- Volume 61 , Number 1
- Page: 82–101
Priorities for the future and prospects for leprosy control
PRIORITIES FOR THE FUTURE
The major priorities for leprosy control services in the near future are: a) to increase multidrug therapy (MDT) coverage; b) to integrate the leprosy control services with the general health services; and c) to prevent disabilities.
Increase of MDT Coverage
The first priority for many leprosy control programs should be to increase MDT coverage. Based on at least one leprosy patient on treatment per 10,000 population, at the beginning of 1992 leprosy was a public health problem in 88 countries. The MDT coverage in these countries at that time, expressed as the percentage of registered patients on MDT, is given in Table 1. 1
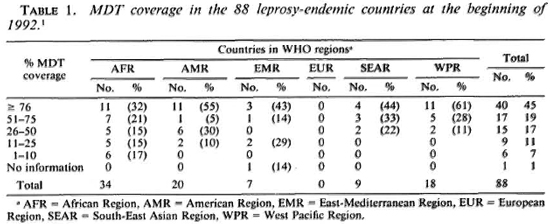
Thus, by the beginning of 1992 the MDT coverage was still below 25% in 15 countries (17%); 11 (73%) of them belonged to the African Region. Of the 57 countries (65%) with MDT coverage of over 50%, only 18 (32%) were situated in the African Region.
The registered number of leprosy cases, the registered number of cases per 10,000 population, the proportion of patients on MDT, and the cumulative MDT coverage (defined as the proportion of patients eligible for MDT brought under MDT since its introduction) at the beginning of 1992, for the different WHO regions, are given in Table 2.
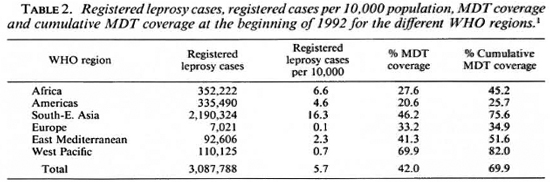
The changes in registered leprosy patients, in registered number of patients per 10,000 population, and in MDT coverage between the end of 1990 and the beginning of 1992 are given in Table 3. Although the MDT coverage in the African Region is still low, the 50% increase is promising. A main reason for the low MDT coverage in the African Region was that Nigeria, with 44.3% of all registered leprosy patients, had attained MDT coverage and a cumulative MDT coverage of only 8.9% and 10.2%, respectively. The situation in the Americas, with the lowest MDT coverage of all the WHO regions, and a 13% decrease in MDT coverage, is particularly worrisome. Brazil, where 77.5% of all leprosy patients of the American Region were registered, had only reached 12.4% MDT coverage, with a cumulative MDT coverage of 14.6%. Although the MDT coverage in the South-East Asia Region, where 71% of all leprosy patients were registered, is one of the highest, the 30% decrease should be a matter of concern. Apparently one or more countries in this region were not able to maintain high MDT coverage. The poor results in the European Region were especially caused by low MDT coverage in the Russian Federation and Turkey, where 86.3% of the patients were registered. In the Russian Federation, both MDT coverage and cumulative MDT coverage were 54.9%, and in Turkey both rates were 16.5%. The most satisfactory results were obtained in the West Pacific Region, which had the highest MDT coverage rate and where MDT coverage continued to increase.
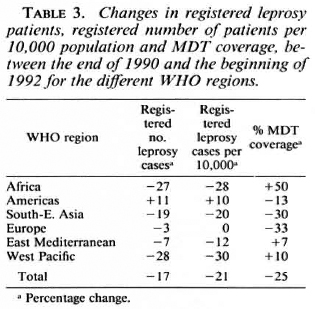
The background for the recommendation of MDT was the increasing problem of dapsone resistance, which was considered a world-wide problem. 2 Although recent data about dapsone resistance are not available, it should be expected that in the areas where the majority of the patients are still treated with dapsone monotherapy, the dapsoneresistance problem has further increased. The 1.8 million registered leprosy patients who were still on dapsone monotherapy by the beginning of 1992 most probably included several hundreds of thousands of patients with dapsone-resistant leprosy. If these patients are not treated effectively, they will continue to increase the dapsone-resistance problem, while their condition may seriously deteriorate.
The present situation, 10 years after the recommendation of MDT, requires immediate and vigorous attention, particularly in those countries which have not been able to attain at least 50% MDT coverage. Several of these countries face major problems, including a poor infrastructure for leprosy control. Others have more pressing public health problems, difficulties in the mobilization of resources, and the preparation of a realistic plan of action for MDT. They should be assisted with the preparation of a plan of action, including the development of strategies for MDT which can be applied under the prevailing circumstances, while making optimal use of the available resources. Furthermore, these countries may need substantial financial assistance, not only for drugs and training but also for operational costs, particularly during the first years of MDT implementation.
Recently the World Health Assembly adopted a resolution on the elimination of leprosy as a public health problem by the year 2000. 3 Its plea to member states to increase their commitment for leprosy may hopefully lead to increased efforts toward MDT implementation.
As a result of MDT implementation the number of patients registered for treatment will rapidly decrease. In several programs a decline in the number of patients on antileprosy treatment of 75% or more was observed some years after the introduction of MDT. 4-5 In the All Africa Leprosy and Rehabilitation Training Center (ALERT) leprosy control program the number of patients on antileprosy treatment declined from 21,338 in July 1982 to 3725 in July 1989. This is a reduction of 82.5% within a period of 7 years. As a consequence of the marked decrease in case- and workload, the organization of leprosy control services should be reconsidered and priorities of these services should be redefined. In this respect the two major priority areas are: a) the integration of the leprosy control services with the general health services, and b) the prevention of disabilities.
Integration of Leprosy Control with General Health Services
So far most of the progress with MDT implementation has been made in nonintcgrated programs. A logical option for these programs will be the integration of leprosy control activities within the general health services. 4, 6, 16-20 Integration has political, professional, operational, technical and financial implications. Although it is the policy of many governments that control of communicable diseases should be integrated within the general health services, its implications have not always been worked out.
There is much confusion about the meaning of integration. It implies that the leprosy control activities will become the responsibility of the permanent multipurpose services. 6, 16, 18, 20 This does not mean that all specialized staff and services should disappear. Specialized staff at each country's Ministry of Health and at defined administrative levels will often remain essential for planning, organization, supervision, distribution of supplies, training, monitoring and evaluation.
The transformation of a nonintegrated into an integrated program is a complex process. It requires careful planning including the identification of areas where problems can be expected and finding the solutions for preventing or solving these problems. The lack of motivation of a general health staff for leprosy control is one of these problems. Others are the absorption of specialized leprosy staff by the general health services and the reluctance of specialized leprosy staff to delegate responsibilities to the general health staff.
While integration may be the most appropriate, efficient, and effective solution in several countries, this may not be the case everywhere. An adequately functioning general health service is considered a prerequisite for integration. 16, 20 This implies that the general health services are adequate in quantity and quality. Thus, the vast majority of the population should have access to the general health services, meaning that they live within 5 km from such a service, and staff should be available who can take responsibility for the leprosy control activities. Where such services do not exist, it will be more effective and efficient to use the leprosy control infrastructure for strengthening the control of other diseases, in particular tuberculosis. It is sometimes recommended that dermatological services should take up the responsibility for leprosy control. These services should certainly be involved in diagnosing patients. However, because they often only exist in towns and concentrate on curative treatment, they arc not the most appropriate units for taking responsibility for the control of leprosy.
Another option is combined leprosy and tuberculosis control which is integrated into the general health services, with specialized staff at various administrative levels. In an increasing number of countries such combined, integrated programs are in use. 7, 15, 21, 22
Which strategy would be most appropriate under the prevailing circumstances also depends on, besides the infrastructure of the general health services, factors such as: a) the extent of the leprosy problem; b) the cost of specialized services; c) the existence of programs for control of other diseases, and possibilities for combined services; and e) the available resources. To illustrate what may be the most appropriate approach, under the prevailing circumstances, the ALERT leprosy control program is given as an example.
ALERT Leprosy Control Program
Leprosy control services
In 1971, at the request of the Ethiopian Government, ALERT took the responsibility for leprosy control in the Shoa Administrative Region, excluding the southern part of the region and Addis Ababa. In 1972 Addis Ababa was added and in 1978, the southern part of Shoa. The area covers about 85,000 sq. km with an estimated population of about 11 million (1989).
The ALERT leprosy control program is a nonintcgrated program. In 1989 the leprosy control activities in the field were carried out by about 60 field workers and 16 supervisors. The field workers were health assistants who had followed a formal general medical training of 1 ½ years, which should equip them with the knowledge and skills to take care of common medical problems in the rural areas. The supervisors were either sanitarians, who had been trained in environmental health, or experienced health assistants. They had over 10 years of experience as intermediate-level leprosy control managers.
