- Volume 61 , Number 1
- Page: 76–81
Search for effective short-course regimens for the treatment of leprosy
Editorial opinions expressed are those of the writers.
For evident reasons the treatment period for infectious diseases should be as short as possible. During the last 15 years we have been involved in studies on short-course regimens for the treatment ofleprosy. Based on published reports 1-12 and ongoing observations on as many patients as possible who entered these studies in the past, some conclusions can be drawn and are presented here.
Several factors influencing the minimal duration of treatment of infectious diseases have been identified as the: a) number of infecting organisms present; b) metabolic state of the organisms; c) extra- or intracellular residence of the infecting organisms; d) bactericidal and postantibiotic effect(s) of the drug(s) administered; e) availability of long-acting drugs; and f) immune competence of the host.
All of these factors have been studied and well illustrated in leprosy. The number of viable bacilli in the multibacillary (MB) forms of the disease may be very high: 10 10 - 10 11 viable. This population of Mycobacterium leprae is composed of three subgroups: 13 a) wild-type, drug-susceptible organisms; b) drug-resistant mutants; and c) dormant bacilli.
The first subgroup, representing more than 99.9% of the total, can be killed by any of the available drugs active against M. leprae. The rapidity and extent of the bacterial killing varies for each drug, and can be studied in experimentally inoculated mice 14-17 and through "short term clinical trials" in man, when serial skin biopsies obtained during treatment are inoculated into normal and/ or immunosuppressed mice. 18-20
At present the available drugs can be classified in decreasing order of bactericidal activity: rifampin (RMP), ofloxacin (OFLO), minocycline (MINO), clarithromycin (CLA), prothionamidc (PTH) or ethionamide (ETH), clofazimine (CLO), and dapsone (DDS). In man, RMP kills 4 log 10 of M. leprae after a few doses,19 OFLO after a month of daily administration,18 CLA and MINO after 2 months of treatment. 21-22 The available techniques do not allow the measurement of the bactericidal effect of drugs beyond this 4 log killing. However, after this first phase of rapid or relatively rapid killing, the remaining viable bacilli most probably follow an asymptotic curve.
The second subgroup of the drug-resistant mutants represents a fraction of 10 9 - 10 6 (depending on the drug) of the wild population, and is the reason why, for MB patients, combined therapy is mandatory, mutants resistant to one of the drugs in the combination (and only mutants resistant to a single drug are mathematically possible) being killed by the other drug(s). In contrast, paucibacillary (PB) patients, who harbor a maximum of 10 6 organisms, can be treated with monotherapy.
The third subgroup consists of so-called "dormant bacilli," a small fraction of the wild-type, drug-susceptible organisms which, although drug susceptible, are unaffected by them.13-20 It is generally assumed that these organisms escape the action of antibiotics because they are not metabolically active. Such organisms are not limited to leprosy but occur in many infectious diseases. They may remain dormant for long periods, are gradually eliminated by the immune defense mechanisms of the host, but may as long as they are present be reactivaled by unknown mechanisms, and give rise to a relapse due to drug susceptible organisms.
In leprosy, dormant bacilli have been shown to exist in numbers between 10 3 - 10 5 in at least 9% of those patients who have a bacterial index (131) = 5 prctrcatment, corresponding to a total of 10 9 - 10 10 viable M. leprae. 20 Dormant bacilli have been documented after RMP-DDS, RMP-thiambutosine, and intensive RMP-PTH-DDS therapy (daily for 2 years). 23-25
In the absence of a laboratory model for the study of these dormant leprosy bacilli, and their possible sccptibility to new drugs in particular, information concerning the optimal treatment of leprosy has to be derived from carefully designed clinical studies. Short-term clinical trials also measure the intracellular activity of the antileprosy drugs as well as any postantibiotic effect or long-term action, e.g., when the drug under study is administered intermittently as has been shown for monthly doses of CLO. The immune deficit in MB leprosy has been well documented, but whether it precludes definite cure of MB leprosy has to be documented by a follow up of patients after treatment.
How short can leprosy therapy be?
Paucibacillary leprosy. In spite of the important bactericidal activity of RMP, and the limited bacterial load in PB leprosy, a single dose of RMP-even in high dose (40 mg/kg) - cannot be advocated in PB leprosy since this regimen is followed by high failure and relapse rates, particularly in patients with three or more skin lesions. 11 This observation shows that the bactericidal activity of a single dose of RMP does not exceed 10 6 organisms.
