- Volume 61 , Number 1
- Page: 59–65
Clarithromycin is bactericidal against strains of Mycobacterium leprae resistant and susceptible to dapsone and rifampin
ABSTRACT
The anti-Mycobacterium leprae activity of clarithromycin when administered alone and in combination with rifampin and dapsone in the diet was determined using the kinetic method of drug evaluation in mice. Clarithromycin when administered at a concentration of 0.1% (w/w) in the diet completely prevented growth of 2 pan-susceptible, 3 dapsone-resistant, 2 rifampin-resistant, and 2 rifampin and dapsone double resistant strains of M. leprae. A 0.03% (w/w) concentration also completely prevented growth of M. leprae in all mice infected with 2 of 7 strains tested, but in only some of the mice infected with the remaining 5 strains. No antagonistic drug interactions were observed between clarithromycin and dapsone or rifampin. The addition of clarithromycin to the currently recommended multidrug regimen should improve the rate of killing of M. leprae and help to prevent the growth of dapsone-resistant and rifampin-resistant strains.RÉSUMÉ
L'activité anti-Mycobacterium leprae de la clarithromyci n administrée seule dans l'alimentation et en combinaison avec la rifampicine et la dapsone a été évaluée chez la souris par la méthode cinétique. La clarithromycine administrée à une concentration de 0.1% (poids/poids) dans l'alimentation empêchait complètement la multiplication de 2 souches de M. leprae sensibles aux deux médicaments, de 3 souches résistant à la dapsone, de 2 souches résistant à la rifampicine et de 2 souches ayant la double résistance à la dapsone et à la rifampicine. Une concentration de 0.03% (poids/poids) empêchait également complètement la multiplication de At. leprae chez toutes les souris infectées par 2 des 7 souches testées, mais seulement chez certaines des souris infectées par les 5 autres souches. Aucune interaction antagoniste n'a été observée entre la clarithromycine et la dapsone ou la rifampicine. L'addition de clarithromycine au régime de polychimiothérapic actuellement recommandé devrait améliorer le pouvoir bactéricide vis-à-vis de M. leprae et aider à prévenir la multiplication de souches résistant à la dapsone et à la rifampicine.RESUMEN
Utilizando el método cinético de evaluación de drogas en el ratón, se determinó la actividad anti-Mycobacterium leprae de la claritromicina administrada sola o en combinación con rifampina y dapsona. La claritromicina administrada en la dieta a la concentración del 0.1% (peso/peso) evitó completamente el crecimiento de 2 cepas de M. leprae resistentes a todo, 3 cepas resistentes a la dapsona, 2 resistentes a la rifampina, y 2 resistentes a la rifampina y a la dapsona. Una concentración del 0.03% también evitó completamente el crecimiento del M. leprae en todos los ratones infectados con 2 de las 7 cepas probadas y en algunos de los ratones infectados con las 5 cepas restantes. No se observaron interacciones antagonistas de las drogas entre la claritromicina, la dapsona y la rifampina. Es posible que la adición de claritromicina al tratamiento con poliquimioterapia recomendado por la OMS, ayude a mejorar su capacidad bactericida y a prevenir el crecimiento de cepas resistentes a la dapsona y de cepas resistentes a la rifampina.Hansen's disease is a chronic, infectious disease that afflicts 10-15 million people worldwide, mainly in developing countries (1). This disease results from an infection with the acid-fast bacillus Mycobacterium leprae and, if left untreated, can result in disability and deformity. The use of sulfones to treat Hansen's disease began in the 1940s, and dapsone has been used widely as monotherapy for over 40 years (2). In response to the appearance of dapsonc-resistant isolates of M. leprae, the World Health Organization (WHO) began recommending multidrug therapy (MDT) with dapsone, rifampin, and clofazimine in 1982 (8). However, rifampin-resistant and dapsone- and rifampin-doubly resistant strains of M. leprae have been reported (8,9). Although reports of clofazimine-resistant isolates of M. leprae are rare and have not yet been confirmed (17), there is a clear need for new anti-M leprae drugs to combat the potential drug-resistant strains. Also, because rifampin is the only powerful bactericidal anti-M leprae agent currently used, new bactericidal drugs are needed to improve the chemotherapeutic regimen.