Of the 329 general health facilities which existed in the rural areas of the Shoa Region, 151 operated leprosy clinics run by a leprosy field worker. The main reasons that services were not provided in all facilities were the unequal distribution of these facilities, as well as the disease: a relatively high number of facilities were located in areas with the lowest leprosy problem. The leprosy control field staff also provided services in 114 socalled "leprosy clinics," which had been established in remote areas where a general health service did not exist. These clinics were conducted in a school, farmers' associations' buildings or in the open field.
About 50% of the clinics where leprosy services were provided were accessible by car during the whole year; 32% were accessible by car during the dry season only and 18% were not accessible by car and had to be reached on foot or by mule. Public transport was hardly available, and due to the mountainous terrain and the existence of only a few tarmac roads, the possibilities for the use of motorcycles and bicycles were very limited. The majority of the leprosy patients lived more than 1 hour's travel from a clinic. Of 1641 leprosy patients who were diagnosed during the period July 1987 to July 1989, 38% lived within 1 hour's travel from a leprosy clinic, 27% within 1-2 hours, 24% within 2-4 hours, and 11% more than 4 hours.
Leprosy control activities
Case detection. Case detection was almost exclusively passive, that is, through voluntary reporting by patients. Of 8224 patients who were diagnosed during the period July 1979 to July 1989, 94.6% were selfreporting, 2.3% were detected through contact examination, 1.5% through examination of school children, and 1.6% through examination of special groups.
Figure 1 shows the annual self-reported leprosy incidence per 10,000 population during the period 1975 to 1989. A decline in the self-reported incidence can be observed starting in 1981. The decline forms a plateau since 1985, with a stabilization at a self-reported incidence rate of about 1 per 10,000 population. Similar trends were observed in other regions of Ethiopia. 23 Because the proportion of new patients with a disability grade of 2 at the time of diagnosis of leprosy decreased from an estimated percentage of over 30 in 1977, 24 to 20.6% in 1984, 25 the decline most probably reflects a decline in incidence of the disease rather than delay in case finding. Data on classification and age of new patients were only collected since 1984. Therefore, a possible increase in the proportion of new patients with lepromatous leprosy and shifts in the relative age distribution of new patients toward higher age groups, which arc indicators of a decline in leprosy incidence rates, 13, 26-29 could not be studied.
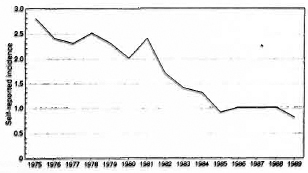
Fig. 1. Annual self-reported leprosy incidence per 10,000 population during the period 1975-1989.
The possible reasons for the decline in incidence of the disease could be a decrease in transmission of leprosy infection due to diagnosis and treatment of patients, protection of healthy individuals by BCG vaccination, or improvement of the socioeconomic conditions of the population. A variable degree of protection by BCG, ranging from 20% to 80%, has been demonstrated in several studies. 30-37 Because in Ethiopia the BCG coverage of newborns was less than 25% in the 1980s 38 and probably not higher in the 1970s and earlier, protection by BCG will not have been a major reason for the decline. If there was any improvement at all in the socioeconomic conditions of the rural population during the last decades, it will have been very marginal. Therefore, the effects of the leprosy control activities appear the most probable reason for the decline in incidence. Because MDT was introduced in 1983, this must have been the effect of treatment with dapsone monotherapy. Others arc, however, of the opinion that there is no strong evidence that chemotherapy-based control attributes to a decline in incidence rates. 26,39
Table 4 presents the annual self-reported incidence rates per 10,000 population from 1982 to 1989 for the 11 rural districts and Addis Ababa separately. Besides a substantial difference in case-detection rates between the districts, a decline in self-reported incidence rates can be observed in most districts, particularly until the end of 1985. A substantial increase in case detection following the implementation of MDT was not observed. If there was some increase, for example, in the Haykoch & B. and Menz & G. districts, it seems to have been very temporary.
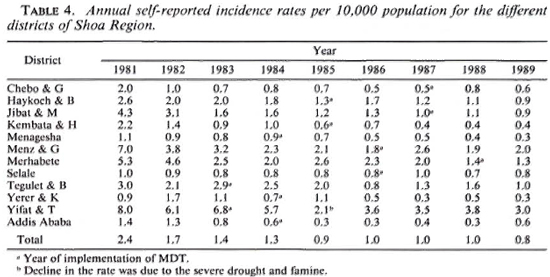
The period between the patients' observation of the first signs of the disease and the time of diagnosis of leprosy is still long. The average period for 1641 patients who were diagnosed during the period July 1987-July 1989 is presented in Table 5.
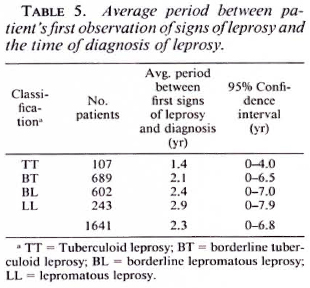
The delay in diagnosis of the patients indicates that there are many undiagnosed patients in the community. Thus, although the program has been effective with respect to treatment results, it has not been effective with regard to case detection. The accessibility of the services is certainly one of the factors contributing to the delay in diagnosis of patients. The data in Table 5 show that the more the average period between the first signs of leprosy and the diagnosis increases, the more the classification is toward the LL pole.
Registered patients. The evolution of the rate of registered leprosy patients per 10,000 population from 1972 until 1989 is presented in Figure 2. The rate gradually increased until 1982, after which, due to implementation of MDT and the release of patients on dapsone monotherapy, it steadily decreased.
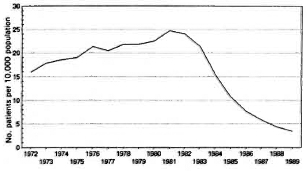
Fig. 2. Evolution in the rate of registered leprosy patients.
General health service
In July 1987 the general health service in the rural areas of Shoa consisted of 10 hospitals, 18 health centers, and 301 health stations-one health care facility for, on the average, 32,000 population, with a range of 12,000 to 49,000 for the different districts.4 0 With respect to integration of the leprosy control services within the general health services, the health stations would be the most important units. During the year 1986/ 1987 the total budget for the hospitals, health centers and health stations in the Shoa Region was approximately US$6,500,000, of which US$962,000 (14.8%) was allocated to the health stations.4 0 Of this amount US$417,000 (43.4%) was for drugs, averaging US$1400 for each health station.
In most of the health stations a health assistant was the officer in charge and might have been assisted by other health assistants or one or more nurses. In the year 1986/ 1987 a total of 588 health assistants were assigned to the 301 health stations in the Shoa Region. The number of health assistants per health station varied from none (6.3%) to one (35.2%), two (37.2%), three (12.0%) and more than three (8.3%).4 0 Thus, a health station, with an average of two health assistants and an average annual budget for drugs of US$1400, was responsible for the health care in an area with an average population of 32,000.
Because of nonavailability of transportation and funds, a referral system from health stations to health centers and hospitals did not exist. The chronic shortage of drugs was certainly an important reason for the fact that health stations were often open only a few hours per day or closed during part of the month (own observation). Further, a system of supervision did not exist in practice. Patients who could afford to pay for transportation often went directly to a hospital. About 60% of the population in the rural areas lived more than 1 hour's travel from a health care facility. There is no evidence that this situation has changed during the last few years.
Tuberculosis problem
Tuberculosis is a major public health problem. In the year 1986/1987 it was the third leading cause for hospitalization and the first leading cause of hospital death.4 0 The estimated annual risk of tuberculous infection is 2%.3 8 This corresponds with an annual incidence of about 100 sputumsmear-positive tuberculosis patients per 100,000 population. For the Shoa Region and Addis Ababa this means that 11,000 persons will develop infectious pulmonary tuberculosis each year. In addition, 11,000 persons will develop noninfectious pulmonary tuberculosis or extra-pulmonary tuberculosis. During the last decades systematic, large-scale tuberculosis control measures have not been undertaken. Due to shortages of drugs in the hospitals, the nonavailability of drugs in the rural areas, and the absence of a system of follow up, the vast majority of patients who were diagnosed with tuberculosis and started on treatment discontinued it within a few weeks.3 8 The tuberculosis problem is definitely increasing due to the increasing problem of infection with the human immunodeficiency virus (HIV). By early 1992, 1818 AIDS patients had been diagnosed,4 1 compared to 348 two years earlier.4 2 Because in most people the cause of death is not established, these patients represent only the tip of the iceberg.
Combined leprosy and tuberculosis control
For the Shoa Region, and probably for Ethiopia as a whole, the most appropriate approach would be to establish combined tuberculosis and leprosy control. Tuberculosis control would greatly benefit from the leprosy control infrastructure, and a justifiable infrastructure could be maintained for leprosy control. Although the number of leprosy patients on treatment has been reduced greatly due to the implementation of MDT, specialized staff at regional and district levels is certainly still required, especially for supervision, training and monitoring.
