- Volume 61 , Number 1
- Page: 66–99
Sparfloxacin is more bactericidal than ofloxacin against Mycobacterium leprae in mice
ABSTRACT
The comparative bactericidal activities of sparfloxacin and ofloxacin against Mycobacterium leprae in mice were determined using the proportional bactericidal test at doses of 12.5 mg/kg-100 mg/kg. Significant bactericidal activity was found at 12.5 mg/ kg sparfloxacin and 25 mg/kg ofloxacin. Sparfloxacin was significantly more bactericidal than ofloxacin at all doses, and the results with 25 mg/kg sparfloxacin were nearly identical to those obtained with 100 mg/kg ofloxacin. These results, together with pharmacokinetic and toxicological data in mice and man, suggest that sparfloxacin may have a higher therapeutic index than ofloxacin in leprosy, and that the tentative standard dosage of 200 mg sparfloxacin daily should be appropriate for a clinical trial.RÉSUMÉ
Les activités bactéricides de la sparlloxacinc et de l'ofloxacine vis-à-vis de Mycobacterium leprae chez la souris ont été comparées et déterminées en utilisant le test bactéricide proportionnel à des doses de 12.5mg/ kg à 100 mg/kg. Une activité bactéricide significative a été observée avec 12.5 mg/kg de sparfloxacine et 25 mg/kg d'ofloxacine. La sparfloxacine était significativcment plus bactéricide que l'ofloxacine à tous les dosages, et les résultats obtenus avec 25 mg/kg de sparfloxacine étaient presque identiques à ceux obtenus avec 100 mg/kg d'ofloxacine. Ces résultats, ainsi que les données pharmacocinétiques et toxicologiques chez la souris et l'homme, suggèrent que la sparfloxacine pourrait avoir un indice thérapeutique plus élevé que l'ofloxacine dans la lèpre, et que la dose expérimentale standard de 200 mg de sparfloxacine administrée quotidiennement devrait être appropriée pour un essai clinique.RESUMEN
Utilizando la prueba bactericida proporcional, se estudió la actividad bactericida de la esparlloxacina y de la ofloxacinia en contra del Mycobacterium leprae inoculado en ratones. Se encontró significante actividad bactericida a 12.5 mg/kg de esparfloxacina y a 25 mg/ kg de ofoxacina. La esparfloxacina fue significativamente más bactericida que la ofloxacina a todas las dosis (12.5 mg/kg-100 mg/kg), y los resultados obtenidos con 25 mg/kg de esparfloxacina fueron prácticamente idénticos a los obtenidos con 100 mg/kg de ofloxacina. Estos resultados, junto con los datos farmacocinéticos y toxicológicos en el ratón y en el hombre, sugieren que la esparfloxacina puede tener un índice terapéutico mayor que la ofloxacina en la lepra, y que una dosis estándar tentativa de 200 mg diarios de esparfloxacina es apropiada para un ensayo clínico.Ofloxacin and pefloxacin were the first fluoroquinolones to have demonstrated activity against Mycobacterium leprae in a mouse model (4,6,13) and were thus subsequently tested in human leprosy (5). Ofloxacin, with a better therapeutic index, has been selected as the "second bactericidal agent" in a recently initiated, multicenter, World Health Organization (WHO)-sponsored trial in leprosy.
A large number of new quinolones were produced by the pharmaceutical industry in the late 1980s. In an in vitro evaluation of 20 such agents, several were found to be more potent than ofloxacin with respect to their activity against M. leprae in the BACTEC system (2). One of these agents, sparfloxacin (AT-4140), was also shown to have more potent in vivo activity against M. leprae in a nude mouse model of leprosy when compared to ofloxacin (15).
In this study we compared the activity of sparfloxacin and ofloxacin against M. leprae in normal immune-competent mice using the proportional bactericidal test (1). In addition, we determined the pharmacokinetics of sparfloxacin for the purpose of determining an appropriate human dosage for leprosy.
MATERIALS AND METHODS
Proportional bactericidal method. Threehundred-sixty, 7-week-old, female BALB/c mice were inoculated in each of their hind foot pads with approximately 30 µl of a nude-mouse-derived inoculum of M. leprae prepared in Hanks' balanced salt solution (HBSS). Groups of 90 mice received 5, 50, 500 or 5000 acid-fast bacilli (AFB) in both foot pads. After 30 days, mice received either ofloxacin (Daiichi Pharmaceutical Co. Ltd., Tokyo, Japan), sparfloxacin (Dainippon Pharmaceutical Co. Ltd., Osaka, Japan) or carboxymethylcellulose (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) by esophageal canula for 8 weeks (5 days/week). The drug suspensions were prepared fresh at weekly intervals in 0.5% sterile carboxymethylcellulose and kept refrigerated and protected from light between use. Groups of 10 mice at each inoculum dosage received either 0.2 ml sparfloxacin or ofloxacin to achieve final dosages of 12.5, 25, 50 and 100 mg/kg. Control mice received 0.2 ml 0.5% carboxymethylcellulose. Dosage adjustments for growth of the mice during the experiment were made by determining mean body weights on a weekly basis.