Interest in the use of macrolides against intracellular pathogens such as M. leprae arose from the observation that erythromycin was active against Chlamydia trachomatis and Legionella pneumophila (12). Macrolides interfere with protein synthesis by blocking ribosomal function (12) which would represent a new target for anti- M. leprae agents. Franzblau and Hastings (5) surveyed several macrolides for activity against M. leprae using an in vitro radiometric assay of metabolic activity. The most active of these was clarithromycin. Clarithromycin differs from erythromycin in the methylation at the 6-hydroxy position of the macrolide ring (7).
In animal studies, clarithromycin was found to have bactericidal activity against pan-susceptible strains of M. leprae (5,6). It has also been reported to be active against M. avium in vitro and in vivo (3,4). We report here the activity of clarithromycin, alone and in multidrug combinations with dapsone and rifampin, against pan-susceptible, dapsone-resistant, rifampin-resistant, and dapsone + rifampin-resistant strains of M. leprae in the mouse foot pad model of infection.
MATERIALS AND METHODS
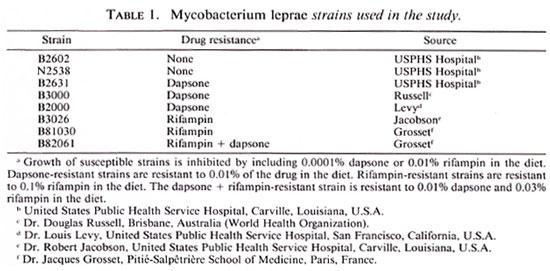
Strains. The strains used in these studies and their sources are listed in Table 1. The pan-susceptible and the dapsone-resistant strains were maintained in regular mouse passage (14) without drug treatment. Strains resistant to rifampin and to dapsone + rifampin were maintained on a diet containing 0.01% rifampin in regular mouse passage.
Antimicrobial agents. Clarithromycin (Abbott-56268; TE-031) was discovered by Taisho Pharmaceutical Co., Japan, and was donated by Abbott Laboratories, Abbott Park, Illinois, U.S.A. Rifampin was purchased from Sigma Chemical Company, St. Louis, Missouri, U.S.A., and dapsone was purchased from ICN Biomedicals, Inc., Plainview, New York, U.S.A. The drugs were mixed with ground commercial mouse chow (Rodent Lab Chow 5001; Purina Mills, Inc., Richmond, Indiana, U.S.A.) in a twinshell blender (Patterson-Kelly Co., East Stroudsburg, Pennsylvania, U.S.A.), and concentrations were expressed as weight/ weight percentages of the diet. A concentration of 0.001% in the diet corresponds to a dosage of about 1.0 mg/kg mouse weight/ day. Control groups were fed diets containing no drug.
Evaluation of drug activities in vivo. The kinetic method for determining drug activity against M. leprae in the mouse foot pad model was used as previously described (14). Briefly, approximately 5000 M. leprae cells were injected into the right hindfoot pad of 3-week-old CFW female mice. Mice were fed a diet containing drugs in various concentrations alone and in multidrug combinations from day 70 after inoculation through day 126, which is approximately the logarithmic phase of growth (14). Growth of the bacilli in the foot pads of mice fed a drug-free diet was monitored monthly beginning at day 70 by pooling foot pad tissue from four mice and enumerating the bacilli. When bacilli in this group reached approximately 106 bacilli per foot pad or a plateau level (similar numbers in two consecutive harvests), foot pads from five mice in each group were removed individually and bacilli were enumerated in each. This first time point varied from 5 to 7 months after challenge. A second assay was done 5-6 months after the first assay, and a third assay was done 3-6 months later if mice were available. The numbers of bacilli per foot pad in the various drug groups were compared statistically to their respective control group using the Wilcoxon rank sum test (15). Differences of p < 0.05 were considered statistically significant. Because the M. leprae had grown to 104 to 105 acid-fast bacilli (AFB) per foot pad by day 70 (the start of drug administration), growth of the M. leprae after drug treatment was considered significant if the foot pads contained > 2 x 105 AFB per foot pad.