At the time a combined leprosy and tuberculosis program will have been introduced in the whole of the Shoa Region, the estimated caseload of patients on treatment will be as follows: a) the number of leprosy patients on MDT will, at any time, be about 2250. This is based on 1000 patients who will be put on MDT each year; about 850 of them new patients and 150 others (relapses, returned defaulters); 50% of them paucibacillary (PB) patients and 50% multibacillary (MB) patients. The period of treatment is 6 months for PB patients and 4 years, on average, for MB patients if MDT is continued until skin-smear negativity. If the period of treatment of MB patients is limited to 2 years, to be completed within an average period of 2Vi years, the number of patients on MDT will be about 1500. b) The number of tuberculosis patients on treatment will, at any time, be about 7300, half of them smear-positive pulmonary tuberculosis patients and half of them smearnegative pulmonary- and extra-pulmonary cases. This is based on the assumption that 50% of the estimated new smear-positive patients and 50% of the smear-negative pulmonary- and extra-pulmonary patients will be diagnosed and put on treatment. The period of treatment with short-course chemotherapy is 8 months. Because the combined program should be implemented gradually, and diagnosis of 50% of the estimated tuberculosis patients will, most probably, not be attained immediately from the start of the program, the above number of patients will be reached after some time. After the program has been well established, gradually a higher proportion of the estimated tuberculosis patients may be diagnosed.
For a combined leprosy and tuberculosis program some supervisors, in addition to the available leprosy control supervisors, will be required. These should be recruited from among staff who are already working in tuberculosis. Combined control of the two diseases should, as much as possible, be integrated within the general health services. However, the leprosy control services which have been established in remote areas should continue to be operational for leprosy and tuberculosis until adequate general health services have been established or alternative solutions, particularly for the treatment of patients, have been found.
In order to monitor the operational aspects, combined tuberculosis and leprosy control initially should be implemented in a limited area. If the results are satisfactory, the combined program should be expanded to other areas. Important indicators for assessing whether results are satisfactory should be the proportion of new sputumsmear-positivc tuberculosis patients who have been cured and the proportion of leprosy patients who completed the prescribed course of MDT.
Prerequisites for combined leprosy and tuberculosis control are: a) a detailed plan of operations for the combined program is prepared; b) the infrastructure which has been established for leprosy control will remain operational; c) the government of Ethiopia will continue its support for leprosy control, and will intensify its commitments to tuberculosis control; d) international leprosy sponsors continue their support for leprosy control; e) additional funding will be secured for the training of staff, antituberculosis drugs, and establishing and up-grading laboratory facilities for tuberculosis. In addition, because hospital inpatient facilities are very limited (in the Shoa Region there is one hospital bed for 12,000 population on average), alternatives for ensuring supervised treatment during the initial intensive phase of treatment of smearpositive tuberculosis patients have to be found. This could be the establishment of "tukuls," traditional houses. At several places, mainly attached to nongovernmental hospitals, "tukuls" are already used as low-care units.
Disability Prevention
A major problem of leprosy control programs, which will continue to be a problem for several years, arc the disabled patients. The seriousness of leprosy comes from the fact that it is mainly a disease of peripheral nerves. Although effective treatment can ensure bacterial cure, it docs not reverse nerve function loss.
It is estimated that there are 2-3 million individuals in the world who are physically disabled as a result of past or present leprosy. 1, 2, 43 There is wide variation in the published data on disabilities in registered leprosy patients. The data range from less than 10% to over 75%. 44-61 The data are difficult to interpret because it is not always specified to which leprosy cases they refer, or they relate to special groups of patients, such as those institutionalized or attending urban clinics. Further, the disability grading recommended by the WHO has been revised a few times, 62-64 and modifications to this grading have been used in several countries. 56, 65-67 Also, very little is known about the extent and pattern of leprosy-attributable disability in populations, and hardly any information has been published on when patients are most at risk of developing disabilities.
A main part of the disability problem is considered the result of failure to incorporate in leprosy control programs activities which are directed toward prevention of disabilities. 11 Prevention of disabilities has definitely not been given due attention in most leprosy programs.
International organizations have given basic recommendations for the prevention of disabilities. There are a number of excellent manuals on the control of disabilities in patients with irreversible nerve function loss. 68-71 However, manuals which cover all major components of disability prevention, which can be applied under routine field conditions, have not yet been published.
Most beds in leprosy hospitals are occupied by patients with severe complications due to nerve function loss. Substantially more resources are spent on the treatment of such patients than on the prevention of disabilities or on the prevention of complications of nerve function loss. Furthermore, the number of patients who require hospital admission often by far exceed the available inpatient facilities. The long-term results of treatment of complications are often poor; ulcers frequently recur, which ultimately lead to loss of bones and soft tissue. Reconstructive surgery, if available at all, can only be of help to a very limited number of patients and requires surgeons trained in specialized techniques, as well as physiotherapists. 72 Because surgery can only correct deformities due to motor paralysis, the possible consequences of loss of sensation remain.
It is obvious that the first priority in the prevention of disabilities should be the prevention of irreversible nerve function loss. The early detection and effective treatment of patients is usually considered the most effective way to prevent disabilities. The early detection and appropriate treatment of leprosy reactions is another important measure.
The next priority is the prevention of complications of irreversible nerve function loss. Once nerve function loss is established, disability has a natural tendency to deteriorate. This tendency is unaffected by chemotherapy and specific measures have to be taken to prevent this. 73
Early diagnosis and effective treatment of patients
In leprosy control programs early diagnosis means diagnosis before disabilities have occurred. Early detection and effective treatment alone, however, will not prevent disabilities. A major problem after starting chemotherapy is the development of leprosy reactions, notably reversal reactions. There is no evidence that the risk of developing a reaction is less in patients who are diagnosed at an early stage of the disease than in those who already have nerve function loss at the time of diagnosis of leprosy. It has been reported that the incidence of reactions is significantly lower among actively detected patients than among self-reporting patients. 74 Because in a proportion of actively detected patients the lesions would have been self-healing if they had not been diagnosed, 75 this group is not comparable with self-reporting patients.
Only if early diagnosis and effective treatment of patients goes hand in hand with appropriate management of leprosy reactions will disabilities be prevented. Health education, training of medical and paramedical staff, and improvement of the delivery of the service at the peripheral level are usually recommended as measures to promote the early diagnosis of leprosy patients. 19
Management of leprosy reactions
This aspect of the prevention of disabilities has been discussed extensively in another paper. 76 Provided that preconditions are fulfilled and precautions are taken, the management of reactions under field conditions should be a major component in the prevention of disabilities. The prevention of irreversible nerve function loss through adequate treatment of reactions is certainly a very efficient and effective measure in the prevention of disabilities.
It is very unfortunate that there has been hardly any progress in the development of methods for the prevention and treatment of leprosy reaction during the last decades. The importance of research on identification of risk factors for the development of leprosy reactions cannot be over-emphasized. Furthermore, there is an urgent need for studies on the treatment of reactions, including identification of drugs other than prednisolone.
Prevention of complications of irreversible nerve function loss
Ideally, no patient should have irreversible nerve function loss at the time of diagnosis of the disease. Although a gradual downward trend in the proportion of patients who are already disabled at the time of diagnosis of leprosy has been observed in some programs, 6, 56, 77 many patients are still diagnosed only at the time they arc already disabled.
Table 6 gives the percentages of patients with a disability grade of 1 and 2 among those diagnosed in the ALERT leprosy control program during the years 1984 to 1989. The disability grading is according to the recommendation of a WHO Expert Committee on Leprosy of 1988. 64 Grade 1 of hands and feet means loss of sensation, but no visible deformity or damage; grade 2 is visible deformity or damage. Grade 1 of eyes means eye problems due to leprosy, but vision not severely affected; grade 2 is severe visual impairment. The data in Table 6 show a gradual downward trend in the percentage of patients with a disability grade of 2. This indicates earlier diagnosis of cases. However, the total percentage of patients with a disability remained the same.
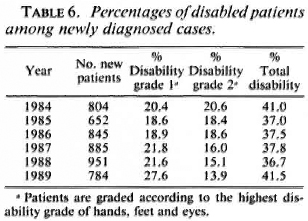
Disabilities of the eyes occur least frequently. However, a substantial proportion of patients with an eye disability also have disabilities of the hands and feet. Of 1643 patients who were diagnosed with leprosy in the ALERT leprosy control program during the period July 1987 to July 1989, 68 patients (4.1%) had an eye disability; 47 of these patients (69.1%) also had a disability of the hands and/or feet, in 27 patients (39.7% of those with an eye disability) both eyes, both hands, and both feet were affected.
The pattern of hand and foot disability of these 1643 patients at the time of diagnosis of leprosy, and that of 1349 patients who were started on MDT during the same period but had been treated with dapsone before MDT, is presented in Table 7.
