- Volume 61 , Number 1
- Page: 51–8
Lymphocyte proliferation, IFN-γ production and limiting dilution analysis of T-cell responses to ICRC and Mycobacterium leprae antigens in leprosy patients
ABSTRACT
Lymphocyte proliferative responses and interfcron-gamma (IFN-γ) production after stimulation with antigens of ICRC, Mycobacterium leprae, and purified protein derivative (PPD) were assessed in leprosy patients and healthy donors. The patients studied were newly diagnosed as having lcpromatous leprosy (LL), multidrug therapy (MDT) responders (MDT-R LL), MDT nonresponders (MDT-NR LL), borderline lepromatous (BL), and borderline tuberculoid/tuberculoid (BT/TT) leprosy. The tuberculoid leprosy patients showed increased lymphocyte proliferation and IFN-γ production in response to stimulation with ICRC, M. leprae, and PPD antigens compared to other groups of LL patients and healthy donors. Although lymphocytes f rom LL patients showed low responses to ICRC and M. leprae antigens, their responses to PPD were not grossly affected. MDT-R LL patients showed higher lymphocyte proliferative responses and IFN-γ production after stimulation with ICRC and PPD but not with M. leprae antigens. Tuberculoid leprosy patients showed higher T-cell frequencies to ICRC and M. leprae antigens compared to MDT-R LL and MDT-NR LL patients. The increased lymphocyte proliferative responses to ICRC observed in the MDT-R LL patients was reflected in the increased T-cell frequency to ICRC compared to M. leprae.
RESUMEN
Se midieron las respuestas linfoproliferativas y la producción de interferon gamma (IFN-γ) por los linfocitos de pacientes con lepra y de donadores sanos, después de su estimulación con antigenos de ICRC, de Mycobacterium leprae, y con PPD. Los pacientes estudiados fueron recién diagnosticados como lcpromatosos (LL), lepromatosos respondedores a la poliquimioterapia (PQT-R LL), lepromatosos no respondedores (PQT-NR LL), lepromatosos subpolares (BL), y tuberculoides/tuberculoides-subpolares (BT-TT). Comparados con los grupos de pacientes LL y con los controles sanos, los pacientes con lepra tuberculoide mostraron aumentadas sus respuestas linfoproliferativas y la producción de IFN-γ en respuesta a la estimulación con ICRC, M. leprae y PPD. Aunque los linfocitos de los pacientes LL mostraron bajas respuestas al ICRC y al M. leprae, sus respuestas hacia el PPD no estuvieron mayormente afectadas. Los pacientes PQT-R LL mostraron altas respuestas linfoproliferativas y mayor producción de IFN-γ después de su estmulacióm con ICRC y con PPD, pero no respondieron a los antigenos del M, leprae. Los pacientes con lepra tubereuloide mostraron mayor proporción de células T reactivas contra ICRC y M. leprae que los pacientes PQT-R LL y PQT-NR-LL. Las incrementadas respuestas proliferativas hacia el ICRC observadas en los pacientes PQT-R LL estuvieron asociadas con niveles aumentados de células T reactivas al ICRC que contra M. leprae.