RESULTS
Activity against pan-susceptible strains. In a preliminary experiment, mice were infected with the pan-susceptible strain N2538 and fed diets containing clarithromycin at concentrations of 0.1%, 0.01%, or 0.001%. Clarithromycin at 0.01% and 0.001% in the diet did not significantly inhibit the growth of the M. leprae at any of the time points (7, 11, or 13 months postchallenge; data not shown). In contrast, no bacilli were detected in the foot pads of mice fed the 0.1% concentration even at 340 days post-inoculation (214 days after cessation of treatment). Therefore, in the subsequent studies, clarithromycin was administered in concentrations of 0.03% and 0.1% in the diet. Growth of the pan-susceptible strain B2602 was inhibited (p < 0.05) at both time points by clarithromycin at a dietary concentration of 0.1% (Table 2). Furthermore, no growth of M. leprae was observed in the foot pads of any of the mice in this treatment group assayed 368 days after inoculation. Clarithromycin when tested at a concentration of 0.3% in the diet (Table 2) significantly inhibited (p < 0.05) the growth of 132602 in the foot pads of all mice assayed at the first time point (day 179), but prevented growth in only 2 of the 5 mice assayed 368 days after inoculation.

Dapsone and/or rifampin (Table 2) included in the diet did not antagonize the ability of clarithromycin at 0.1 % in the diet to completely prevent growth of this pansusceptible strain. Interestingly, growth was observed at the day 368 time point in foot pads of 2 of the 5 mice fed diets containing 0.01% dapsone + 0.01% rifampin, but not in the foot pads of any of the five mice fed diets containing only 0.01% rifampin. This is consistent with the suggested antagonism between dapsone and rifampin (13).
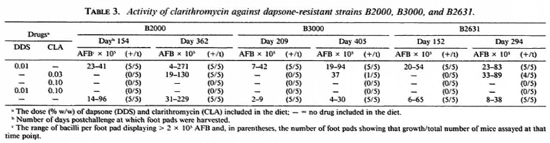
Activity against dapsone-resistant strains. As expected, the dapsone-resistant strains (B2000, B2631, B3000) grew in the foot pads of mice fed a diet containing 0.01 % dapsone (Table 3). Dapsone at this concentration in the diet did not interfere with the ability of clarithromycin at 0.1 % in the diet to prevent growth of strain B3000 in the foot pads of all mice assayed. When tested at the 0.03% concentration, clarithromycin prevented growth of this strain in 4 of the 5 mice assayed 405 days postchallenge.
In vivo growth of two of the dapsone-resistant strains, B2631 and B2000, was rapid and already at detectable numbers when treatment started on day 70. Since bacilli are cleared very slowly from the foot pad (16), anti- M. leprae activity is assessed in this case by the absence of an increase in the number of bacilli per foot pad. Clarithromycin when fed at a concentration of 0.1 % alone or in combination with 0.01% dapsone prevented any significant increase (p < 0.05) in the numbers of strain B2631 (day 294) or strain B2000 (day 362). Clarithromycin when fed at a concentration of 0.03% significantly inhibited (p < 0.05) the growth of strains B2631 and B2000 in the foot pads of all mice assayed at the first time points (day 152 and 154) but in none of the foot pads of five mice infected with strain B2000 and assayed 362 days postchallenge, and in the foot pad of only 1 of 5 mice infected with strain B2631 and assayed 294 days postchallenge. In addition, growth of strain B2631 was not inhibited in the foot pads of any of four mice assayed 480 days postchallenge (data not shown).
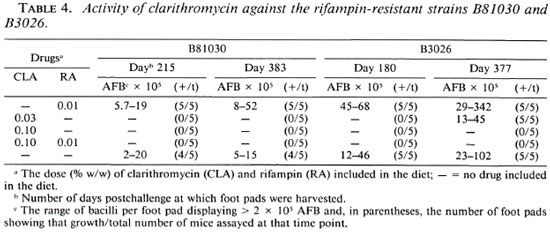
Activity against rifampin-resistant strains. Rifampin-resistant strains B3026 and B81030 grew in the foot pads of mice fed a diet containing 0.01% of the drug (Table 4). Clarithromycin at a concentration of 0.1%, alone or in combination with 0.01% rifampin, prevented growth of these rifampin-resistant strains in the foot pads of all mice assayed. When tested at a concentration of 0.03%, clarithromycin significantly inhibited growth (p < 0.05) of B3026 in the foot pads of all five mice at the first time point (day 180) but did not inhibit growth in the foot pads of any of the mice assayed 377 days postchallenge. This concentration of clarithromycin in the diet also completely prevented the growth of 1381030 since no bacilli were found in any of the five foot pads of the mice assayed 383 days postchallenge (Table 4) or in the foot pads of the two mice assayed 459 days postchallcnge (data not shown).