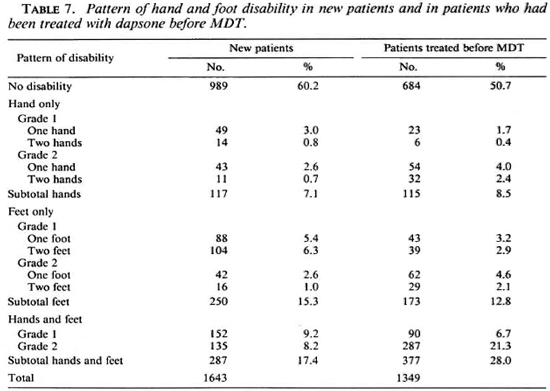
In both groups of patients, particularly those who had been treated with dapsone before MDT, both the hands and feet were most commonly affected, followed by the feet only. Of the new patients, 24.7% had disability grade 1 and 15.1% had disability grade 2, compared with 14.9% and 34.4%, respectively, of the patients who had been treated with dapsone. Whether the latter group already had a higher disability at the time of diagnosis of leprosy, with respect to the grade and the number of limbs affected, or the disability increased during treatment is not known. Because the management of leprosy reactions was grossly inadequate before 1987 and measures toward prevention of the increase of disability were hardly or poorly applied, it is most probable that in part of the patients the disability either developed or increased during treatment.
The data in Table 7 show that the disability problem is an immense problem. It is also a very complicated problem. The social stigma attached to the disease is an important cause for late self-reporting by patients. Complications due to peripheral nerve damage in turn contribute to maintaining the stigma.
There is wide variation in recommended approaches regarding care for patients with disabilities.11, 19, 20, 73, 78-82 These range from medical, social and economic rehabilitation during and after treatment, provided or initiated by the leprosy control services, to the promotion of community-based rehabilitation. Medical, social and economic rehabilitation schemes are usually small-scale initiatives.
In several programs patients are provided with appliances, especially footwear, to protect insensitive limbs. Such provision is often limited to patients who attend a leprosy institution. Rather than supplying appliances, the importance of promotion of selfcare by patients is more and more recognized. Even if funds would be available, which is mostly not the case, the organization of a service which provides footwear without interruption and for life to patients with insensitive soles is extremely difficult, if at all possible. 78
Community-based rehabilitation is another recommended way to deal with the disability problem. 11, 81 This approach aims at promoting awareness, self-reliance, and responsibility for rehabilitation in the community. Compared to institution-based services, which take care of the physical condition of usually a very limited number of patients only, community-based rehabilitation, which is expected to take into account the social and economic condition of the patient as well, is a much more appropriate approach. However, whether this is a feasible approach in rural communities which face many other problems besides leprosy is questionable, particularly if one also takes into consideration the stigma which is attached to the disease. Although with increased MDT coverage the number of patients on antileprosy treatment steadily decreases, the disability problem in past, present and future patients will remain a tremendous one for many years. We do not know how to deal with it effectively and efficiently.
As long as the social and economic conditions of leprosy patients and their communities do not improve, it is unlikely that medical interventions will decrease the problem of leprosy-related disabilities and complications due to this disease. They may help some individual patients, but at a cost which is not affordable for most countries.
The following experiences in the ALERT leprosy control program illustrate the many difficulties in dealing with the disability problem.
Early detection of patients was promoted through health education in the communities and the provision of diagnosis and treatment through a network of clinics. However, as shown in Table 6, a substantial proportion of the patients was already disabled at the time of diagnosis of the disease. The introduction of management of leprosy reactions in the field during 1987 was an important step toward the prevention of disabilities.
In order to prevent complications of nerve function loss, the leprosy field workers had to teach patients how to take care of insensitive and/or paralyzed limbs. They had to promote self-care by patients with complications, notably wounds on soles and palms and eye problems. The results of these measures were not evaluated systematically. However, during field visits it was observed that in most patients with established nerve function loss the disability deteriorated. Only rarely were wounds healed, or stiffness of joints improved, or impairment of vision prevented. Part of this could certainly be attributed to the attitude and teaching methods of the staff, which often did not appear to encourage patients to practice selfcarc. However, a major problem was that many patients, because of their social and economic situation, had hardly any opportunities to practice self-care. The loss of sole sensation and sole wounds were major problems. Most patients either could not afford to buy footwear or they used inappropriate footwear. Because of the scarcity of water, proper care of wounds was often not possible, and patients were often not in the position to rest their limbs in case of wounds. It was estimated in 1988 that about 8500 patients in the field were in need of protective footwear-7000 of them needed footwear with soft innersoles and 1500 patients, with advanced foot disability, needed more specialized footwear.
The ALERT hospital had to provide specialized services to patients who could not be managed in the field. These services included the treatment of patients with septic wounds, provision of protective footwear, and reconstructive surgery for patients with motor paralysis. The hospital could, however, not meet the requirements and limited its services almost exclusively to patients who had easy access to them. Most of these patients lived in the "leprosy village" in the vicinity of the ALERT compound. Over 75% of the leprosy patients lived in the rural areas, and their access to the hospital services was very limited. Of patients with septic wounds not more than 5% could be admitted. Of those in need of reconstructive surgery, less than 15% received surgery. The orthopedic workshop covered only about 10%-15% of the requirements for protective footwear.
In order to provide protective footwear to patients in the rural areas, a Sandal Production Unit under the responsibility of the leprosy control program was established in 1988. This unit had to prepare standard sandals for patients in the field. The initial experience with the production and distribution of sandals was that footwear which was suitable on medical grounds but which could be identified as "leprosy footwear" was not acceptable to many patients. Further, it appeared very difficult to make standard footwear which is suitable for different terrains and climatological conditions. The durability of the standard footwear was poor; many of the distributed sandals needed simple repairs within the first few months. Because there were no provisions for repair in the field, the sandals had to be brought to ALERT, leaving the patients without footwear for 1 month or more. The cost of production of one pair of sandals was about USS8; most patients could not afford or were not prepared to share US$1 per pair in the cost.
PROSPECTS FOR LEPROSY CONTROL
The forty-fourth World Health Assembly adopted in May 1991 a resolution on the elimination of leprosy as a public health problem by the year 2000. 3 Elimination was defined as "attaining a level of prevalence below one case per 10,000 population." The resolution referred to elimination on a global scale. It was adopted after the Assembly noted the significant progress which was made with MDT and the subsequent reduction in prevalence of the disease.3 The resolution also urged member states to increase their political commitment for leprosy, and requested the Director-General of the World Health Organization to strengthen the technical support to member states and to continue to mobilize and coordinate resources from nongovernmental organizations to achieve the goal.
What Does This Resolution Imply in Practical Terms?
Firstly, it does not mean eradication of leprosy. By definition, a residual leprosy problem should be accepted. Less than one leprosy case per 10,000 population refers to patients registered for treatment and not to new and undetected cases which occur in the communities. It is hoped that, as a consequence of MDT, early self-reporting of patients will increase and, therefore, the gap between the registered and estimated cases will steadily decrease, and that the residual unregistered cases will consist of mainly early minimal and mostly self-healing PB cases. 3 Gradually, the registered cases will then consist mainly of incident cases. Although the introduction of MDT may have led to an increase in early self-reporting by patients in some programs, there are hardly any published data which substantiate this, certainly not in the long term. The definition of elimination in relation to registered cases may be appropriate in some programs, especially those which rely on vigorous active case-finding. However, as was shown in Table 5, the patients who were diagnosed in the ALERT leprosy control program represent only a proportion of the existing cases, and this is likely to be the case in most leprosy control programs.
Certainly, in programs which depend on self-reporting by patients, an indicator of delay in case detection should be included. The proportion of patients with a leprosyrelated disability at the time of diagnosis of the disease would be an appropriate indicator. For example, only if at the time the rate of patients registered for treatment is less than 1 per 10,000 population, and less than 5% of the patients have a leprosy-related disability at the time of diagnosis of the disease, will elimination have been attained.
Secondly, because the resolution refers to global elimination, the strategies for achieving this (implementation of MDT and effective case detection) should be implemented rather evenly in leprosy endemic countries. The wide variation in coverage with MDT shows that much work still needs to be done. Even if efforts to implement MDT would be intensified during the next few years, it is very unlikely that global elimination will be reached by the year 2000.
Annually about 600,000 new leprosy patients are diagnosed in the world. There is no evidence that this number will substantially decrease in the near future. On the contrary, it is expected that, because of increased self-reporting, the number of diagnosed patients will increase. Let us assume that the number of diagnosed patients will remain 600,000 annually, of whom 60% will be classified as PB and 40% as MB. With a treatment period of 6 months for PB patients and 2 years for MB patients (which will be completed during an average period of 2 ½ years), at any time there will be about 800,000 patients on treatment in the world. With a world population of 5.4 billion, this would mean about 1.5 patient per 10,000 population, being the contribution of newly diagnosed patients only. An increase in the world population to 6 billion by the year 2000 will still not bring the number of patients registered for treatment under 1 per 10,000. In addition, there were 1.8 million registered leprosy patients at the beginning of 1992 who had not been put on MDT.
