- Volume 61 , Number 1
- Page: 70–5
Animal model to study the mechanism of nerve damage in leprosy - a preliminary report
ABSTRACT
Intraneural injection of 10-20 x 106 viable Mycobacterium leprae into the sciatic nerve of normal, unsensitized, Swiss white mice gives rise to a tuberculoid type of granulomatous response in 2 weeks. The same dose of viable M. leprae when injected into the sciatic nerves of unsensitized immunosuppressed mice (T200 x 5R) elicited a macrophage response. When macrophages were systemically immobilized using an intraperitoneal injection of silica quartz dust in normal mice, the lesion produced was of the lepromatous type, suggesting a role for the macrophage in the induction of the tuberculoid type of granulomatous response. In all of these in situ experiments, M. leprae failed to enter the Schwann cells.RÉSUMÉ
L'injection intra-neurale de 10-20 x 106 organismes de Mycobacterium leprae viables dans le nerf sciatique de souris blanches Swiss normales et non sensibilisées donne lieu dans les deux semaines à une réponse granulomateusc de type tuberculoide. La même dose de M. leprae viables injectée dans le nerf sciatique de souris non sensibilisées mais ayant subi une immunosuppression (T200 x 5R) a produit une réponse macrophagique. Quand les macrophages ont été systématiquement immobilisés par l'injection intraperitoneal de poussière de quartz de silice chez la souris normale, la lésion produite était du type lépromateux, ce qui suggère que le macrophage a un rôle dans la production de la réponse granulomatcusc de type tuberculoide. Dans aucune de ces expérimentations in situ, M. leprae n'est entré dans les cellules de Schwann.RESUMEN
La inyección intraneural de 10-20 millones de bacilos de la lepra viables en el nervio sciático de ratones Swiss-white normales, origina en dos semanas una respuesta de tipo granulomatosa. La misma dosis de Mycobacterium leprae viables inyectada en los nervios sciáticos de ratones inmunosuprimidos (T200 x 5R) indujo un respuesta macrofágica. Cuando los macrófagos se inmobilizaron sistemicamente inyectando intraperitonealmcnte polvo de silicato de cuarzo en ratones normales, la lesión producida fue del tipo lepromatoso sugiriendo un papel de los macrófagos en la inducción de la respuesta granulomatosa de tipo tubereuloide. En todos estos experimentos in situ nunca se observó que el M. leprae penetrara en las células de Schwann.Since 1881 over 30 animal species have been tried for the experimental induction of leprosy, and today we arc using a few strains of rats (6) and mice (17), nude mice (8), three species of armadillos (19), the Korean chipmunk (9), and four nonhuman primates (10) in this successful list. Nerve lesions are documented in all of the animal models with minor variations related to route of infection and with susceptibility/resistance factors playing a role (7). A major limitation has been that none of the animal models really depict the spectral disease of human leprosy, and the slow progressive nerve damage seen in these models is representative, at best, of borderline disease. Thus, the search is still on (4) for a better animal model to use in studying nerve damage during the normal course of the leprosy infection and during the various reactional states.
Our present study aims at developing an animal model for studying the kinetics of nerve damage induced by the tuberculoid and lepromatous types of granulomatous responses and also the effect of selective deletion of macrophages on the granulomatous response and nerve damage in a timebound manner. For obvious reasons, such studies cannot be undertaken on naturally occurring lesions.
In a preliminary study we have used intraneural injection of Mycobacterium leprae, a technique followed by Wisniewski and Bloom (18) to bypass the blood-nerve barrier. Using this technique Mshana, et al. (11) and Cowley, et al. (3) have induced granulomatous responses within the sciatic nerves of rabbits and guinea pigs presensitized to mycobacterial antigens.
Unlike these researchers, we choose to use viable M. leprae in unsensitized, normal and immunomodulated mice since there is evidence suggesting that the molecular level response to viable M. leprae may be different from that of nonviable M. leprae, particularly in the neural environment (13). The results obtained were encouraging and arc the subject of this report.
MATERIALS AND METHODS
A total of 20 right and left sciatic nerve biopsies obtained from ten mice were studied in two sets of experiments using a protocol as shown in The Table. Normal, immunosuppressed (T200 x 5R) and silica-treated, Swiss white female mice were injected intraneurally with 10-20 x 10''viable M. leprae suspended in normal saline.