RÉSUMÉ
Les réponses de prolifération lymphocytaire et la production d'interferon-gamma (IFN-γ) après stimulation avec des antigènes d'ICRC, Mycobacterium leprae, et la protéine purifiée derivative (PPD) ont été évalués chez des malades de la lèpre et des donneurs en bonne santé. Les patients étudiés étaient des patients nouvellement diagnostiqués comme présentant une lèpre lépromateusc (LL), des patients répondant à la polychimiothérapic (PCT-R LL), des patients ne répondant pas à la polychimiothérapic (PCT-NR LL), et des patients borderline tuberculoides et tuberculoides (BT/TT). Les patients tuberculoides ont montré une augmentation de la prolifération lymphocytaire et de production d'IFN-γ en réponse à la stimulation avec RICRC, M. leprae et les antigènes PPD, comparés aux autres groupes de patients LL et aux donneurs en bonne santé. Bien que les lymphocytes des patients LL aient montré des réponses plus faibles à l'ICRC et aux antigènes de M. leprae, leur réponse vis-à-vis du PPD n'était à peu près pas affectée. Les patients PCT-R LL ont montré des réponses de prolifération lymphocytaire et une production d'IFN-γ plus élevée après stimulation avec ICRC et PPD, mais pas avec les antigènes de M. leprae. Les patients présentant une lèpre tuberculoide ont montre des fréquences plus élevées de cellules T à la stimulation par l'ICRC et les antigènes de M. leprae comparés aux patients PCT-R LL et PCT-NR LL. L'accroissement des réponses de prolifération lymphocytaire était reflété par une fréquence plus élevée de cellules T pour l'ICRC par rapport à M. leprae.RESUMEN
Se midieron las respuestas linfoproliferativas y la producción de interferon gamma (IFN-γ) por los linfocitos de pacientes con lepra y de donadores sanos, después de su estimulación con antigenos de ICRC, de Mycobacterium leprae, y con PPD. Los pacientes estudiados fueron recién diagnosticados como lcpromatosos (LL), lepromatosos respondedores a la poliquimioterapia (PQT-R LL), lepromatosos no respondedores (PQT-NR LL), lepromatosos subpolares (BL), y tuberculoides/tuberculoides-subpolares (BT-TT). Comparados con los grupos de pacientes LL y con los controles sanos, los pacientes con lepra tuberculoide mostraron aumentadas sus respuestas linfoproliferativas y la producción de IFN-γ en respuesta a la estimulación con ICRC, M. leprae y PPD. Aunque los linfocitos de los pacientes LL mostraron bajas respuestas al ICRC y al M. leprae, sus respuestas hacia el PPD no estuvieron mayormente afectadas. Los pacientes PQT-R LL mostraron altas respuestas linfoproliferativas y mayor producción de IFN-γ después de su estmulacióm con ICRC y con PPD, pero no respondieron a los antigenos del M, leprae. Los pacientes con lepra tubereuloide mostraron mayor proporción de células T reactivas contra ICRC y M. leprae que los pacientes PQT-R LL y PQT-NR-LL. Las incrementadas respuestas proliferativas hacia el ICRC observadas en los pacientes PQT-R LL estuvieron asociadas con niveles aumentados de células T reactivas al ICRC que contra M. leprae.Leprosy displays a spectrum of clinical manifestations correlating with the level of cell-mediated immunity in the patients (21,25). Lepromatous leprosy (LL) patients show Mycobacterium leprae -specific T-cell anergy, while tuberculoid leprosy (TT) patients mount strong cell-mediated immune responses to M. leprae antigens. Several reasons have been put forth to explain the M. leprae -specific T-cell anergy in LL patients. These include: deficiency of cytokine production (14,15), presence of suppressor cells (20), paucity of M. leprae -reactive T cells (13,18), and defective macrophage functions (22). Although the disease may be cured by effective chemotherapy, the T-cell tolerance to M. leprae antigens persists for life (3).
The ICRC antilcprosy vaccine developed in our laboratory brings about persistent lepromin conversion in 95% of healthy, lepromin-negative contacts and 55% of LL patients, associated with upgrading of tissue responses in the latter (9,10). Studies conducted earlier have demonstrated antigenic relatedness between ICRC and M. leprae antigens (11,12). Using a panel of sera from leprosy patients across the clinical spectrum and contacts, we have demonstrated that ICRC shares crossreactive antigens with M. leprae (6). The study further demonstrated the presence of species-specific immunodominant antigens on these mycobacteria.
In the present communication, we have analyzed the ability of ICRC, M. leprae, and purified protein derivative (PPD) antigens to induce lymphocyte proliferation and interferon-γ (IFN-γ) production in leprosy patients and healthy donors. The patients under study included newly diagnosed LL patients, treated LL patients responding to multidrug therapy (MDT-R LL), LL patients nonresponsive to multidrug therapy (MDT-NR LL), borderline lepromatous (BL), and a combined group of borderline tuberculoid and tuberculoid (BT/TT) patients. Using the technique of limiting dilution analysis (LDA), the frequency of ICRC and M. leprae -reactive T cells have been assessed in MDT-R LL, MDT-NR LL, and BT/TT patients.
Our results indicate that BT/TT patients showed increased lymphocyte proliferation and IFN-γ production in response to stimulation with ICRC, M. leprae, and PPD antigens compared to responses observed in BL and LL patients and healthy donors. Lymphocyte responses to ICRC and M. leprae antigens within each group of leprosy patients and healthy donors were comparable. Our results demonstrate that lepromatous leprosy patients with high bacterial load (BL, new LL, and MDT-NR LL) showed low lymphocyte responses to ICRC and M. leprae antigens. However, LL patients responding to MDT showed increased lymphocyte proliferation and IFN-γ production in response to stimulation with ICRC antigens compared to M. leprae antigens.