Further, it is only realistic that the global goal will be translated into national goals. At the time the goal has been reached, governments may decide to reallocate the resources for leprosy, irrespective of the quality and effectiveness of case finding and treatment.
Thirdly, the elimination strategy implies that MDT will continue to be effective. Although the relapse rates so far have been very low, the duration of follow up is most probably too short to draw final conclusions. Because the results were mainly obtained in well-established, nonintcgratcd programs, the findings may well be quite different in areas with a less favorable infrastructure, depending on poorly supervised staff.
Fourthly, the question arises whether the elimination of leprosy can be achieved with the presently available MDT alone. An increasing proportion of the patients who reside in areas where MDT has not yet been implemented will have developed or been infected with dapsone-resistant bacilli. As a result of improper application of MDT or inadequate treatment, there is potentially a risk of development of multiple drug resistance. In particular, resistance to rifampin could become a major problem.
Fifthly, it is questionable whether the elimination of leprosy through detection of cases at an early stage of the disease, and treating these patients with MDT, is feasible. The uncertainties about the route of transmission of infection, the insidious onset of the disease, the unknown period of infectivity, and the need for long-term treatment (although the period of treatment has substantially been decreased with MDT) are important limiting factors. Furthermore, at the time the rate of registered patients is close to 1 per 10,000 population, the efforts required to further reduce this rate arc likely to increase.
Developments in the following fields could have a considerable bearing on the prospects of possible elimination of leprosy: a) primary prevention through vaccination against leprosy; b) better treatment regimens, especially from the point of view of operational applicability; c) tests for the identification of individuals infected with M. leprae, and the prediction of development of the disease; and d) effects of the HIV pandemic on the leprosy problem.
Vaccination Against Leprosy
Primary prevention, through vaccination, has been a main issue for several years. Considerable efforts have been made to develop a vaccine which is highly efficacious, cost-effective and acceptable. Trials on the protective value of BCG vaccination against leprosy have been carried out in Uganda, Myanmar (Burma), India, and Papua New Guinea. 30-36 The efficacy of BCG vaccines against leprosy varied from 20% in Myanmar to 80% in Uganda. In particular, the Papua New Guinean trial showed evidence of protection against the muitibacillary forms of leprosy. 30 It is considered unlikely that the variations are due to methodological artefacts. 83 Possible reasons for the variability in protection by BCG vaccines could be the differences in exposure of the population to M. leprae and other mycobacteria, differences in immunogenetic characteristics of the population, different strains of M. leprae, and the variance in BCG strains with respect to their protective effect. 32-34
From a public health point of view, an ideal vaccine should be able to prevent particularly the muitibacillary forms which are responsible for transmission of the infection. In view of the low incidence of muitibacillary leprosy and its long incubation period, field trials which include large populations over long time periods have to be carried out to measure the protective effect of a possible vaccine against multibacillary leprosy. In attempts to find a vaccine that gives greater and more consistent protection against leprosy than BCG vaccine alone, BCG with and without killed M. leprae was compared in trials in Venezuela and in Malawi. 34, 84 During the first 5 years of follow up of the Venezuelan trial there was no evidence that BCG plus killed M. leprae offers substantially better protection than BCG alone. 84 Continued follow up of the trial population over an additional 10 years is considered important.
Research is also being done on the development of BCG into a multivaccine vehicle. Hope has been expressed about the possibility of testing the feasibility of immunizing with different protective antigens which are expressed in recombinant BCG in experimental systems within the next few years. 85 However, such hope may only be realistic if protective leprosy antigens will be identified.
Even if a very effective vaccine would be developed, its application will, most probably, be limited. Problems in the application of even the most effective tools in control activities often appear more difficult to surmount than the problems in the development of such tools. 86 The comment of Antia and Birdie should be well taken: "Meanwhile, the enthusiasm of the scientist and the associated wide publicity given to the vaccine as the final solution for leprosy should be tempered with caution since, unfortunately, it has tended to divert and dilute the attention from other priority areas such as early detection, drug research and ensuring regularity of treatment. It should also be remembered that even if a vaccine was available, its delivery, especially if it requires repeated injections, may prove an insurmountable problem with the existing machinery for delivery of health services. This has been well demonstrated in the case of tetanus, where an effective vaccine has been available for over half a century yet tetanus continues to be the second largest killer in India today." 87
If it would be possible to identify clusters with high transmission of infection, and provided that an effective vaccine against leprosy would become available, selective vaccination could aid the elimination of leprosy. Although the observed protective effect of BCG vaccination against leprosy varies considerably, because of its wide use in many leprosy-endemic countries it is likely to have protected thousands of individuals from getting leprosy. At present the active support by the leprosy control services of a wider application of BCG vaccination appears an appropriate approach in the prevention of leprosy.
New Treatment Regimens
At present the most effective tool for individual patients as well as the control of leprosy in the community is chemotherapy. From experience with tuberculosis, bactericidal drugs should be used as much as possible. Such drugs allow a shorter duration of treatment and, when used in combination, there is less chance of the development of resistant mutants. In the present MDT regimens only one strong bactericidal drug, rifampin, is included. A combination of drugs must consist of at least two effective drugs, each of which must work by a different mechanism. New regimens should prevent the causes of failure of dapsone monotherapy, notably poor compliance, the emergence of resistance and the phenomenon of bacterial persisters. So far the results of MDT are very encouraging. Significant improvements in the compliance have been reported from many parts of the world. The relapse rates during the first years after stopping MDT are very low. The problem of bacterial persisters has not yet been solved. Although it has been reported that persisters may survive for 20 years, very little is known about their significance in the development of relapses.
With the apparent successes of well implemented MDT the question arises whether new drugs and other drug regimens arc still needed. The answer should certainly be yes, for the following reasons: a) Only four antileprosy drugs [dapsone, rifampin, clofazimine and thioamidc (prothionamide/ ethionamide)] with activities that act by different mechanisms arc available. Because thioamidc may cause hepatotoxicity, especially when combined with rifampin, this should not be used under routine field conditions. Therefore there is no alternative to the present MDT regimen for MB patients, b) Resistance to each of the antileprosy drugs has been documented. 88 Therefore, there is certainly a threat of multiple-resistant strains in the near future. Strains which are resistant to both dapsone and rifampin have already been identified in areas where rifampin monotherapy was given to patients with dapsone-resistant leprosy. 89 The treatment of these double-rcsistant patients is extremely difficult. 90 c) Although we do not know if the development of rifampin resistance in patients treated with MDT will be significant, this should certainly be considered, d) Alternative regimens are needed for patients who do not tolerate the present regimens or object to clofazimine (because of skin discoloration) and for those who cannot comply with the monthly supervised treatment, for example, because they live in unacccssible areas, e) The present MDT regimens are certainly not ideal. The recommended duration of the treatment, particularly for MB patients, is still rather long.
Ideally, the treatment should have the following characteristics: tablet formulation, preferably in a combined preparation; long periodicity or self-administration if frequent doses are required; short duration, a few weeks to at most a few months; effective; low toxicity; and low cost. Furthermore, the regimen should destroy "persistent" organisms and be the same for all patients.
The prospects for new drugs are promising. Especially the fluoroquinolones, pefloxacin and ofloxacin, and minocycline (a tetracycline), show strong bactericidal effects against M. leprae. 88, 90-96 It has been demonstrated in a clinical trial that 22 daily doses of either pcfloxacin or ofloxacin kills 4 logs of viable M. leprae. 94 This is about the number of rifampin-resistant mutants which are expected to be present in a lepromatous patient before the start of treatment. It is, therefore, very well possible that the combination of ofloxacin or pcfloxacin and rifampin will considerably shorten the required duration of MDT.
A 1-month regimen of daily rifampin and ofloxacin will be compared with the standard WHO/MDT regimens in a multiccnter double-blind trial. 90 Both PB and MB patients will be treated with the 1-month regimen. In order to be able to demonstrate that the regimen is at least as good as or better than the current regimens, the trial requires quite a large number of patients and a long period of follow up (the period of follow up after completion of treatment will be 5 years for PB patients and 7 years for MB patients). Therefore, the results of this trial will not be available before the end of the century. Besides the relapse rate, the tolerance, side effects and feasibility of the regimen will also be evaluated. Clinical trials in which the bactericidal activity of minocycline is evaluated are presently being carried out. 90
Because the new drugs are very expensive, their use in leprosy-endemic countries will probably remain limited unless the cost is reduced drastically. It is likely that the current MDT regimens will have to be used for many more years. The major constraint at present is not the effectiveness of the available regimens, but their application under routine field conditions.
A main problem with regimens of very short duration will be the development of leprosy reactions and nerve damage after stopping treatment. This is already a problem with the current regimens, especially in PB patients.7 6 In evaluating new treatment regimens, the incidence rate of reactions during and after chemotherapy is certainly as important as the relapse rate.
Tests for Identification of Leprosy Infection and Prediction of Disease Development
One of the present challenges of leprosy research is the identification of past or present infection with M. leprae as distinct from clinical disease. 97 This could provide the answers to important questions, such as the sources of infection in the communities, the duration of the incubation period, and the identification of risk factors for infection and for disease. 98 All of these questions are highly relevant for the elimination goal.