In experiment 1, the M. leprae used for intraneural injection were obtained from a fresh nodule biopsy of an untreated lepromatous patient. In experiment 2, M. leprae were obtained from a frozen armadillo liver. The tissues were homogenized using the technique of Shepard (17). The bacterial suspension thus obtained was used for intraneural injection within 24 hr.
Immunosuppression of mice. Three-tofour-week-old, Swiss white female mice were thymectomized and irradiated with five graded doses of 200 rads each x irradiation at biweekly intervals (T200 x 5R) (2). These mice were used for intraneural injection 2 weeks after the last dose of irradiation.
Treatment of mice with silica for macrophage inactivation. Adult, Swiss white female mice were given intraperitoneal injections of 1 ml of 20% silica quartz dust (size 80% of particles, 1-5 µm; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) suspension in 0.5% buffered saline solution (BSS), 1 day prior to intraneural injection of M. leprae and/or a crush injury to the sciatic nerve. The silica dose was repeated two times per week thereafter until biopsy. Treatment with silica quartz dust selectively inhibits macrophage function in situ (1). Thus, in the present study, normal mice with a crush injury to the sciatic nerve (standard model for Wallerian degeneration) and mice treated with silica (+Si) followed by a crush injury to the sciatic nerve were used as controls to assess the effect of M. leprae in the nerve with or without a crush injury in silica-treated mice (protocol as in The Table).
Intraneural injection procedure. Mice were anesthetized with an intraperitoneal injection of 1% pentabarbitone. Using a dissecting microscope, both the right and left sciatic nerves were exposed asceptically from under the biceps femoris, lifted from the surrounding tissue, and 0.5 µl of M. leprae suspension containing 10-20 x 106 viable M. leprae were injected into the endoneurial space using a 30-gauge needle and a Hamilton syringe. A cyclomixer was used to get an even bacterial suspension.
For inducing a crush injury, a fine watchmaker's forcep was used. In group 4 (The Table) the nerve was first crushed, then immediately injected with the M. leprae suspension, and the wound was sutured.
Biopsy. The sciatic nerve biopsies from all of the mice were obtained using pentabarbitone anesthesia 2 weeks after the intraneural injection of M. leprae and/or a crush injury. The nerves were fixed in 2.5% glutaraldehyde, postfixed in osmium, dehydrated, and embedded in araldite. Onejtm-thick, semithin sections were stained with toludine blue and studied under the light microscope. Subsequent ultrathin sections stained with uranyl acetate and lead citrate were studied using a JEOL 100S transmission electron microscope.
RESULTS
Normal mice with intraneural M. leprae. One-µm-thick resin sections stained with toludine blue revealed patchy areas of fiberloss. There were cuffs of lymphocytes around the dilated blood vessels, and a few aggregates of epithelioid cells and giant cells were also seen (Fig. 1A).
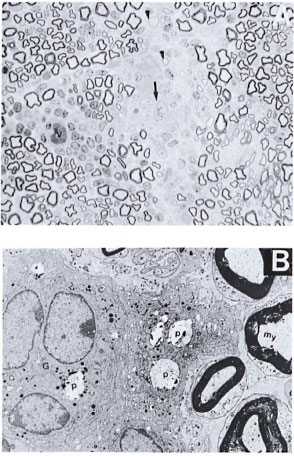
Fig. 1. A. Part of the sciatic nerve from a normal mouse injected intraneurally with viable M. leprae suspension 2 weeks prior to biopsy showing patchy areas of fiber loss. Note the presence of lymphocytic infiltrate around one of the blood vessels (v). Several pale, nucleated epithelioid cells and one giant cell are also seen (arrow). A few thinly myelinated regenerative fibers arc seen (arrowhead) on an edematous background. This lesion is comparable to an early human tuberculoid type of nerve lesion (semithin transverse section stained with toludine blue x 750). B. Electron photomicrograph of a part of the Figure 1A nerve showing one multinucleated giant cell (G) surrounded by a few epithelioid macrophages in the endoneurial area. A few phagosomes (P) with bacterial debris are also seen within these cells. My = myelinated fiber (uranyl acetate plus lead citrate stain x6000.)
Scanning the sections under the electron microscope confirmed the light-microscopic findings (Fig. IB). A carpet of epithelioid cells and a few giant cells were seen in the endoneurium. Occasional macrophages were seen with bacilli, single or in small clusters. No bacilli were traced to Schwann cells. Myelin debris in various stages of disintegration was seen mostly in the macrophages. Schwann cells appeared active and increased in number. A few regenerating fibers were also seen. The lesions seen in these nerves compared well with the human tuberculoid type of lesions as per the Ridley-Jopling classification (15).