Higher T-cell frequencies to ICRC and M. leprae antigens were observed in the peripheral blood compartment of BT/TT patients compared to MDT-R LLand MDTNR LL patients. As observed in bulk lymphocyte proliferation assays, MDT-R LL patients showed increased T-cell frequencies to ICRC compared to M. leprae.
MATERIALS AND METHODS
Patients Leprosy patients attending the outpatient clinic at the Richardson Leprosy Hospital, Miraj, India, were included in the study. The group consisted of newly diagnosed cases of lepromatous leprosy (N = 19), MDT-R LL patients on MDT for 3-4 years (N = 14), LL patients clinically classified as nonresponders to MDT (MDT-NR LL, N = 20) who showed no drop in their tissue bacterial indices (Bis) despite 3-4 years of MDT (average Bl = 2.8 +), BL (N = 7), BT/ TT patients (N = 13), and healthy donors who were mostly laboratory personnel (N = 11).
Antigens
ICRC antigens. ICRC bacilli were cultivated in Dubos' modified medium supplemented with 10% human AB scrum as described by Chirmule, et al. (7). The bacilli were harvested, washed, and killed by γ-irradiation at 500 Krads. The ICRC bacilli were used at a concentration of 1 X 106 bacilli/50 µl/well in the lymphocyte proliferation assays.
Sonicate of ICRC bacilli was prepared as described by Chirmule, et al. (7). The bacterial extract was centrifuged at 218,200 X g x 1 hr at 4ºC. The supernatant referred to as "soluble ICRC" was collected and used at a concentration of 500 ng/50 µl/well.
M. leprae antigens. Whole M. leprae bacilli (Batch CD 102) and soluble M. leprae (Batch CD 122) were obtained as a gift from Dr. R. J. W. Rees (U.K.) through the WHO-IMMLEP program, and were used at concentrations of 1 x 106 bacilli/50 µl/well and 1 MG/50 µl/well, respectively. PPD was obtained from the Statens Serum Institute (Denmark) and used at a concentration of 2 AIG/50 µI/well.
Lymphocyte transformation assay. Peripheral blood lymphocytes (PBL) were separated from heparinized blood by layering on a Ficoll-Hypaque gradient. Separated PBL were suspended in RPMI 1640 (GIB-CO Laboratories, Grand Island, New York, U.S.A.) supplemented with 10% heat-inactivated human AB serum, glutaminc (4 mM), 2-mercaptocthanol (5 X 10-5 M), and antibiotics. The cells were plated at 2 X 106/ ml (0.1 ml/well) in triplicate sets in microliter plates (Nunc, Denmark). PBL were stimulated with whole/soluble antigens of ICRC, M. leprae, and PPD. PBL with medium alone served as controls. The cultures were incubated for 5 days at 37ºC, and were pulsed with 0.5 µCi of3H-thymidinc (BRIT, Bombay; specific activity 6-9 Ci/mM) during the last 18 hr. The cells were harvested onto glass fiber filters (Flow Labs, Finland), and the radioactivity incorporated in the cells was measured in a liquid scintillation counter (LKB, Sweden). Results were expressed as net counts per minute (cpm) which were calculated as the mean cpm of stimulated cultures - cpm of control cultures. Lymphocyte proliferative responses to antigens within each group were compared using the Student's t test. From parallel sets of cultures, supernatants were collected 24 hr after initiation of the experiment for assessing the IFN-γ production.
Interferon-γ assay. IFN-γ production was assayed in the lymphocyte culture supernatants according to the method described by Borden and Leonhardt (4). WISH cells (3 X 104/100 µl in Iscove's modified Dulbecco's medium (IMDM) containing 10% fetal calf serum (GIBCO) were plated in flat-bottom microtiter plates (Nunc). After the WISH cells had formed a monolayer, 0.1 ml volumes of lymphocyte culture supernatants were added to the microtiter plates in varying dilutions ranging from 1:2 to 1:4096. The cultures were incubated at 37ºC for 24 hr. The medium in the wells was flicked off, the monolayer of cells was washed with plain IMDM medium, and 0.1 ml of vesicular stomatitis virus (VSV, 1:200; obtained as a gift from the National Institute of Virology, Pune, India) was added to each well of the microtiter plate. The cells with medium alone served as the "cell control," while WISH cells incubated with VSV alone served as the "virus control." The cultures were incubated at 37ºC for 24-48 hr. When 75% destruction of the monolayer was observed in the virus control wells, the medium was discarded and the cells were fixed in absolute methanol. The cells were then incubated with 200 µl (0.072%) neutral red dye in Hanks' balanced salt solution at 37ºC for 2 hr. The dye was cluted using 100 mM glacial acetic acid and absolute cthanol (1:1), and optical density (OD) was read at 540 nm on an ELISA plate reader (Organon-Teknika, Belgium). The titers of IFN-γ obtained were expressed as units/ml after comparing with standard reference human IFN-γ. The IFN-γ nature of the cytokine was confirmed using polyclonal anti-IFN-γ scrum (Bochringcr Mannheim, Germany) in the assay.