For the treatment to be effective in PB leprosy, a dose of RMP should be associated with several other doses of RMP, either daily for 6 days, or weekly for 8-12 weeks or monthly for 6 months, or with daily DDS for 1 year. 8-12 Thus, the combination of RMP with a 200-mg dose of the long-acting drug MINO deserves to be studied or a combination of RMP-OFLO-CLA-MINO shown to be very bactericidal in the mouse. 22, 26-29
Multibacillary leprosy. In view of the bacterial populations present at the start of therapy in MB leprosy, there can be no doubt about the need for combined therapy to prevent the selection of drug-resistant mutants. Thus, the questions remain: Which drugs should be administered, with what frequency and for how long, to kill the population of actively multiplying M. leprae and to prevent the resurgence of the dormant bacilli? Although many theoretical considerations may be formulated, proof of the efficacy of regimens, in terms of definite cure without relapse, can only result from prospective trials.
In our studies on the treatment of MB leprosy, the duration of treatment varied from 104 weeks to 52, 34, 13 and 4 weeks. 3, 4, 6, 10, 12, 19, 26, 28, 29 Some of these studies were controlled clinical trials, other were pilot trials studying only one regimen (Table 1).

Except for one double-drug regimen studied in 1976 1 (regimen A: RMP + DDS) and the 4-week regimen with four drugs, 7 all regimens were triple-drug regimens consisting of RMP + PTH (or ETH) + either DDS or CLO; the latter replacing DDS in relapsing patients who had been treated previously for 5 years or more with DDS monotherapy and were thus at risk for harboring DDS-resistant M. leprae. RMP was administered once monthly (WHO regimen) or twice weekly (six regimens: A, B, RED, REC, 13w RED,13w REC). In four regimens RMP was administered daily for 26 weeks (regimens RED/D, RED/ED, REC/C, REC/EC grouped in Table 2 as 26 + 26).3 In two regimens RMP was administered daily for 8 weeks, followed by a once-weekly administration for 44 weeks (regimen 8 + 44) 2 or none (regimen 8 + 26). 6 ETH and DDS or CLO were always administered daily. All treatments were supervised.
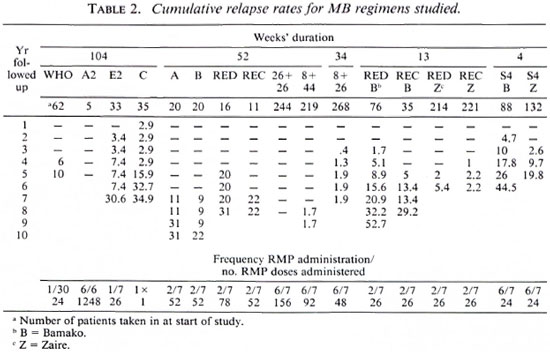
The diagnosis of disease and relapse was based on clinical, bacteriological and histopathological evidence. During follow up the patients were examined at least yearly. Whenever possible, M. leprae strains responsible for relapses were isolated in mouse foot pads and tested for drug sensitivity. The cumulative relapse rates were calculated by the life table method up to the time when more than five patients were seen. Comparisons were made by the logrank test.
In all regimens the BI continued to decrease after the end of therapy to reach 0, except in the 4-wcck regimen, where the BI decreased considerably more slowly. 7
In almost all regimens relapses did occur although with great differences in incidence and in the disease-free intervals between the end of therapy and the diagnosis of relapse. Relapse in MB leprosy may be the result of three different mechanisms: from treatment failure, resulting from insufficient killing of the original wild-type population which starts to multiply again very soon after stopping treatment; from the selection of drugresistant organisms; or from reactivation of dormant organisms. Since all M. leprae isolated from relapses were fully drug sensitive, relapses must have resulted from treatment failure or from reactivation. Furthermore, it may be expected that relapses resulting from treatment failure occur earlier than those resulting from reactivation.
Table 2 presents the information concerning the relapse rates for the different regimens. The mean length of follow up differs widely among the regimens, from 3.7 to 9.7 years. Among the 11 treatment groups followed for at least 7 years, regimens E2, C, A, B, RED, REC, 13w RED-B, 13w REC-B, 26 + 26, 8 + 44 and 8 + 26, the latter three regimens stand out for their very low relapse rates of 0.07% (95% C.I.: 0.06-0.08), 1.7% (95% C.I.: 1.1-2.3) and 1.9% (95% C.I.: 1.13-2.67), respectively. In all other regimens of this group, the cumulative relapse rates reached 21% to 52%.