Strain B82061, which is resistant to both dapsone and rifampin, grew well in the foot pads of mice fed diets containing 0.01% dapsone and 0.01% rifampin (Table 2). Growth of this doubly resistant strain was prevented in the foot pads of all mice assayed by clarithromycin at 0.1% in the diet alone or in combination with dapsone and/ or rifampin. Clarithromycin, when fed at a concentration of 0.03%, significantly inhibited growth (p < 0.05) in the foot pads of all mice at the first time point (day 176) but in only 2 of 5 mice assayed 368 days postchallenge. Similarly, growth was prevented in only 1 of 4 foot pads assayed 501 days postchallengc (data not shown).
DISCUSSION
In the kinetic method of drug evaluation, drugs arc administered only from day 70 through day 126 after inoculation. A drug that is bacteriostatic will delay the growth of M. leprae for the period that the drug is present in the foot pad tissue in effective concentrations. A drug that kills all of the bacilli will prevent any growth after removal of the drug. Killing a fraction of the bacilli will cause a growth delay greater than the period during which the drug is present. The length of the growth delay is roughly proportional to the number of bacilli killed. Using the kinetic method of drug evaluation in the mouse foot pad model, Gelber, et al. (6) showed that 0.1% clarithromycin in the diet prevented multiplication of a pansusceptible M. leprae strain in the foot pads of all mice assayed as long as 11 months after inoculation. In addition, using the proportional bactericidal technique (6), they reported that a dietary concentration of 0.1% clarithromycin was 96% ± 2% bactericidal since 0 of 10 foot pads inoculated with 103 M. leprae had detectable bacilli after 1 year. Ji, et. al., also using the proportional bactericidal technique in mice (10), reported strong bactericidal activity of clarithromycin against M. leprae when administered by gavage as 20 daily doses at levels of 12.5 to 50 mg/kg of body weight (87.4%-94.9% bactericide). In our studies, 0.1% clarithromycin in the diet completely prevented growth of each of the eight strains tested. Assuming that growth begins when drug administration is stopped (day 126) and that the generation time of M leprae is 12.5 days (14), the failure to observe growth by day 350 in our experiments indicates that no viable bacilli were present in the foot pad on day 126. If so, this suggests that the 0.1% clarithromycin treatment was > 99.9% bactericidal because there were 104 to 105 bacilli per foot pad at the beginning of treatment. Similarly, our data suggest that 0.03% clarithromycin was > 99.9% bactericidal for four strains (B2602, B3000, B81030, and B82016), approximately 99% bactericidal for strain B2631, and approximately 90% bactericidal for strains B2000 and B3026. However, it must be noted that this apparent bactericidal activity may reflect contributions both from drug activity and from a possible immune response since the experiments involved immunocompetent mice (11).
Neither 0.01% nor 0.001% clarithromycin in the diet inhibited the growth of the one strain (N2538) tested with these concentrations. In contrast, Franzblau and Hastings (5) reported that a dietary concentration of 0.01% clarithromycin was fully bactericidal against M. leprae when assayed using a minor modification of the kinetic method; that is, no bacilli (i.e., < 1.6 x 104/foot pad) were found in foot pads from six mice assayed 5 months after discontinuation of drug treatment. The differences in our observations may reflect variations in the susceptibility of individual strains to clarithromycin, such as seen with B3026 and B3000 in the present study. Overall, the data indicate that a dietary concentration of 0.1% clarithromycin (approximately 100 mg/kg/ day/mouse) is necessary to ensure full bactericidal activity against all M. leprae strains.
Previous studies have suggested that dapsone can antagonize the bactericidal activity of rifampin (13). Our observation of the growth of strain B2602 by day 368 in the foot pads of 2 of 5 mice fed diets containing 0.01% rifampin + 0.01% dapsone, but no growth in the foot pads of any of the 5 mice fed diets containing 0.01% rifampin alone is consistent with such antagonism but our observed difference is not statistically significant. The addition of 0.1% clarithromycin to the diet containing 0.01 % rifampin + 0.1 % dapsone prevented growth of B2602 in the foot pads of all five mice assayed on day 368. Also, no antagonism was observed between clarithromycin and dapsone or rifampin in any of the combinations tested. This suggests that the addition of a second bactericidal drug, clarithromycin, to the currently recommended multidrug regimen may improve the killing of M. leprae bacilli even in the presence of a bacteriostatic drug such as dapsone.