LDA for estimating frequencies of antigen-reactive T cells. Limiting dilution analysis (LDA) was performed according to the method of Brett, et al. (5) with some modifications. T cells were purified from PBL by separation on nylon wool columns. The eluted T-cell population was > 90% positive for the surface CD3 marker. T cells in doubling dilutions ranging from 2 x 104 to 0.0157 X 104 cells in 0.1 ml of medium were seeded in each well of the microtiter plates as 24 replicates of each dilution. Autologous γ-irradiated (2500 rads) PBL (1 X 104/100 µl) were added to the wells along with antigens (either whole ICRC or whole M. leprae, 1 X 10V50 µl/well) and recombinant interleukin-2 (rIL-2, 2.5 µl/20 /ul/well; gift from Cetus Corporation, U.S.A.). Parallel sets of cultures containing only T cells, γ-irradiated PBL, and rIL-2 served as controls. The cultures were incubated at 37ºC for 6 days. On the sixth day rIL-2 (2.5 U/20 µl/well) was again added to the control and test plates, and the cultures were incubated further for 72 hr at 37ºC. -3H-Thymidine (0.5 µCi/10 µl/well) was added to all of the cultures, and the plates were incubated at 37ºC for 18 hr. The cultures were harvested and the radioactivity incorporated was measured as described earlier. Positive cultures were determined by scoring the test cultures (containing IL-2 + antigen), in which the 3H-thymidine incorporation exceeded the mean cpm + three standard deviations of control cultures (containing no antigen) at the corresponding cell number. The frequency of antigen-reactive T cells was calculated as described by Lefkovits and Waldmann (17) and Taswell (29), using the Poisson distribution formula with the help of a computer program. Graphs of the number of T cells/culture against the fraction of nonresponding cultures (Fo) were plotted to represent the frequency of antigen-reactive T cells.
RESULTS
Baseline studies across the leprosy spectrum. In the present investigations, we have analyzed lymphocyte proliferative responses and IFN-γ production in leprosy patients across the clinical spectrum and healthy donors after stimulation with antigens of M. leprae and ICRC. As seen in Figure 1, lymphocytes from BT/TT patients showed increased proliferative responses to whole ICRC (12,792 ± 3450 cpm), soluble ICRC (2291 ± 786 cpm), whole M. leprae (10,188 ± 2575 cpm), soluble M. leprae (8888 ± 2767 cpm), and PPD (40,332 ± 5726 cpm) compared to responses observed in BL, newly diagnosed LL, LL patients responding to MDT (MDT-R LL), LL patients not responding to MDT (MDT-NR LL), and healthy donors. The lymphocyte proliferation responses to soluble antigens of ICRC and M. leprae were low but comparable in all groups of LL patients.
These results were further supported by limiting dilution experiments where frequencies of T cells to ICRC and M. leprae in the peripheral blood of BT/TT patients were found to be higher compared to LL patients (The Table). It was interesting to observe that lymphocytes of BT/TT patients showing increased proliferative responses to ICRC and M. leprae antigens also produced increased levels of IFN-γ after stimulation with these antigens (Figs. 2 and 3). A higher number of BT/TT patients produced increased levels of IFN-γ after stimulation with whole ICRC (N = 8; range 61 U/ml to 600 U/ml; mean 210 ± 75 U/ml) compared to whole M. leprae (N = 4; range 18 U/ml to 159 U/ml; mean 89 ± 24 U/ml; Fig. 2). All groups of LL patients and healthy donors exhibited a similar pattern of response (Fig. 2). Lymphocytes from new LL and BT/TT patients produced increased amounts of IFN-γ after stimulation with soluble ICRC compared to soluble M. leprae (Fig. 3). Although lymphocyte proliferative responses to PPD were significantly higher in BT/TT patients compared to BL/LL patients, IFN-γ production varied within these groups of patients (Figs. 1 and 3).