Regimen C (1 dose of RMP, followed by 2 years of daily DDS) gave rise to early relapses which remained stable until year 5, suggesting early relapses could be interpreted as treatment failures and later relapses as reactivations. The same phenomena seemed to occur in regimen E2. Relapses in regimens A and B were diagnosed very late, after 9 and 10 years. In the two regimens with very low relapse rates (8 + 44 and 8 + 26), the relapses appeared 8 and 3 years after the end of treatment; in the 8 + 26 regimen, the relapse rate remained stable between years 5 to 8 post-treatment. Three other patient groups have been followed for 6 years: 13w RED-Z, 13w REC-Z and 4w B. The relapse rate for them is still increasing. The remaining patient groups have been followed for 4-5 years only (WHO, A2, S4-Z).
There is an important difference between the 13w RED-B and the 13w RED-Z at 6 years: 15.6% versus 5.4% relapses (logrank test (0.01 < p < 0.05). However, the Bis at the start of treatment in the patients in Bamako were significantly higher than those in the patients in Zaire: 72% of the patients had a BI of 5 in Bamako against 47% in Zaire (p = 0.002). Overall, relapses occur more frequently among patients with a higher BI at the start of treatment (Table 3). There is no significant difference at 5 years between the 4-weck regimens applied in Bamako and Zaire, but in the latter country the diagnosis of relapses was made somewhat later.

The regimens with the best results had a follow up of at least 7 years, all had a duration of 8 or 12 months (regimens 26 + 26, 8 + 44 and 8 + 26), and involved at least a period of daily administration of RMP, 6 days a week, the minimum being 8 weeks in regimen 8 + 26. In terms of the number of doses of RMP administered, this regimen may be compared with regimens A, B and REC (in which 52 doses of RMP were administered intermittently, twice a week, for 26 weeks) and in regimens B and REC in combination with 6 months of ETH. These results illustrate that daily administration of RMP is more bactericidal than intermittent administration, although it must be mentioned that the only patient treated with regimen A2 and seen at 8 years did relapse. At first sight, the greater efficacy of the daily administration of RMP is surprising. However, it has been observed once in experiments in mice by Grosset and Guelpa-Lauras. 30 This would also mean that the WHO regimen when applied to highly bacilliferous cases will be followed by relapses. Indeed, the WHO regimen applied to previously untreated, highly bacilliferous patients (45 of 62 or 72.5% of the patients had a BI = 5 at the start) is followed by a cumulative relapse rate of 10% at 5 years. From these observations the following conclusions can be drawn:
a) To evaluate treatment regimens in MB leprosy, it is necessary to follow patients until 9-10 years after the end of treatment.
b) The risk of relapse increases with time.
c) Relapses occurring during the first 3 years of follow up at a rate higher than 1% may be a first indication of a later important incidence of relapses. However, the absence of relapses during the first 3 years does not exclude high relapse rates later on.
d) Relapses occur probably more often among patients with a higher BI at the start of treatment.
e) There are indications that daily administration of RMP for 8 weeks or more is more bactericidal than intermittent administration.
f) The combination of this powerful bactericidal with an "intermediate" bactericidal, such as thioamide, both administered daily for 4 weeks is insufficient and leads to early relapses (points d and e should be the subjects of new studies).
As a result of its hepatotoxicity, 31-33 the combination of RMP and a thioamide cannot be advocated, except under close medical supervision. Thus, a combination of RMP together with one or (possibly, preferably?) more of the powerful recently discovered bactericidals, administered daily, could be able to shorten therapy below 8 months.
Is there any effect of the newer bactericidals on the dormant bacilli? The only answer to this question is through clinical trials with follow-up periods of 9-10 years! For the time being it seems logical to administer to those patients who have been treated in the past with whatever combined regimen and who are "inactive," a short course of a few days of a bactericidal drug such as RMP, once a year, for 10 years, after the end of treatment to eliminate any possible bursts of dormant bacilli.
But what level of relapse is acceptable? A figure of 1% per year has been proposed for leprosy since this figure can be obtained after 20 years of supervised DDS monotherapy. 34 In tuberculosis it has been shown that a cure rate of 80% has an impact on the transmission and, if the effort can be sustained, tuberculosis would cease to be a public health problem by the year 2000. 29 Regimens 8 + 44 and 8 + 26 would satisfy this requirement. The discovery of other drugs highly bactericidal for M. leprae. 22-26-29 opens perspectives for still shorter treatment regimens in MB leprosy.
