers were related with the disease activity, as has been observed in the serological studies (6,23). In relation to the specific treatment, we observed that the IgM anti-PGL-I level was higher in the untreated borderline group than in the treated group, but we did not find any difference among lepromatous patients. There was no difference between the patients with or without leprosy reaction, perhaps because the previous treatment with corticosteroids and/or thalidomide might change the CIC levels.
The presence of antibodies against mycobacterial antigens was found in the sera as well as in the isolated CIC. However, a reciprocal relationship between circulating antibodies and CIC was not apparent. Although sequestration of the antibodies by CIC formation was not demonstrated herein, it is possible that because of low solubility the immune complexes may be deposited in the tissues, as suggested by Chakrabarty, et al. (4). Although it has been proposed that formation of immune complexes in the vascular and extravascular compartments contribute to erythema nodosum leprosum (ENL), we found no evidence for this in our series of cases. In spite of the high levels of CIC precipitate, we should not forget the possibility of free immunoglobulins' co-precipitation, that could raise the test titration. This problem was previously reported by other researchers using this method (5,8). Since we did not observe any apparent relationship between CIC levels and leprosy reactions, we think that a prospective study using this technique should be conducted by assaying CIC during the course of ENL. This should be of interest to determine whether the glycolipid antigens also participate in the pathogenesis of ENL.
Acknowledgment. We thank Prof. Neil Ferrara Novo and Prof. Yara Juliano for invaluable and expert assistance in the statistical analysis.
REFERENCES
1. AGNELLO, V., WINCHESTER, R. J. and KUNKEL, H. G. Precipitin reactions of Clq component of complement with aggregated gama-globulin and immune complexes in gel diffusion. Immunology 19(1970)909-919.
2. BRENNAN, P. J. and BARROW, W. W. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int. J. Lepr. 48(1980)382-387.
3. CHAKRABARTY, A. K., YASHYAP, A., SEHGAL, V. N. and SAHA, K. Solubilization of preformed immune complexes in sera of patients with type 1 and type 2 lepra reactions. Int. J. Lepr. 56(1988)559-565.
4. CHAKRABARTY, A. K., MAIRE, M., SAHA, K. and LAMBERT, P. H. Identification of components of immune complexes purified from human sera: II. Demonstration of mycobacterial antigens in immune complexes isolated from sera of lepromatous patients. Clin. Exp. Immunol. 5(1983)225-231.
5. CREIGHTON, W. D., LAMBERT, P. H. and MIE-SCHER, P. A. Detection of antibodies and soluble antigen-antibody complexes by precipitation with polyethylene glycol. J. Immunol. 111(1973) 1219- 1227.
6. CRUAUD, P., YAMASHITA, J. T., CASABONA, N. M., PAPA, F. and DAVID, H. L. Evaluation of a novel 2, 3-diacyl-trehalose 21-sulphate (SL-IV) antigen for case finding and diagnosis of leprosy and tuberculosis. Res. Microbiol. 141(1990)679-694.
7. DAFFE, M., PAPA, F., LASZLO, A. and DAVID, H. L. Glycolipids of recent clinical isolates of Mycobacterium tuberculosis: chemical characterization and immunoreactivity. J. Gen. Microbiol. 135(1989)2759-2766.
8. DIGEON, M., IAVER, M., RIZA, J. and BACH, J. F. Detection of circulating immune complexes in human sera by simplified assays with polyethylene glycol. J. Immunol. Meth. 16(1977)165-183.
9. INTERNATIONAL CONGRESS OF LEPROSY. Madrid 1953: report of the Committee on Classification. Int. J. Lepr. 21(1953)504-516.
10. LOUZIR, H., TERNYNCK, T., GORGI, Y., AYED, K. and AVRAMEAS, S. Enzyme immunoassay analysis of antibody specificities present in the circulating immune complexes of selected pathological sera. J. Immunol. Meth. 114(1988)145-153.
11. LURHUMA, A. Z., CAMBIASO, C. L., MASSON, P. L. and HEREMANS, J. F. Detection of circulating antigen-antibody complexes by their inhibitory effect on the agglutination of IgG coated particles by rheumatoid factor or Clq. Clin. Exp. Immunol. 25(1976)212-226.