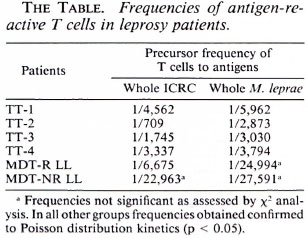
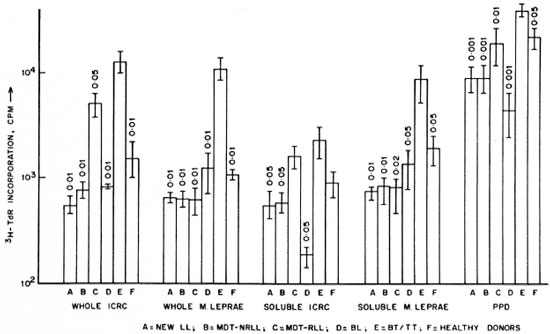
Fig. 1. Proliferative responses of lymphocytes from leprosy patients and healthy donors to antigens of ICRC, M. leprae, and PPD. A = newly diagnosed LL patients (N = 19); B = LL patients not responding to MDT (MDT-NR, N = 20); C = LL patients responding to MDT (MDR-R, N = 14); D = BL patients (N = 7); E = BT/TT patients (N = 13); F = healthy donors (N = 11). Differences in lymphocyte proliferation between each group and group E were analyzed statistically by the Student's / test; p values obtained are indicated above each histogram.
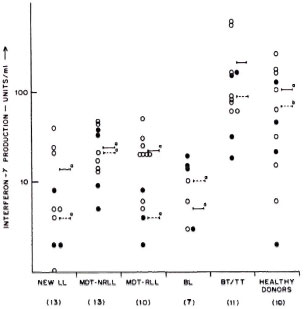
Fig. 2. Interferon-y production by lymphocytes from leprosy patients and healthy donors in response to stimulation with whole ICRC (o) and whole M. leprae 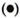 , showing mean values of IFN-γ production in response to stimulation with whole ICRC (-) and whole M. leprae (---). Figures in parentheses indicate total numbers of patients/healthy donors. Numbers of patients/healthy donors showing detectable levels of IFN-γ production are represented on the graph. Differences in IFN-γ production between each group and BT/TT patients were analyzed statistically by Student's t test; p values obtained are represented in the figure as: a) p < 0.001 and b) p < 0.02.
, showing mean values of IFN-γ production in response to stimulation with whole ICRC (-) and whole M. leprae (---). Figures in parentheses indicate total numbers of patients/healthy donors. Numbers of patients/healthy donors showing detectable levels of IFN-γ production are represented on the graph. Differences in IFN-γ production between each group and BT/TT patients were analyzed statistically by Student's t test; p values obtained are represented in the figure as: a) p < 0.001 and b) p < 0.02.
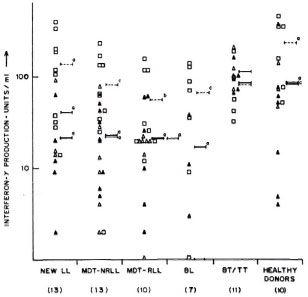
Fig. 3. Interferon-y production by lymphocytesfrom leprosy patients and healthy donors in responseto stimulation with soluble ICRC  , soluble M. leprae
, soluble M. leprae  , and PPD
, and PPD  . Mean values of IFN-γ production in response to stimulation with soluble ICRC (-), soluble M. leprae
. Mean values of IFN-γ production in response to stimulation with soluble ICRC (-), soluble M. leprae  , and PPD (---) are indicated. Figures in parentheses indicate total numbers of patients/healthy donors. Numbers of patients/healthy donors showing detectable levels of IFN-γ production are represented on the graph. Differences in IFN-γ production between each group and BT/TT patients were analyzed statistically by Student's t test; p values obtained are represented in the figure as: a) p < 0.001; b) p < 0.02, and c) not significant.
, and PPD (---) are indicated. Figures in parentheses indicate total numbers of patients/healthy donors. Numbers of patients/healthy donors showing detectable levels of IFN-γ production are represented on the graph. Differences in IFN-γ production between each group and BT/TT patients were analyzed statistically by Student's t test; p values obtained are represented in the figure as: a) p < 0.001; b) p < 0.02, and c) not significant.