Finally, it should be emphasized that most MB patients are diagnosed when their Bis = 5 or 4. If they could be detected at an earlier stage, the number of their dormant bacilli would be smaller and the possibility for late relapses would be much reduced, allowing shorter courses of treatment.
A posteriori, during the two decades of the 1970-1980s two misleading arguments were widespread: one was that RMP was a very expensive drug and would not become much cheaper; the second was that RMP was such a powerful bactericidal drug that nothing could be gained by administering it as frequently as daily. Both of these arguments have been refuted.
- Stefaan R. Pattyn, M.D.
Institute for Tropical Medicine and University of Antwerp
Antwerp, Belgium
1. Pattyn, S. R., Saint André, P., Ferracci, C. and Baquillon, G. Comparative study of two regimens of combined chemotherapy of one year duration in multibacillary leprosy; results after four and five years follow-up. Int. J. Lepr. 52(1984)297-303.
2. Pattyn, S. R., Bourland, J., Grillone, S., Grocnen, G. and Ghys, P. Combined regimens of one year duration in the treatment of multibacillary leprosy. I. Combined regimen with rifampin administered during one year. Lepr. Rev. 60(1989)109-117.
3. Pattyn, S. R., Groenen, G., Janssens, L., Deverchin, J. and Ghys, P. Combined regiments of one year duration in the treatment of multibacillary leprosy. II. Combined regimens with rifampin administered during 6 months. Lepr. Rev. 60(1989)118-123.
4. Pattyn, S. R., Bourland, J., Deverchin, J., Ghys, P., Grillone, S., Groenen, G., Janssens, L. and Kuykens, P. Status of the multibacillary leprosy patients treated with combined regimens of 1 year duration, after a mean follow-up of more than 5 years. Lepr. Rev. 62(1991)337-339.
5. Pattyn, S. R., Groenen, G., Janssens, L., Kuykens, L. and Mputu, L. B. Treatment of multibacillary leprosy with a regimen of 13 weeks duration. Lepr. Rev. 63(1992)41-46.
6. Pattyn, S. R., Bourland, J. and Kazeze, C. Ambulatory treatment of multibacillary leprosy with a regimen of 8 months duration. Lepr. Rev. 63(1992)36- 40.
7. Jamet, P. and Ji, B. for the Marchoux Chemotherapy Study Group. Relapses in multibacillary leprosy patients after stopping treatment with rifampincontaining combined regimens. Int. J. Lepr. 60(1992)525-535.
8. Warndorfr, J., Bourland, J. and Pattyn, S. R. Follow-up on short course 2 months' rifampin treatment of paucibacillary leprosy. Lepr. Rev. 53(1982)9-17.
9. Pattyn, S. R., Groenen, G., Bourland, J., Grillone, S., Janssens, L. and the Collaborative Study Group for the Treatment of Leprosy. A controlled therapeutic trial in paucibacillary leprosy comparing a single dose of rifampin followed by one year of daily dapsone with ten weeklv doses of rifampin. Lepr. Rev. 58(1987)349-358.
10. Pattyn, S. R., Bacquillon, G., Husser, J. A., Maiga, M. and Jamet, P. Evaluation of five treatment regimens, using either dapsone monotherapy or several doses of rifampin in the treatment of paucibacillary leprosy. Lepr. Rev. 61(1990)151-156.
11. Pattyn, S. R., Groenen, G., Janssens, L., Kuykens, P. and Mputu, L. B. A controlled therapeutic trial in paucibacillary leprosy comparing a single dose of rifampin with a single dose of rifampin followed by one year of daily dapsone. Lepr. Rev. 62(1991)179- 185.
12. Pattyn, S. R., Groenen, G., Bourland, J., Grillone, S., Kuykens, L. and Stes, P. Eight years of follow-up of paucibacillary patients treated with short course regimens. Lepr. Rev. 63(1992)80-82.
13. Ellard, G. A. Chemotherapy of leprosy. Br. Med. Bull. 44(1988)775-790.
14. Colston, M. J., Hilson. G. R. F. and Bannerjee, D. K. The "proportional bactericidal test," a method for assessing bactericidal activity of drugs against Mycobacterium leprae in mice. Lepr. Rev. 49(1978)7-12.