One year after infection all mice were sacrificed, and smears were prepared from tissue homogenates of individual foot pads. A total of 60 high-power fields were examined on Ziehl-Neelsen-stained smears for the presence of AFB. The observation of any AFB resulted in the scoring ofthat foot pad as positive.
Statistical analysis was performed as described by Shepard (14).
Pharmacokinetics. Mice were administered sparfloxacin at 12.5 mg/kg as described above. Immediately prior to administration and again at 20, 40 and 60 min and 2, 4, 8 and 24 hr post-administration, three mice were bled from the brachial plexus. Equal volumes of sera from the three mice were pooled and then frozen until analyzed by high-performance liquid chromatography (HPLC) (courtesy of Dainippon). Other mice were administered repeated doses of sparfloxacin at 24-hr intervals, and groups of three mice were bled at 30 min and 24 hr postadministration.
RESULTS AND DISCUSSION
Pattyn (12) an d Grosset, et al. (4), using the proportional bactericidal and kinetic methods, respectively, had previously reported ofloxacin to be bactericidal against M. leprae in the mouse at 50 mg/kg, the lowest dose tested. In this study ofloxacin produced a significant bactericidal effect at dosages of25-100 mg/k g (The Table). Sparfloxacin, however, demonstrated significant bactericidal activity at 12.5 mg/kg. Moreover, it was significantly more bactericidal than ofloxacin at each dose (p < 0.001 at 12.5, 25 and 50 mg/kg; p = 0.029 at 100 mg/kg). The results obtained with 25 mg/ kg Sparfloxacin were nearly identical to those obtained with 100 mg/kg ofloxacin.
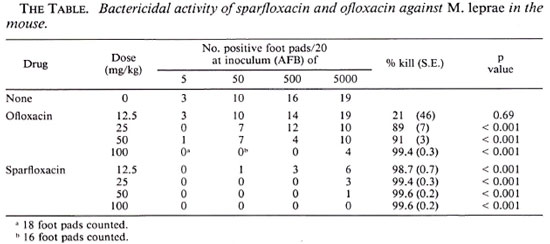
Tsutsumi and Gidoh also found Sparflox acin to be more active than ofloxacin against M. leprae in their nude mouse model (15). They recently reported Sparfloxacin to be bactericidal in the nude mouse model at 10 mg/kg and bacteriostatic at 5 and 2 mg/kg (3), corroborating our finding here of bactericidal activity at 12.5 mg/kg. Considering that the LD5 0 in mice (po administration) of Sparfloxacin (> 5000 mg/kg) (7) is at least equal to that of ofloxacin (5355 mg/kg) (11), Sparfloxacin would be expected to have a higher therapeutic inde x fo r leprosy.
The estimated maximum serum concentration of Sparfloxacin in mice following a single oral dose of 12.5 mg/kg was 1.74 µg/ ml. This may be a slight underestimate since this was the concentration obtained on the first sampling at 20 min postadministration. The repeated daily administration of 12.5 mg/kg sparfloxacin (as was done for the proportional bactericidal experiment) did not result in higher serum levels (data not shown). The dosage required in humans to obtain 1.74 µg/ml sparfloxacin in serum is approximately 400-600 mg (10). The halflife of sparfloxacin in this study was 1.2 hr in contrast to 16 hr in humans; therefore, it would be more appropriate to use the area under the serum concentration curve (AUC) in selecting a suitable dosage for a human trial. At 12.5 mg/kg, the AUC in mice was 2.99 µg hr ml while an equivalent value in humans would be obtained at a dosage of less than 100 mg (9). The manufacturer is currently considering the standard dosage of sparfloxacin to be 200 mg daily (personal communication, M. Ishizaki, Dainippon, 1992). According to the data obtained in this study, this dose should be appropriate for a clinical trial in human leprosy.