Immunosuppressed (T20 0 x 5R) mice with intraneural M. leprae. There was a more diffuse and moderate loss of nerve fibers. There was only macrophage infiltrate in these nerves. Occasional lymphocytes were seen, but the epithelioid type of macrophages or giant cells were not seen. The rest of the changes seen were similar to normal mice with intraneural M. leprae. Here, again, M. leprae were seen only in the macrophages.
Silica-treated mice with a crush injury. There was no macrophage infiltrate in these nerves. Fairly intact myelin rings were seen around collapsed and degenerated axons within the Schwann tubes. No activation or proliferation of Schwann cells nor any regeneration or remyelinating activity was seen in these nerves (Fig. 2A and 2B).
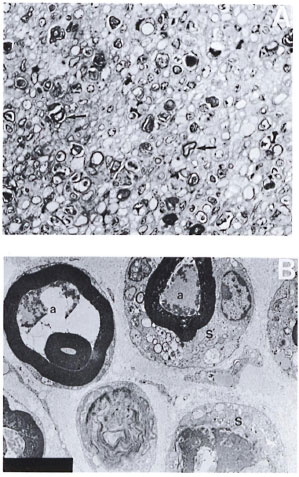
Fig. 2. A. Part of the sciatic nerve with a crush injury in a silica-treated mouse from a biopsy obtained 2 weeks after a crush injury. No normal-looking myelinated fibers, macrophages, or activation of Schwann cells arc seen. Regeneration and remyelinating fibers are also not seen in this nerve. Note the presence of large numbers of darkly stained myelin rings (arrow) (semithin transverse section stained with toludine blue x 750). B. Electron photomicrograph of a part of the crushed nerve from the silica-treated mouse in Figure 2A. Note the degenerate and collapsed axons (a) and fairly intact myelin rings lying within the Schwann cells (S). Some membraneous inclusions arc also seen in the Schwann cell cytoplasm. Note the absence of macrophages and regenerative activity in this nerve (uranyl acetate plus lead citrate stain x 5000).
In contrast, nerves with only a crush injury (group 6) revealed macrophage infiltrate, phagocytosis, and a breakdown of myelin by the macrophages. There was Schwannian proliferation and regeneration of fibers, suggesting that in silica-treated mice the normal process of degeneration and regeneration following a crush injury was retarded in the absence of macrophages.
Silica-treated mice with/without a crush injury and M. leprae. When M. leprae were injected along with a crush injury in the silica-treated mice (group 4), the lesion obtained was very similar to that seen with a crush alone, although the extent of the macrophage infiltrate was less. Myelin degradation was not as effective as in the mice without silica but regenerative and remyelinative activity was very good (Fig. 3). The epithelioid type of macrophages and lymphocytes were not seen in these nerves, and bacilli were seen only in the macrophages.
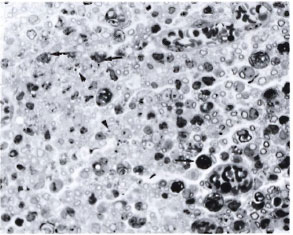
Fig. 3. Part of the sciatic nerve with a crush injury plus intraneural M. leprae in a silica-treated mouse. There are uniformly distributed, small, thinly myelinated fibers suggesting regeneration. Most of the darkly stained structures (arrow) arc the myelin debris, some within macrophages and some within Schwann cells. Note the presence of large numbers of bacilli within some of the macrophages (arrowhead) (semithin transverse section stained with toludine blue x 750).
When M. leprae were injected intraneurally without crushing the nerve in silicatreated mice (group 3), the lesion obtained was similar to that of TR200 x 5R mice with M. leprae. Several macrophages carrying large numbers of M. leprae were seen within the endoneurium (Fig. 4A and 4B).