Several studies have been carried out on the seroprevalence of M. leprae-specxhe antibodies, especially phenolic glycolipid-I (PGL-I)-IgM antibodies, in leprosy endemic-communities and in household contacts of leprosy patients. 99-109 In studies among household contacts, a statistical association between serological positivity and leprosy risk was observed, showing that a seropositive individual had a 21-40 times higher chance of developing leprosy than a sero negative person. 101, 103, 106, 109 However, the majority of seropositives (85% or more) did not develop the disease within the timeframe of these studies, which was 2-3 years. Even if there is a strong association between antibody level and the subsequent risk of developing leprosy, it is very unlikely that serological screening would be a useful method of detecting persons at risk. In one study in which two cases of MB leprosy were detected among ten contacts with the highest levels of PGL-I antibodies, 9500 sera were screened to identify these ten persons. 109 Fine commented on the test specificity in relation to the rarity of leprosy. He calculated that, even if a test is 99% specific, and the true risk of leprosy is 1 per 1000, 10 out of 11 (90.9%) of the positive tests will be false, that is, not resulting in disease. 110 The studies showed that the assay of antibodies to PGL-I is not a sensitive or specific test for infection with M. leprae. Further, its value, if there is any, in the identification of those at risk of developing leprosy is very limited.
In terms of sensitivity, serological tests have been surpassed by the development in molecular biology of the polymerase chain reaction (PCR) which was discovered in 1987. 111 Because of the inability to cultivate M. leprae in vitro, this technique may especially be valuable for the detection of small numbers of M. leprae. Results from studies indicate that the PCR technique can specifically detect as few as 10-100 organisms. 112-115
A potential use of the PCR technique is for the study of the transmission of leprosy and the development of the disease in infected persons. 113, 116 Other possible uses are the diagnosis of patients thought to have leprosy but with negative skin smears, and the differentiation between a reversal reaction and a relapse in PB patients, provided that PCR positivity is shown to be related to viable bacilli. 113, 116 Studies in untreated patients gave positive PCR results in 92%-100% of skin-smear-positive patients and 56%-61% in skin-smear-negative patients. 115 The test still has a number of pitfalls which limit its possibilities for routine use. These include contamination of samples or reagents producing false-positive results, and its cost (US$3-$3.50 per test). 113 PCR at present is mainly a research tool which may give answers to specific problems. Its practical value in the control of leprosy has yet to be determined.
Leprosy and HIV
The control of leprosy might be jeopardized by the increasing problem of HIV infection, particularly on the African continent. HIV infection constitutes a major risk factor for the development of clinical tuberculosis in persons infected with M. tuberculosis. 117 So far there is no evidence for an association between HIV infection and leprosy incidence. 118, 119 Studies have shown that the prevalence of HIV antibodies is identical in leprosy patients and controls and no difference in the prevalence of HIV infection in lepromatous and tuberculoid patients was found. 120, 121 It has been suggested that infection with HIV may increase the incidence of leprosy among individuals with subclinical infection with M. leprae, either through shortening the incubation period or by increasing disease penetrance. 117 Similarly, clinical leprosy may accelerate the course of HIV disease. Possible effects of HIV infection may be an increase in the detection of tuberculoid patients, or a decline in case detection rates if patients with lepromatous leprosy are unrecognized because they die before the onset of clinical symptoms. 122 Further, the relapse rates after MDT may be high in HIV-positive individuals.
Whether, how, and to what extent leprosy will be affected by HIV infection has to be determined in studies on the epidemiology, clinical manifestations, response to antileprosy treatment, and immunological responses of patients dually infected with M. leprae and HIV. Particularly in Africa there will be many such individuals.
CONCLUSIONS
In conclusion, it is most probable that, at least during the next decade, our tools in the control and ultimately the elimination of leprosy will continue to be the early detection of patients and their treatment with the presently available MDT regimens. Although it is not known whether the elimination of leprosy can be achieved with these tools only, major emphasis should be put on using them as widely, as effectively, and as efficiently as possible. The availability of new drug regimens of shorter duration may facilitate implementation of MDT. Research in the field of immunology and molecular biology may ultimately provide tools which could aid in the elimination of leprosy. If there will be a practical use of such tools in field programs, their application will, most probably, be limited to selected areas. They are unlikely to provide answers to most of the present pressing problems in leprosy control which, in particular, concern ensuring early detection of patients and their completion of MDT, and the prevention of disabilities. Field research on the effective application of available methods for the control of leprosy, under different field conditions, should be given high priority. In addition, there is an urgent need for research on the prevention of disabilities and on the risk factors for the development of leprosy reactions.
- Marijke Becx-Bleumink,
M.D., D.T.P.H., Ph.D.
Plasweg 15
3768 AK Soest
The Netherlands
1. World Health Organization. Weekly Epidemiological Record 67(1992)153-160.
2. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982, Tech. Rep. Ser. 675.
3. Noordeen, S. K. Elimination of leprosy as a public health problem. Lepr. Rev. 63(1992)1-4.
4. Becx-BIcumink, M. New developments in the ALERT leprosy control programme and the issues of integration. Ethiopia J. Health Dev. 1(1984)49-55.
5. Becx-Bleumink, M. Implementation of multidrug therapy in the ALERT leprosy control programme in the Shoa region of Ethiopia. First results with paucibacillary patients. Lepr. Rev. 57(1986)111-119.
6. Becx-BIcumink, M. Operational aspects of multidrug therapy. Int. J. Lepr. 57(1989)540-551.
7. Chum, H. J. The impact of MDT implementation in the Tanzanian National TB-Leprosy Programme. Lepr. Rev. 57 Suppl. 3(1986)63-66.
8. Ekambaram, V. and Rao, M. K. Changing picture of leprosy in North Arcot district, Tamil Nadu, after MDT. Indian J. Lepr. 61(1989)31-43.
9. Gilbody, J. S. Impact of multidrug therapy on the treatment and control of leprosy. Int. J. Lepr. 59(1991)458-478.
10. Jesudasan, K., Vijayakumaran, P., Pannikar, V. K. and Christian, M. Impact of MDT on leprosy as measured by selective indicators. Lepr. Rev. 59(1988)215-223.
11. Noordeen, S. K. A look at world leprosy. Lepr. Rev. 62(1991)72-86.
12. Revankar, C. R., Pawar, P. L., belurkar, L. S., Rashmi, R. P. and Ganapati, R. Reduction in caseload after multidrug therapy in an urban leprosy control programme-a retrospective study in Bombay. Lepr. Rev. 62(1991)44-48.
13. Rose, P. Changes in epidemiological indices following the introduction of WHO MDT into the Guyana leprosy control programme. Lepr. Rev. 60(1989)151-156.
14. Sundar Rao, P. S. S., Jesudasan, K., Pannikar, V. K. and Christian, M. Epidemiological impact of multidrug therapy in Gudiyatham control area, Karigiri. (Abstract) Int. J. Lepr. 57(1989)331.
15. Wheate, H. W. and Harris, G. F. Operational problems in leprosy programmes when the endemicity declines. Lepr. Rev. 58(1987)1-5.
16. Feenstra, P. and Tcdla, T. A broader scope for leprosy control. World Health Forum 9(1988)53-58.
17. Loretti, A. Leprosy control: the rationale of integration. Lepr. Rev. 60(1989)306-316.
18. McDougall, A. C. and Georgiev, G. D. Priority in leprosy control. Lepr. Rev. 60(1989)1-7.
19. World Health Organization. A Guide to Leprosy Control. 2nd ed. Geneva: World Health Organization, 1988.
20. World Health Organization. Report of a consultation on implementation of leprosy control through primary health care. Geneva: World Health Organization, 1986. WHO/CDS/LEP/86.3.
21. Alvarenga, A. E. Report of the Joint Leprosy-Tuberculosis Project in Paraguay. Lepr. Rev. 57 Suppl. 3(1986)53-59.
22. de Rijk, A. J. Combining tuberculosis and leprosy services in one programme. Ethiopian J. Health Dev. 1(1984)37-43.
23. Berhe, D., Haimanot, R. T., Tedla, T. and Tadcsse, T. Epidemiological patterns of leprosy in Ethiopia: a review of the control programmes. Lepr. Rev. 61(1990)258-266.
24. Becx-Bleumink, M. Assessment of the disability problem in the ALERT Leprosy Control Programme, unpublished data, 1988.
25. ALERT, Annual Reports 1984-1989. Addis Ababa: All Africa Leprosy and Rehabilitation Training Center.
26. Boerrigter, G. and Ponnighaus, J. M. Ten years' leprosy control work in Malawi (Central Africa) -II. Patterns of endemicity since 1973. Lepr. Rev. 57(1986)221-236.