12. MOWBRAY, J. F., HOLBOROW, E. J., HOFFBRAND, A. V., SEAH, P. P. and FRY, L. Circulating immune complexes in dermatitis herpetiformis. Lancet 1(1973)400-401.
13. NYDEGGER, U. E., LAMBERT, P. H., GERBER, H. and MIESCHER, P. A. Circulating immune complexes in the serum in systemic lupus erythematosus and in carries of hepatite B antigen. Quantitation by binding to radiolabeled Clq. J. Clin. Invest. 54(1974)297-309.
14. ONYEWOTU, I. I., HOLSOROW, E. J. and JOHNSON, G. D. Detection and radioassay of soluble circulating immune complexes using guinea pig peritoneal exudate cells. Nature 248(1974)156-159.
15. PAPA, F., CRUAUD, P. and DAVID, H. L. Antigenicity and specificity of selected glycolipid fractions from Mycobacterium tuberculosis. Res. Microbiol. (Inst. Pasteur) 140(1989)569-578.
16. PAPA, F., LASZLO, A. and DAVID H. L. Specificity of Mycobacterium tuberculosis phenolic glycolipid (PGL-Tbl) antiserum. Ann. Inst. Pasteur Microbiol. (Paris) 139(1988)535-545.
17. PATIL, S. A., SINHA, S. and SENGUPTA, U. Detection of mycobacterial antigens in leprosy serum immune complex. J. Clin. Microbiol. 24(1986)169-171.
18. PENTTINEN, K., VAHERI, A. and MYLLYIA, G. Detection and characterization of immune complexes by the platelet aggregation test. I. Complexes formed in vitro. Clin. Exp. Immunol. 8(1971)389-397.
19. RAMANATHAN, V. D., PARKASH, O. M., RAMU, G., PARKER, D., CURTIS, J., SENGUPTA, U. and TURK, J. L. Isolation and analysis of circulating immune complexes in leprosy. Clin. Immunol. Immunopathol. 32(1984)261-268.
20. REMINGTON, R. D . and SCHORK, M. A. Statistics with Applications to the Biological and Health Sciences. Englewood Cliffs, New Jersey: Prentice-Hall Inc., 1970.
21. RIDLEY, M. J. and RIDLEY, D. S. The immunopathology of erythema nodosum leprosum: the role of cxtravascular complex. Lepr. Rev. 54(1983)95-107.
22. SAHA, K., CHAKRAHARTY, A. K., SIIARMA, V. K. and SEHGAL, V. N. Polyethylene glycol precipitates in scrum during and after erythema nodosum leprosum. Study of their composition and anticomplementary. Int. J. Lepr. 52(1984)44-48.
23. SCHWERER, B., MEEKER, H. C, SERSEN, G. and LEVIS, W. R. IgM antibodies against phenolic glycolipid I from Mycobacterium leprae in leprosysera: relationship to bacterial index and erythema nodosum leprosum. Acta leprol. (Geneve) 2(1984)395-402.
24. SEHGAL, S. and KUMAR, 13. Circulating and tissue immune complexes in leprosy. Int. J. Lepr. 49(1981)294-301.
25. SIEGEL, S. Estadística no Parametrics. Mexico City: Editora Trillas, 1975.
26. WEMAMBU, S. N. C, TURK, J. L., WATERS, M. F.R. and REES, R. J. W. Erythema nodosum leprosum: a clinical manifestation of the Arthus phenomenon. Lancet 2(1969)933-935.
1. M.D.; Departamento de Dermatologia, Escola Paulista de Medicina, 740 Rua Botucatu, 04023 São Paulo, Brazil.
2. M.D., Ph.D., Departamento de Dermatologia, Escola Paulista de Medicina, 740 Rua Botucatu, 04023 São Paulo, Brazil.
3. M.Sc.Pharm., Hôpital Jean Verdier, 93140 Bondy, France.
4. M.Sc.Pharm.; Unite de la Tuberculose et des Mycobactéries, Institut Pasteur, 75724 Paris 15, France.
5. M.D., Ph.D., Unite de la Tuberculose et des Mycobactéries, Institut Pasteur, 75724 Paris 15, France.
Reprint requests to Dr. David.
Received for publication on 2 January 1992.
Accepted for publication in revised form on 22 October 1992.