Effect of MDT on anergy in lepromatous leprosy patients. LL patients (newly diagnosed LL, MDT-NR LL) exhibited low lymphocyte proliferative responses to whole ICRC and M. leprae antigens (Fig. 1). However, significant differences in lymphocyte reactivity to ICRC and M. leprae antigens were observed in MDT-R LL patients. Lymphocytes of MDT-R LL patients showed significantly increased (p < 0.01) proliferative responses after stimulation with whole ICRC (5132 ± 1365 cpm) compared to whole M. leprae (518 ± 183 cpm; Fig. 1). Nine out of ten MDT-R LL patients showed higher levels of IFN-γ production in response to stimulation with whole ICRC (22 ± 5 U/ml). On the contrary, only 3 out of 10 MDT-R LL patients showed marginal levels of IFN-γ production (4 ± 1 U/ml) after stimulation with whole M. leprae (Fig. 2). Although increased lymphocyte proliferative responses to soluble ICRC compared to soluble M. leprae antigens were observed in MDT-R LL patients, the IFN-γ levels were comparable (Figs. 1 and 3).
The differences observed in the lymphocyte proliferative responses to ICRC and M. leprae were further confirmed by analyzing the pool of antigen-reactive T cells in the peripheral blood by limiting dilution analysis (The Table). MDT-NR LL patients (BI 3.0+) showed very low frequencies of T cells to whole ICRC (1/22,963) as well as to whole M. leprae (1/27,591). On the other hand, MDT-R LL patients exhibited a higher frequency of T cells to whole ICRC (1/6675) compared to whole M. leprae (1/24,994), further supporting the bulk lymphocyte proliferation data.
Thus, using various parameters such as lymphocyte proliferation, IFN-γ production, and estimation of T-cell frequencies, we observed comparable lymphocyte responses to ICRC and M. leprae antigens in each group of LL and TT patients. Our results also demonstrated that although M. leprae- specific anergy persists in LL patients despite MDT, the lymphocyte responses to another antigenically related mycobacterium, such as ICRC, appear to have improved.
DISCUSSION
The ICRC antileprosy vaccine is currently undergoing field trials in India. The present investigation forms a baseline study for analyzing in vitro T-cell responses of leprosy patients across the clinical spectrum to ICRC and M. leprae antigens.
Our results demonstrated that lepromatous leprosy patients (new LL, MDT-NR LL) exhibited low lymphocyte proliferative responses to whole and soluble antigens of both ICRC and M. leprae. As reported by others (1,3), we observed that the T-cell anergy is largely restricted to M. leprae antigens in LL patients since cell-mediated immune responses to PPD were not affected. In our studies, lymphocyte responses to ICRC and M. leprae antigens were comparable in each group of leprosy patients (new LL, MDT-NR LL, BL and BT/TT patients). Lymphocytes from BT/TT patients showed increased proliferative responses to ICRC, M. leprae and PPD antigens compared to BL/LL patients. High lymphocyte proliferative responses to M. leprae and PPD antigens in BT/TT patients have also been reported by several others (2,8,16,19,21).
In our studies we have observed that MDT-R LL patients (MDT treatment 3-4 years) showed increased proliferative responses to ICRC and PPD antigens but not to M. leprae antigens, indicating that M. leprae -specific anergy could not be reversed despite long-term MDT. Tan Trao, et al. (28) earlier reported increased lymphocyte proliferative responses to M. leprae only in short-term MDT-treated LL patients, but not in untreated or long-term dapsonctreated LL patients. Our observations were further confirmed using limiting dilution analysis which revealed higher T-cell frequency to whole ICRC but not to whole M. leprae antigens in MDT-R LL patients. Stanford (26) has earlier reported that nonspecific unresponsiveness is reversible with clinical improvement in leprosy patients.
IFN-γ is a product of activated lymphocytes and an important mediator of antimicrobial activity and hydrogen peroxide production in macrophages (21). As reported by Nogueira, et al. (24) and Kaplan, et al. (16), we also observed that lymphocytes from BT/TT patients showed increased levels of IFN-γ production in response to stimulation with M. leprae antigens compared to LL patients. BT/TT patients also produced higher amounts of IFN-γ compared to BL/ LL patients after stimulation with ICRC antigens. Increased levels of IFN-γ were observed in MDT-R LL patients in response to stimulation with whole ICRC compared to whole M. leprae, further confirming our data on increased lymphocyte proliferation and the frequency of T cells to ICRC antigens observed in these patients.