27. Irgens, L. M. Leprosy in Norway. Lepr. Rev. 51 Suppl. 1(1980)1-130.
28. Irgens, L. M. Secular trends in leprosy: increase in age at onset associated with declining rates and long incubation periods. Int. J. Lepr. 53(1985)610-617.
29. Irgens, L. M., Melo Caeiro, F. and Lechat, M. F. Leprosy in Portugal 1946-80. Epidemiologic patterns observed during declining incidence rates. Lepr. Rev. 61(1990)32-49.
30. Bagshawe, A., Scott, G. C, Russel, D. A., Wigley, S. C, Mcrianos. A. and Berry, G. BCG vaccination in leprosv; final results of the trial in Karimui, Papua New Guinea. Bull. WHO 67(1989)389-399.
31. Fine, P. E. M. The Kcllersberger Memorial Lecture 1985: the role of BCG in the control of leprosy. Ethiopian Med. J. 23(1985)179-191.
32. Fine, P. E. M. BCG vaccination against tuberculosis and leprosy. Br. Med. J. 55(1988)691-703.
33. Fine, P. E. M. Vaccination against leprosy: neglecting the obvious? In: Health Cooperation Papers, Proceedings of the VI Leprosy Symposium on "Leprosy Research, "Santa Margherita Ligure-Genoa, Italy, October 3-7 1990. Associazione Italiana "Amici di Raoul Follercau," 1992, pp. 211-215.
34. Gupte, M. D. Report of a pre-Congress workshop on "Vaccine trials." XHIth International Leprosy Congress, The Hague, The Netherlands, 11-17 September 1988. Lepr. Rev. 59(1988)296-300.
35. Lwin, K., Sundaresan, T., Gyi, M. M., Bechelli, L. M., Tamondong, C, Garbajosa, P. G., Sansarricq, H. and Noordeen, S. K. BCG vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull. WHO 63(1985)1069-1078.
36. Stanley, B. J., Howland, C, Stone, M. M. and Sutherland, I. BCG vaccination of children against leprosy in Uganda. Final results. J. Hyg. (Cambridge) 87(1981)233-248.
37. Tripathy, S. P. The case for BCG. Ann. Natl. Acad. Med. Sci. (India) 19(1983)11-21.
38. Abite, M., Head, National Tuberculosis ControlProgramme, Ethiopia, personal communications, 1987- 1989.
39. Meade, T. W. How effective is the treatment of leprosy? Lepr. Rev. 48(1977)3-8.
40. People's Democratic Republic of Ethiopia, Ministry of Health. Comprehensive Health Service Directory 1979 E.C (1986/1987 G.C.). Planning and Programming Bureau, Addis Ababa, August 1988.
41. World Health Organization. Weekly Epidemiological Record 67(1992)97.
42. World Health Organization. Weekly Epidemiological Record 65(1990)133.
43. World Health Organization. Report of a consultation on disability prevention and rehabilitation in leprosy. Geneva: World Health Organization, 1987. WHO/CDS/LEP/87.3.
44. Bechclli, L. M. and Martinez Dominquez, V. The leprosy problem in the world. Bull. WH O 34(1966)811-826.
45. Bechclli, L. M. and Martinez Dominquez, V. Further information on the leprosy problem in the world. Bull. WHO 46(1972)523-536.
46. Becx-Bleumink, M. Operational aspects of the implementation of multidrug therapy at ALERT, Ethiopia. Lepr. Rev. 57 Suppl. 3(1986)115-123.
47. Boerrigter, G. and Ponnighaus, J. M. Preliminary evaluation of the effect of WHO-MDT on disabilities in leprosy patients in Malawi (Central Africa). Lepr. Rev. 57 Suppl. 3(1986)101-105.
48. Bravo, L. L. and Ratard, R. C. Leprosy disabilities in the New Hebrides. Lepr. Rev. 48(1977)247-260.
49. Chum, H. J. and Otsyula, Y. Leprosy disability in Yimbo and its economic effects. East Afr. Med. J. 47(1970)389-394.
50. Hasan, S. A survey of leprosy deformities among the patients of Hyderabad city. Lepr. India 49(1977)393-398.
51. Kaur, P. and Sing Gurmehan. Deformities in leprosy patients attending urban leprosy clinics at Varanasi. Indian J. Lepr. 57(1985)178-182.
52. Keeler, R. and Ryan, M. A. The incidence of disabilities in Hansen's disease after the commencement of chemotherapy. Lepr. Rev. 51(1980)149-154.
53. Kushmah, S. S., Gorila, A. K. and Kushwah, J. Disabilities among leprosy patients attending leprosy clinics in Gralior, an epidemiological study. Lepr. India 53(1981)240-247.
54. Martinez-Dominguez, V.. Bechclli, L. M. and Patwary'. K. M. WH O surveys of disabilities in leprosy in Northern Nigeria (Katsina), Cameroon and Thailand (Khon Kaen). Int. J. Lepr. 34(1966)244-254.
55. Noordeen, S. K. and Srinivasan, H. Deformity in leprosy: an epidemiological study. Indian J. Med. Res. 57(1969)175-181.
56. Ponnighaus. I. M., Boerrigter, G., Fine, P. E. M., Ponnighaus, J. M. and Russel, J. Disabilities in leprosy patients ascertained in the total population survey in Karonga District, Northern Malawi. Lepr. Rev. 61(1990)366-374.
57. Prasad, S. Deformities in leprosy, a survey in a closed community. Lepr. India 53(1981)626-633.
58. Rao, P. S. S., Karat, S., Karat, A. B. A. and Furness, M. A. Prevalence of deformities and disabilities among leprosy patients in an endemic area. Part 1. General findings. Int. J. Lepr. 38(1970)1-11.
59. Rao, P. S. S., Karat, S., Karat. A. B. A. and Furness, M. A. Prevalence of disabilities in children affected with leprosy. Int. J. Lepr. 41(1973)577-583.
60. Reddy, B. N. and Bansal, R. D. N. An epidemiological study of leprosy disability in a leprosy endemic rural population. Indian J. Lepr. 56(1984)191-199.
61. Sehgal, V. N. and Sharwa, P. K. Patterns of deformities/disabilities in urban leprosy. Indian J. Lepr. 57(1988)183-192.
62. WHO Expert Committee on Leprosy. Second report. Geneva: World Health Organization, 1960. Tech. Rep. Scr. 189.
63. WHO Expert Committee on Leprosy. Fourth report. Geneva: World Health Organization, 1970. Tech. Rep. Scr. 459
64. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
65. Brandsma, J. W., de Jong, N. and Tjcpkema, T. Disability grading in leprosy; suggested modifications to the WHO disability grading forms. Lepr. Rev. 57(1986)361-369.
66. Hasan, S. An appraisal of use of the classification of disabilities resulting from leprosy in field work and suggestions for improvement. Lepr. India 54(1982)135-142.
67. Kulkarni, U. P., Kulkarni, V. N. and Jogaikar, D. G. Classification of disabilities as followed by Poona district leprosy committee. Lepr. India 56(1984)269-279.
68. Kelly, E. D. Physical therapy in leprosy for paramedicals. Levels I & II and levels I & II & III. 3rd ed. Elmwood Park, New Jersey, U.S.A.: American Leprosy Missions, 1981.
69. Nevile, P. J. A footwear manual for leprosy control programmes. Part 1 and Part 2. Addis Ababa: All Africa Leprosy and Rehabilitation Training Centre.
70. Watson, J. M. Preventing disability in leprosy patients. London: The Leprosy Mission International, 1986.
71. Watson, J. M. Essential action to minimise disability in leprosy patients. London: The Leprosy Mission International, 1986.
72. Bourrel, P. Surgical rehabilitation. Lepr. Rev. 62(1991)241-254.
73. Watson, J. M. Disability control in a leprosy control programme. Lepr. Rev. 60(1989)169-177.
74. Boerrigter, G., Ponnighaus, J. M. and Fine, P. E. M. Preliminary appraisal of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
75. Ekambaram, V. and Sithambaram, M. Self-healing in non-lepromatous leprosy in an area of the ELEP Leprosy Control Project Dharmapuri (Tamil Nadu). Lepr. India 49(1977)387-392.
76. Becx-Bleumink, M. and Berhe, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
77. Cruz, M. D. The future of leprosy in the Dominican Republic: experience with multidrug therapy. Lepr. Rev. 57 Suppl. 3(1986)127-133.
78. Becx-Bleumink, M., Berhe, D. and' t Mannetje, W. The management of nerve damage in the leprosy control services. Lepr. Rev. 61(1990)1-11.
79. Brand, M. Report of a pre-Congress workshop on "Rehabilitation." Xlllth International Leprosy Congress, The Hague. The Netherlands, 11-17 September 1988. Lepr. Rev. 59(1988)304-305.
80. Duerksen, F. Report of a pre-Congress workshop on "Prevention and management of impairment in leprosy." Xlllth International Leprosy Congress, The Hague, The Netherlands, 11 -17 September 1988. Lepr. Rev. 59(1988)295-296.