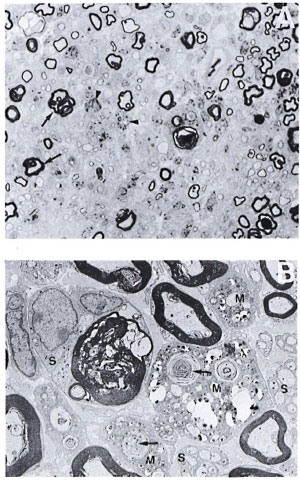
Fig. 4. A. Part of the sciatic nerve injected intraneurally with viable M. leprae in a silica-treated mouse from a biopsy obtained 2 weeks after intraneural injection of M. leprae. Some of the myelinated fibers show extensive folding of the myelin (arrow), and there are several thinly myelinated regenerating libers. Macrophages are also seen, many of them with bacilli (arrowhead), but no lymphocytic infiltrate (semithin transverse section stained with toludine blue x750). B. Electron photomicrograph of the same Figure 4A nerve showing three macrophages (M) carrying large numbers of bacilli and myelin fragments (arrow) lying in separate compartments. No bacilli arc seen in the adjacent Schwann cells (S). One of the Schwann cells is seen with a large myelin ring within its cytoplasm (uranyl acetate plus lead citrate stain x4000).
DISCUSSION
This study is a preliminary attempt to establish an experimental leprosy model primarily to get tuberculoid and lepromatous types of granulomatous responses in the nerve. Our results show that it is possible to observe a spectrum of immune responses by immunomodulating the animal in relation to the nerve damage. There was no significant difference observed in the two groups of experiments carried out using human- and armadillo-derived M. leprae. The kinetics of the cells migrating into the lesion remain to be explored.
The observation that M. leprae were not seen within the Schwann cells even in immunosuppressed mice further raised our curiosity to extend our observations to silicatreated mice in order to immobilize the macrophages. However, our results in this experimental group proved more interesting.
In Swiss white mice on silica treatment following a crush injury there was more or less total retardation of the regenerative activity of the nerve fibers at 2 weeks. This was unlike what has been reported by Muller and Minwegan (14) in a rat model. At 4- 5 weeks these authors reported a macrophage infiltrate plus nerve regeneration but poor myelin clearance. This difference may be due to the dose of silica used, the time span of study, or the animal species itself.
In our experiment, the addition of M. leprae seemed to negate the silica effect, to invite a (sub)population of macrophages into the lesions, and to activate the normal course of de-/regenerative activity of the nerve. What was intriguing was the observation of the lack of lymphocytes and epithelioid type of response in these lesions. This observation suggests a role for a specific group of (silica sensitive ?) macrophages in bringing about a tuberculoid type of granulomatous response.
We failed to see M. leprae in the morphologically identifiable Schwann cells even in silica-treated mice. It is difficult to explain why in situ Schwann cells did not take up any bacteria even though they were viable. In one of our earlier studies, a piece of highly positive lepromatous nerve was grafted onto the sciatic nerve of an immunosuppressed mouse. Although there was good axon innervation into the grafted human nerve, no bacilli were seen in the host Schwann cells even after 6 months of grafting (17). However, in the in vitro system, both in organized dorsal root ganglion cultures and in monolayer cultures of Schwann cells of mice and humans (12,16), Schwann cells do phagocytose M. leprae. In a renewed effort, we injected a second higher dose of M. leprae (30 x 106) into the sciatic nerve of one of the immunosuppressed, silicatreated mice and studied the nerve 5 weeks after the first dose of M. leprae. Even in this nerve, although there were carpets of macrophages with M. leprae (Fig. 5) lying next to denervated Schwann cells, none were located within the Schwann cells. This is contrary to what we see in human leprous nerves.
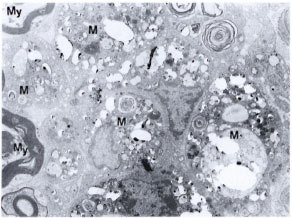
Fig. 5. Electron photomicrograph of part of the sciatic nerve injected intraneurally with two doses of M. leprae (10-20 x 106 and 30 x 106) at a 2-week interval. This mouse was immunosuppressed (T200 x 5R) as well as silica treated. Biopsy was obtained 5 weeks after first dose of M. leprae. Note the presence of bacilli-laden macrophages (M) showing foamy cytoplasmic changes. Myelinated nerve fibers (My) are seen on one side. This lesion is comparable to a lepromatous-type nerve lesion (uranyl acetate plus lead citrate stain x4000).
In a study of the cell population in Wallerian degeneration, Gibson (5) found no evidence for the once quite widely held view that Schwann cells transform into macrophages. Hence, it is unlikely that the macrophages seen in these nerves were transformed Schwann cells. To find the answer to what prevents M. leprae from entering the Schwann cells of mice in situ would be an interesting area for further study.