Thus, from the above study, it appears that ICRC and M. leprae antigens elicit comparable lymphocyte responses as gauged by lymphocyte proliferation, IFN-γ production and frequency analysis of T cells in leprosy patients. In our studies, although effective MDT did not reverse the M. leprae -specific anergy observed in LL patients, the lymphocyte responses to ICRC and PPD appeared to have improved. Stanford and Rook (27) have suggested that mycobacterial antigens which afford protection are those common to all mycobacterial species rather than those unique to individual species. These observations have encouraged us to analyze T-lymphocyte reactivities in leprosy patients to antigens of ICRC, a cultivable mycobacterium, which shares crossreactive antigens with M. leprae. Moreover, these studies would prove to be useful while assessing immune responses in leprosy patients following vaccination with ICRC.
Acknowledgment. The authors wish to acknowledge the financial support by the Indian Council of Medical Research under the project "Immunoprophylaxis trials of ICRC anti-leprosy vaccine" and Indo-EC under the project "Immunology and immunoprophylaxis of leprosy."
REFERENCES
BACH, M.-A., CHATENOUD, L., WALLACH, D., PHAN DINH TUY, F. and COTTENOT, F. Studies on T cell subsets and functions in leprosy. Clin. Exp. Immunol. 44(1981)491-500.
BACH, M.-A., HOFFENBACH, A., LAGRANGE, P. H., WALLACH, D. and COTTENOT, F. Mechanism of T cell unresponsiveness in leprosy. Ann. Immunol. (Inst. Pasteur) 134D(1983)75-84.
BLOOM, B. R. and MEHRA, V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80(1984)5-28.
BORDEN, E. C. and LEONHARDT, P. H. A semiquantitative scmimicro, semiautomated colorimctric assay for interferon. J. Lab. Clin. Med. 89(1977)1036-1042.
BRETT, S. J., KINGSTON, A. E. and COLSTON, M. J. Limiting dilution analysis of human T cell response to mycobacterial antigens from BCG vaccinated individuals and LEPROSY PATIENTS. CLIN. EXP. IMMUNOL. 68(1987)510-520.
CHIPLUNKAR, S. V., KUDALKAR, J. L., BUTLIN, R., SAMSON, P. D., DEO, M. G. and GANGAL, S. G. Major proteins of ICRC and Mycobacterium leprae identified by antibodies in sera from leprosy patients and contacts. J. Clin. Microbiol. 30(1992)336-341.
CHIRMULE, N. B., CHATURVEDI, R. M. and DEO, M. G. Immunogenic "subunit" of the ICRC antileprosy vaccine. Int. J. Lepr. 56(1988)27-35.
CLOSS, O., REITAN, L. J., NEGASSI, K. J., HARBOE, M . and BELEHU, A. vitro stimulation of lymphocytes in leprosy patients, healthy contacts of leprosy patients and subjects not exposed to leprosy. Scand. J. Immunol. 16(1982)103-115.
DEO, M. G., BAPAT, C. V., BHALERAO. V., CHATURVEDI, R. M., CHULAWALLA, R. G. and BHATKI, W. S. Antileprosy potentials of the ICRC vaccine: a study in patients and healthy volunteers. Int. J. Lepr. 51(1983) 540-549.
DEO, M. G., BAPAT. C. V.. CHULAWALLA. R. G. and BHATKI, W. S. Potential anti-leprosy vaccine from killed ICRC bacilli-a clinicopathological study. Indian J. Med. Res. 74(1981)164-177.
EMMRICH, F. and KAUFMANN, S. H. E. Human T cell clones with reactivity to Mycobacterium leprae as tools for the characterization of potential vaccines against leprosy. Infect. Immun. 51(1986)879-883.
GANGAL, S. G. and KHANOLKAR, V. R. Delayed hypersensitivity in vitro to an acid fast mycobacterium cultivated from human lepromatous leprosy. Indian J. Med. Res. 62(1974)290-296.
GODAL, T., MYKELSTAD, B.. SAMUEL, D. R. and MYRVANG, B. Characterization of the cellular immune defective in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae reactive lymphocytes. Clin. Exp. Immunol. 9(1971)821-831.
HAREGEWOIN, A., GODAL. T., MUSTAFA, A. S., BELEHU, A. and YEMANEBERHAN, T. T cell conditioned media reverse T cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
HORWITZ, M. A., LEVIS, W. R. and COHN, Z. A. Defective production of monocyte activating lymphognes in lepromatous leprosy. J. Exp. Med. 159(1984)666-678.