An important result was that clarithromycin was effective when used alone against each of the rifampin-resistant and dapsoneresistant strains tested. In addition, clarithromycin was equally effective against these six drug-resistant strains when given in combination with dapsone and/or rifampin as might be expected since each drug is thought to affect a different cellular process. This strongly suggests that clarithromycin would be a useful addition to the multidrug regimen to prevent the growth of dapsonercsistant or rifampin-resistant bacilli.
Acknowledgment. Wc would like to thank Abbott Laboratories for providing clarithromycin and to thank Dr. R. C. Good for his helpful discussions.
REFERENCES
1. BLOOM, B. R. and GODAL, T. Selective primary health care: strategics for control of disease in the developing world. V. Leprosy. Rev. Infect. Dis. 5(1983)765-780.
2. CENTERS FOR DISEASE CONTROL. Increase in prevalence of leprosy caused by dapsone-resistant Mycobacterium leprae. Morbid. Mortal. Weekly 30(1982)637-638.
3. DAUTZENBERG, B., TRUFFOT, C, LEGRIS, S., MEYOHAS, M., BERLIE, H. C, MERCAT, A., CHEU-RET, S. and GROSSET, J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome; a controlled clinical trial. Am. Rev. Respir. Dis. 144(1991)564-569.
4. FERNANDES, P. B., HARDY, D. J., MCDANIEL, D., HANSON, C. W. and SWANSON, R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob. Agents Chemother. 33(1989)1531-1534.
5. FRANZBLAU, S. G. and HASTINGS, R. C. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32(1988)1758-1762.
6. GELBER, R. H., SIU, P., TSANG, M. and MURRAY, L. P. Activities of various macrolide antibiotics against Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)760-763.
7. HARDY, D. J., HENSEY, D. M., BEYER, J. M., VOJTKO, C, MCDONALD, E. J. and FERNANDES, P. B. Comparative in vitro activities of new 14-, 15-, and 16-membered macrolides. Antimicrob. Agents Chemother. 32(1988)1710-1719.
8. JACOBSON, R. R. and HASTINGS, R. C. Rifampin resistant leprosy. Lancet 2(1976)1304-1305.
9. JI, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
10. JI, B., PERANI, E. G. and GROSSET, J. H. Effectiveness of clarithromycin and minocycline alone and in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
11. LEVY, L. Superinfection in mice previously infected with Mycobacterium leprae. Infect. Immun. 11(1975)1094-1099.
12. MALMBORG, A.-S. The renaissance of erythromycin. J. Antimicrob. Chemother. 18(1986)293-295.
13. MILLAN, J. P. and MOULIA-PELAT, J. P. Antagonism between dapsonc and rifampicin in experimental Mycobacterium leprae infection in mice. Res. Microbiol. 140(1989)143-150.
14. SHEPARD, C. C. A kinetic method for the study of activity of drugs against Mycobacterium leprae in mice. Int. J. Lepr. 35(1967)429-435.
15. SHEPARD, C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae. Int. J. Lepr. 50(1982)96-101.
16. SHEPARD, C. C. and CHANG, Y. T. Effect of DDS on established infections with Mycobacterium leprae in mice. Int. J. Lepr. 35(1967)52-57.
17. WHO EXPERT COMMITTEE ON LEPROSY. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
18. WHO STUDY GROUP. Chemotherapy of leprosy for control programs. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. B. S.; Division of Bacterial and Mycotic Diseases, Mail Stop G35, Centers for Disease Control, 1600 Clifton Road NE, Atlanta, Georgia 30333, U.S.A.
2. M.A.; Division of Bacterial and Mycotic Diseases, Mail Stop G35, Centers for Disease Control, 1600 Clifton Road NE, Atlanta, Georgia 30333, U.S.A.
3. Ph.D., Division of Bacterial and Mycotic Diseases, Mail Stop G35, Centers for Disease Control, 1600 Clifton Road NE, Atlanta, Georgia 30333, U.S.A.
Received for publication on 13 October 1992.
Accepted for publication in revised form on 7 January 1993.