Acknowledgment. 1 am grateful to Dr. N. H. Antia, Trustee and Director of the Foundation for Medical Research, for supporting this work; to Drs. Tannaz Birdi and Nergcs Mistry for helpful suggestions, and to Miss Suchitra Khadc for technical assistance.
REFERENCES
1. BROSNAN, C. F., BORNSTEIN, M. B. and BLOOM, B. R. The effects of macrophage depletion on the clinical and pathological expression of experimental allergic encephalomyelitis. J. Immunol. 126(1981)614-620.
2. COLSTON, M. J. Application of thymectomized irradiated mouse to the detection of persisting Mycobacterium leprae. Int. J. Lepr. 50(1982)83-89.
3. COWLEY, S. A., BUTLER, C, VERGHESE, S., CURTIS, J. and TURK, J. L. Nerve damage induced by mycobacterial granulomas in guinea pig sciatic nerves. Int. J. Lepr. 56(1988)283-290.
4. ENGERS, H. and Ji, B. Promotion of research on leprosy reactions and nerve damage. TDR Newsletter 25 Winter/Spring 1988.
5. GIBSON, J. D. The origin of the neural macrophages: a quantitative ultrastructural study of cell population changes during Wallerian degeneration. J. Anat. 129(1979)1-19.
6. HILSON, G. E. R. Observations on the inoculation of M. leprae in the footpads of the white rat. Int. J. Lepr. 33(1965)662-665.
7. JOHNSTONE, P. A. S. The search for animal models of leprosy. Int. J. Lepr. 55(1987)535-547.
8. KOHSAKA, K., MORI, T. and ITO, T. Lepromatoid lesion developed in nude mouse inoculated with Mycobacterium leprae: animal transmission of leprosy. Lepro 45(1976)177-187. (Abstract) Int. J. Lepr. 45(1977)403-404.
9. LEW, J., YANG, Y. T. and PYUN, W. S. Experimental infection in the Korean chipmunk with M. leprae. Int. J. Lepr. 42(1974)193-202.
10. MARTIN, L. N., GORMUS, B. J., WOLF, R. H. and WALSH, G. P. Animal models of leprosy. In: Microbiology 1984. Leive, L. and Schlessingcr, D., cds. Washington, D.C.: American Society for Microbiology, 1984, pp. 307-311.
11. MSHANA, R. N., HUMBER, D. P., HARBOE, M. and BELEHU, A. Nerve damage following intraneural injection of Mycobacterium leprae into rabbits presensitized to mycobacteria. Clin. Exp. Immunol. 52(1983)441-448.
12. MUKHERJEE, R., MAHADEVAN, P. R. and ANTIA, N. H. Organized nerve culture I. A technique to study the effect of M. leprae infection. Int. J. Lepr. 48(1980)183-188.
13. MUKHERJEE, R., MAHADEVAN, P. R. and ANTIA, N. H. Organized nerve culture II: DNA synthesis in Schwann cells in the presence of M. leprae. Int. J. Lepr. 48(1980)189-192.
14. MULLER, H. W. and MINWEGAN, P. Nonresident macrophages in peripheral nerve of rat: effect of silica on migration, myelin phagocytosis and apolipoprolein expression during Wallerian degeneration. J. Neurosci. Res. 81(1987)222-229.
15. RIDLEY, D. S. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
16. SAMUEL, N. M., CRAWFORD, C. L. and GRANGE, J. M. Ultrastructurc of human foetal Schwann cells in tissue culture infected with Mycobacterium leprae. Lepr. Rev. 59(1988)17-24.
17. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112(1960)445-454.
18. SHETTY, V. P. and GEORGE, V. Xenograft studies in leprous neuropathy. Int. J. Lepr. 54(1986)133-135.
19. STORRS, E. E., WALSH, G. P., BURCHFIELD, H. P. and BINFORD, C. H. Leprosy in the armadillo: new model for biomedical research. Science 183(1974)851-852.
20. WISNIEWSKI, H. M. and BLOOM, B. R. Primary demyclination as a nonspecific consequence of a cell mediated immune reaction. J. Exp. Med. 111(1975)346-359.
1. Dr. (Mrs.) Senior Research Officer, The Foundation for Medical Research, 84-A, R. G. Thadani Marg, Worli, Bombay 400018, India.
Received for publication on 3 June 1992.
Accepted for publication in revised form on 17 November 1992.