15. Shepard, C. C. A kinetic method for the study of the activity of drugs against Mycobacterium leprae in mice. Int. J. Lepr. 35(1967)429-435.
16. Shepard, C. C, van Landingham, R. M. and Walker, L. L. Recent studies on antileprosy drugs. Lepr. Rev. 54(1983)23S-27S.
17. Styblo, K. and Chum, H. J. Treatment results of smear positive tuberculosis in Tanzania National Tuberculosis and Leprosy Programme: standard and shortcourse chemotherapy. Proc. XXVI World Conference on Tuberculosis and Respiratory Diseases, Singapore, 4-7 November 1986. Tokyo: Professional Postgraduate Services, 1987, pp. 122-126.
18. Grosset, J. H., Ji, B., Guelpa-Lauras, C. C, Perani, E. G. and N'Deli, L. N. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281-295.
19. Levy, L., Shepard, C. C. and Fasal, P. The bactericidal effect of rifampin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
20. Subcommittee on Clinical Trials of the Chemotherapy (Thelep) Scientific Working Group of the UNDP/World Bank/WHO Special Programme for Research and Training of Tropical Diseases. Persisting Mycobacterium leprae among Thelep trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
21. Gelber, R. H., Murray, L., Sin, P., Tsang, M., Iranmancsh, A. and Rca, T. H. Bactericidal antibiotics of three different classes emerge to treat leprosy: recent developments from the laboratory to the patient. Int. J. Lepr. 59(1991)7-18.
22. Gelber, R. H., Sin, P., Tsang, M. and Murray, L. Activities of various macrolide antibiotics against Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)760-763.
23. Rees, R. J. W., Waters, M. F. R.. Pearson, J. M. H., Helmey, H. S. and Laing. A. B. O. Long-term treatment of dapsone-rcsistant leprosy with rifampin: clinical and bacteriological studies. Int. J. Lepr. 44(1976)159-169.
24. Waters, M. F. R., Recs, R. J. W., McDougall, E. C. and Weddcll. A. G. M. Ten years of dapsonc in lepromatous leprosy: clinical, bacteriological and histological assessment and the finding of viable leprosy bacilli. Lepr. Rev. 45(1976)288-298.
25. Waters, M. F. R., Recs, R. J. W., Pearson, J. M. H., Laing, A. B. G., Helmy, H. S. and Gelber, R. H. Rifampin for lepromatous leprosy: nine years experience. Br. Med. J. 1(1978)133-136.
26. Grosset, J. H., Guclpa-Lauras, C. C., Perani, E. G. and Beoletto, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
27. JI. B., Perani. E. G., Petinon, C. and Grosset, J. H. Bactericidal activities of single or multiple doses of various combinations of new antileprosy drugs and/or rifampin against Mycobacterium leprae in mice. Int. J. Lepr. 60(1992)556-561.
28. Pattyn, S. R. Activity of ofloxacin and pefloxacin against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 31(1987)671-672.
29. Pattyn. S. R. Anu-Mycobacterium leprae activity of several quinolones studied in the mouse. Int. J. Lepr. 59(1991)613-617.
30. Grosset, J. H. and Guelpa-Lauras. C. C. Activity of rifampin in infections of normal mice with Mycobacterium leprae. Int. J. Lepr. 55(1987)847-851.
31 Cartel, J. L., Mallau, J., Guelpa-Lauras, C. C. and Grosset, J. H. Hepatitis in leprosy patients treated with a daily combination of dapsone, rifampin and a thioamide. Int. J. Lepr. 51(1983)461-465.
32. Cartel, J. L., Naudillon, Y., Arthus, J. C. and Grosset, J. H. Hepatotoxicity of the daily combination of 5 mg/kgprothionamide + 10 mg rifampin. Int. J. Lepr. 53(1985)15-18.
33. Pattyn, S. R., Janssens, L., Bourland, J., Saylan, T., Davies, E., Grillone, S., Ferracci, C. and the Collaborative Study Group for the Treatment of Leprosy. Hepatotoxicity of the combination of rifampin-ethionamide in the treatment of multibacillary leprosy. Int. J. Lepr. 52(1984)1-6.
34. Waters, M. F. R., Rees, R. J. W. L., Laing, A. B. G., Fah, K. K., Meade, T. W., Parikshak, N. and North W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulfone therapy. Lepr. Rev. 57(1986)101-109.