KAPLAN, G., WEINSTEIN, D. E., STEINMAN, R. M., LEVIS, W. R., PATTAROYO, M. E. and COHN, Z. A. An analysis of in vitro T cell responsiveness in leprosy. J. Exp. Med. 162(1985)917-929.
LEFKOVITS, I. and WALDMANN, H. Limiting dilution analysis. In: Limiting Dilution Analysis of Cells in the Immune System. Lefkovits, I. and Waldmann, H., eds. Cambridge: Cambridge University Press, 1979, pp. 60-82.
LIM, S. D., Kisziss, D. F., JACOBSON, R. R., CHOI, Y. S. and GOOD, R. A. Thymus dependent lymphocytes of peripheral blood in leprosy patients. Infect. Immun. 9(1974)394-399.
LOCNISKAR, M., MCENIRY, D. W., MUDD, D. W., ROSE, P., LUCAS, D. C, LARRICK. J. and MCADAM, K. P. W. J. Assessment of the immune defect in leprosy patients and the effect of recombinant IL-2 in vitro. Int. J. Lepr. 55(1987)249-259.
MEURA, V., MASON, L. H., FIELDS, J. P. and BLOOM, B. R. Lepromin induced suppressor cells in patients with leprosy. J. Immunol. 123(1979)1813-1817.
MYRVANG, B., GODAL, T., RIDLEY, D. S., FRO-LAND, S. S. and SONG, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
NATHAN, C. F., KAPLAN, G., LEVIS, W. R., NUSARAT, W., WILMER, M. D., SHERWIN, S. A., JOB, C. K., HORWITZ, C. R., STEINMAN, R. M. and COHN, Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
NATHAN, C. and YOSHIDA, R. Cytokines: interferon^. In: Inflammation: Basic Principles and Clinical Correlates. Gallin, J. I., Goldstein, I. M. and Snyderman, R., eds. New York: Raven Press Ltd., 1988, pp. 229-251.
NOGUEIRA, N., KAPLAN, G., LEVY. E., SARNO, E. N., KUSHNER, P., GRANELLI-PIPERNO, A., VIEIRA. L., COLOMER GOULD, V., LEVIS, W. R., STEINMAN, R., YIP, K. and COHN, Z. A. Defective-γ-interferon production in leprosy: reversal with antigen and interleukin-2. J. Exp. Med. 158(1983)2165- 2170.
RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
STANFORD, J. L. Immunologically important constituents of mycobacteria: antigens. In: The Biology of the Mycobacteria, Vol, 2. Ratledge, C. and Stanford. J. L.. eds. London: Academic Press, 1983, pp. 85-127.
STANFORD, J. L. and ROOK, G. A. W . Environmental mycobacteria and immunization with BCG. In: Immunization Against Bacterial Disease, Medical Microbiology, Vol. 2. Easmon, C. S. F. and Jeljaszewicz, J., eds. New York: Academic Press. 1983, pp. 43-69.
TAN TRAO, V., HUONG, P. L. T., THUAN. A. T., LONG, H. T., TRACH, D. D. and WRIGHT, E. P. Responses to Mycobacterium leprae by lymphocytes from new and old leprosy patients; role of exogenous lymphokincs. Ann. Inst. Pasteur/Immunol. 139(1988)121-133.
TASWELL, C. Limiting dilution assay for the determination of immunocompetent cell frequencies: I Data Analysis. J. Immunol. 126(1981)1614-1619.
1. Ph.D.; Immunology Division, Cancer Research Institute, Tata Memorial Center, Parel, Bombay 400012, India.
2. Ph.D.; Immunology Division, Cancer Research Institute, Tata Memorial Center, Parel, Bombay 400012, India.
3. M.D., Ph.D.; Immunology Division, Cancer Research Institute, Tata Memorial Center, Parel, Bombay 400012, India.
4. Ph.D., Immunology Division, Cancer Research Institute, Tata Memorial Center, Parel, Bombay 400012, India.
5. M.B.Bch.; Richardson Leprosy Hospital, Miraj, India.
6. M.B.B.S., M.P.H., Richardson Leprosy Hospital, Miraj, India.
Reprint requests to Dr. Chiplunkar.
Received for publication on 28 May 1992.
Accepted for publication in revised form on 8 January 1993.