81. Gershon, W. and Srinivasan, G. R. Communitybased rehabilitation: an evaluation study. Lepr. Rev. 63(1992)51-59.
82. Neville, J. After multidrug therapy (MDT) who is responsible for continuing care? Lepr. Rev. 59(1988)1-3.
83. Smith, P. G. Epidemiological methods to evaluate vaccine efficacy. Br. Med. J. 44(1988)679-690.
84. Convit, J., Sampson, C, Zuniga, M., Smith, P. G., Plata, J., Silva, J., Molina, J., Pinandi, M. E., Bloom, B. R. and Salgado, A. Immunoprophylactic trial with combined M. leprae /BCG vaccine against leprosy: preliminary' results. Lancet 339(1992)446-450.
85. Bloom, B. R. An ordinary mortal's guide to the molecular biology of mycobacteria. Int. J. Lepr. 58(1990)365-375.
86. Noordeen, S. K. Vaccination against leprosy; recent advances and practical implications. Lepr. Rev. 56(1985)1-3.
87. Antia, N. H. and Birdi, T. J. Leprosy vaccine-a reappraisal. Int. J. Lepr. 56(1988)310-313.
88. Baker, R. J. The need for new drugs in the treatment and control of leprosy. Int. J. Lepr. 58(1990)78-97.
89. Grosset, J.-H., Guelpa-Lauras, C.-C, Bobin, P., Bruckcr, G., Cartel, J.-L., Constant-Desportes, M., Flaguel, B., Frederic, M., Guillaume, J.-C. and Millan, J. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int. J. Lepr. 57(1989)607-614.
90. Ji, B. and Grosset, J.-H. Recent advances in the chemotherapy of leprosv. Lepr. Rev. 61(1990)313- 329.
91. Gelber, R. H. Activity of minocycline in M. leprae -infected mice. J. Infect. Dis. 186(1987)236-239.
92. Grosset, J.-H. Recent developments in the field of multidrug therapy and future research in chemotherapy of leprosy. Lepr. Rev. 57 Suppl. 3(1986)223-234.
93. Grosset, J.-H., Guelpa-Lauras, C.-C, Pcrani, E. G. and Beoletto, C. Activity of ofloxacin against M. leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
94. M Grosset, J.-H., Ji, B., Guelpa-Lauras, C.-C, Perani, E. G. and N'Deli, L. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281-295.
95. Guelpa-Lauras, C.-C, Pcrani, E. G., Giroir, A. M. and Grosset, J.-H. Activities of pcfloxacin and ciprofloxacin against M. leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
96. N'Deli, L., Guelpa-Lauras, C.-C, Pcrani, E. G. and Grosset, J.-H. Effectiveness of pcfloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)12-18.
97. Fine, P. E. M. Problems in the collection and analysis of data in leprosy studies. Lepr. Rev. 52 Suppl. 1(1981)197-206.
98. Fine, P. E. M. Leprosy: the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982)161-188.
99. Bagshawe, A. F., Garsia, R. J., Baumgart, K. and Astbury, L. IgM serum antibodies to phenolic glycolipid-1 and clinical leprosy; two years' observation in a community with hyperendemic leprosy. Int. J. Lepr. 58(1990)25-30.
100. Cartel, J.-L., Chanteau, S., Boutin, J.-P., Plichart, R., Richez, P., Roux, J.-F. and Grosset, J.-H. Assessment of anti-phenolic glycolipid-I IgM levels using an ELISA for detection of M. leprae infection in populations of the South Pacific islands. Int. J. Lepr. 58(1990)512-517.
101. Chanteau, S., Cartel, J.-L., Guidi, C, Plichart, R. and Bach, M.-A. Seroepidemiological study on 724 household contacts of leprosy patients in French Polynesia using disaccharide-octyl-BSA as antigen. Int. J. Lepr. 55(1987)626-632.
102. Chujor, C. S. N., Bemhcimer, H., Levis, W. R. and Schwerer, B. Serum IgAl and IgM antibodies against M. leprac-dcnvcd phenolic glycolipid-I: a comparative study in leprosy patients and their contacts. Int. J. Lepr. 59(1991)441-449.
103. Douglas, J. T., Ccllona, R. V., Abalos, R. M., Madarang, M. G. and Fajardo, T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Cebu, The Philippines. Int. J. Lepr. 55(1987)718-721.
104. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. C. Seroepidemiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugatc antigen. Int. J. Lepr. 56(1988)243-254.
105. Groenen, G., Pattyn, S. R., Ghys, P., Tshilumba, K., Kuykcns, L., Colston, M. J. and the Yalisombo Study Group. A longitudinal study of the incidence of leprosy in a hyperendemic area in Zaire with special reference to PGL-antibody results. Int. J. Lepr. 58(1990)641-650.
106. Klatser, P. R., de Wit, M. Y. L. and Kolk, A. H. J. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
107. Krishnamurthy, P., Rao, P. S., Reddy, B. N., Subramanian, M., Dhandayudapani, S., Bhatia, V., Nee-Ian, P. N. and Dutta, A. Seroepidemiological study of leprosy in a highly endemic population of South India based on an ELISA using synthetic PGL-I. Int. J. Lepr. 59(1991)426-431.
108. Soebono, H. and Klatser, P. R. A seroepidemiological study of leprosy in high- and low-endemic Indonesian villages. Int. J. Lepr. 59(1991)416-425.
109. Ulrich, M., Smith, P. G., Sampson, C, Zuniga, M., Centeno, M., Garcia, V., Manrique, X., Salgado, A. and Convit, J. IgM antibodies to native phenolic glycolip-l in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-415.
110. Fine, P. E. M. Immunological tools in leprosy control. Int. J. Lepr. 57(1989)671-686.
111. Mullis, K. B. and Fallona, F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzcd chain reaction. Meth. Enzymol. 155(1987)335-350.
112. Fiallo, P., Williams, D. L. and Gillis, T. P. The dctcction of At. leprae from formalin-fixed and paraffin-embedded tissues using PCR amplification. In: Health Cooperation Papers, Proceedings of the VI Leprosy Symposium on "Leprosy Research," Santa Alargherita Ligure-Genoa, Italy, October 3-7 1990. Associazione Italiana "Amici di Raoul Follereau," 1992, pp. 171-172.
113. Gillis, T. P. and Williams, D. L. Polymerase chain reaction and leprosy. Int. J. Lepr. 59(1991)311-316.
114. Hartskeerl, R. A., de Wit, M. Y. L. and Klatser, P. R. Polymerase chain reaction for the detection of Ai. leprae. J. Gen. Microbiol. 135(1989)2357-2365.
115. Klatser, P. R., de Wit, M. Y. L, Faber, W. R., Krieg, S. R., Douglas, J. T., Lucas, S. B., Montrewasewat, N., Pattyn, S. R. and Hartskeerl, R. A. Application of a polymerase chain reaction for the detection of A4. leprae in infected tissues. In: Health Cooperation Papers, Proceedings of the VI Leprosy Symposium on "Leprosy Research, " Santa Margherita Ligure-Genoa, Italy, October 3-7 1990. Associazione Italiana "Amicidi Raoul Follereau," 1992, p. 173.
116. Klatser, P. R., Ellard, G. A., Fiallo, P., Williams, D. L. and Gillis, T. P. Working Group on PCR. In: Health Cooperation Papers, Proceedings of the VI Leprosy Symposium on "Leprosy Research. " Santa Alargherita Ligure-Genoa, Italy, October 3-7 1990. Associazione Italiana "Amici di Raoul Follereau," 1992, p. 291.
117. Styblo, K. The global aspects of tuberculosis and HIV infection. Bull. IUATLD. 65(1990)28-32.
118. Miller, R. A. Leprosy and AIDS: a review of the literature and speculations on the impact of CDA+ lymphocyte depletion on immunity to Al. leprae. Int. J. Lepr. 59(1991)639-644.
119. Ponnighaus, J. M., Mwanjasi, L. J., Fine, P. E. M., Shaw, M.-A., Turner, A. C., Oxborrow, S. M., Lucas, S. B., Jenkins, P. A., Sterne, A. C. and Bliss, L. Is HIV infection a risk factor for leprosy? Int. J. Lepr. 59(1991)221-228.
120. Leonard, G., Sangare, A., Vcrdier, M., Sassou-Gusseau, E., Petit, G., Milan, J., M'Boup, S., Rey, J.-L., Dumas, J.-L., Hugen, J., N'Gaporo, 1. and Denis, F. Prevalence of HIV infection among patients with leprosy in African countries and Yemen. Immune Defic. Syndr. 3(1990)1109-1113.
121. Tekle-Haimanot, R., Frommel, D., Tadcsse, T., Verdier, M., Abebe, M. and Denis F. A survey of HTLV-1 and HIVs in Ethiopian leprosy patients. AIDS 5(1991)108-110.
122. Turk, J. L. and Rees, R. J. W. AIDS and leprosy. Lepr. Rev. 59(1988)193-194.