Acknowledgment. We thank Dr. David Heilbronn and Dr. Robert Gelber for assistance with the statistical evaluation of the data. We also thank Dainippon Pharmaceutical Co. Ltd. for assistance in determining the sparfloxacin pharmacokinetics. This investigation received financial support from the UNDP/World Bank/ WHO Special Programme for Research and Training in Tropical Diseases (TDR).
REFERENCES
1. COLSTON, M. J., HILSON, G. R. F. and BANERJEE, D. K. The "proportional bactericidal test," a method for assessing bactericidal activity of drugs against Mycobacterium leprae in mice. Lepr. Rev. 49 (1978)7-15.
2. FRANZHLAU, S. G. and WHITE, K. E. Comparative in vitro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob. Agents Chemother. 34(1990)229-231.
3. GIDOH, M. and TSUTSUMI, S. Activity of Spar floxacin against Mycobacterium leprae inoculated into foopads of nude mice. Lepr. Rev. 63(1992)108-116.
4. GROSSET, J. H., GUELPA-LAURAS, C. C, PERANI, E. G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. Lepr. 56(1988)259-264.
5. GROSSET, J. H., JI, B., GUELPA-LAURAS, C. C, PERANI, E. G. and N'DELI, L. N. Clinical trail of Pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281-295.
6. GUELPA-LAURAS, C. C, PERANI, E. G., GIROIR, A. M. and GROSSET, J. H. Activity of Pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
7. HASHIMOTO, M, MINAMI, A., NAKATA, K., SAKAGUCHI, Y., KOJIMA, T., FUJIMOTO, K., YOSHIDA, H., NAKAMURA, M., NAKAMURA, S., OHNISHI, K. and SHIMIZU, M. Antibacterial and toxicological properties of AT-4140. (Abstract 1488) Program and Abstracts of the Twenty-Eighth Intcrscience Conference on Antimicrobial Agents and Chemotherapy, 23-26 October 19SS. Washington, D.C.: American Society for Microbiology, 1988, p. 375.
8. JOHNSON, J. H., COOPER, M. A., ANDREWS, J. M. and WISE, R. Pharmacokinetics and inflammatory fluid penetration of sparfloxacin. Antimicrob. Agents Chemother. 36(1992)2444-2446.
9. KANAMURA, M., NAKASHIMA, M., UEMATSU, T. and TAKIKUCHI, Y. Pharmacokinetics and safety of a new quinolonc, AT-4140, in healthy volunteers. (Abstract 1490) Program and Abstracts of the Twenty-Eighth Intcrscience Conference on Antimicrobial Agents and Chemotherapy, 23-26 October 1988. Washington, D.C.: American Society for Microbiology, 1988, p. 375.
10. MONTAY, G., BRUNO, R., THEBAULT, J. J., VERGNIOL, J. C, CHASSARD, D., EBMEIER, M. and GAILLOT, J. Dose-dependent pharmacokinetic study of sparfloxacin (spfx) in healthy young volunteers. Abstracts of the 30th Intcrscience Conference on Antimicrobial Agents and Chemotherapy, Atlanta, 1990, p. 294 (abstract no. 1248).
11. OHNO, H., et al. On toxicity of ofloxacin. Chemotherapy (Tokyo) Suppl. 1 (1984) 1084 as cited in The Merck Index. Rahway, N.J., U.S.A.: Merck & Co., Inc., 1989, p. 1071.
12. PATTYN, S. R. Activity of ofloxacin and Pefloxacin against Mycobacterium leprae in mice. (Letter) Antimicrob. Agents Chemother. 31(1987)671-672.
13. SAITO, H., TOMIOKA, H. and NAGASHIMA, K. in vitro and in vivo activity of ofloxacin against Mycobacterium leprae infection induced in mice. Int. J. Lepr. 54 (1986) 560-562.
14. SHEPARD, C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae. Int. J. Lepr. 50(1982) 96-101.
15. TSUTSUMI, S. and GIDOH, M. Studies on the deelopment of novel antileprous chemotherapeu tics using nude mice with special reference to a new quinolonc carboxylic acid, AT-4140. Jpn. J. Lepr. 58(1989)250-258.
1. Ph.D., Laboratory' Research Branch, GWL Hansen's Disease Center at Louisiana State University, P.O. Box 25072, Baton Rouge, Louisiana 70894, U.S.A.
2. B.S.; Research Institute for Tropical Medicine, Alabang, Metro Manila, The Philippines.
3. M.S., M.D., Research Institute for Tropical Medicine, Alabang, Metro Manila, The Philippines.
Received for publication on 8 October 1992.
Accepted for publication on 9 December 1992.
1. Wayne M. Meyers, M.D., Ph.D., served as Acting Editor.