- Volume 60 , Number 4
- Page: 667–75
News and notes
This department furnishes information concerning institutions, organizations, and individuals engaged in work on leprosy and other mycobacterial diseases, and makes note of scientific meetings and other matters of interest.
Cuba. International course in Cuba. The Pedro Kouri Institute of Tropical Medicine and the Cuban Ministry of Public Health announce an international course on Advanced Quantitative Techniques in Epidemiology to be held in January of each year in Havana. The program will include conferences, seminars, practice classes, computer laboratory sessions, and demonstration visits. The topics to be covered will include:
Approaches to the application of measurements in epidemiology Measurement of frequency of association and potential impact Causality in epidemiology Design of a cohort study Design of a controlled case study (with cases and controls); bias in controlled case studies Sampling in the various types of epidemiological study design Screening and evaluation of diagnostic tests Evaluation of risk factors Evaluation of risk factors adjusted for a single variable Review of simple and multiple regression Logistic regression: general principles and applications in epidemiology
The official language of the course will be Spanish. Further information may be obtained from: Dr. Nereyda Cantelar, Subdirectora Docente, "Pedro Kouri" Institute of Tropical Medicine, Apartado Postal #601, Zona Postal Area Marianao 13, Havana, Cuba. Telex 511902, 512341, telephones 204913, 204926, fax 5-37-215957.-PAHO Epidemiol. Bull. 13 (1992) 13-14
Ethiopia. ALERT seeks candidates for two positions. The All Africa Leprosy and Rehabilitation Training Center (ALERT) is seeking a Senior Field Research Medical Officer (SFRMO) (a renewable, 3-year contract) who should continue an important longitudinal research project on reactions, neuritis and nerve function impairment in leprosy patients treated with the WHO-MDT regimen. The successful candidate must have: a basic medical degree; a postgraduate diploma/degree in public health preferably in epidemiology; at least 4 years' experience in leprosy control, preferably with research activities in that field -or- substantial experience with field studies of another chronic disease and at least 2 years' experience in leprosy control; teaching experience; a record of publications in reputed journals.
ALERT is also seeking a Director of its Leprosy and Tuberculosis Field Control Program. The successful candidate will have, in addition to a basic medical degree, a recognized postgraduate qualification, preferably in Public Health or Epidemiology, as well as at least 5 years' experience managing similar programs elsewhere -preferably in Africa. Since ALERT is an international training center, experience in a medical training program will be a definite advantage. Some international travel will be required, as will the ability to establish good professional relationships with people from different cultures and nationalities. The combination of tuberculosis with leprosy is a major innovation for ALERT, so the ability to design, implement and monitor programs of applied research in training and epidemiology would be a distinct advantage.
Internationally competitive salary and benefits, including furnished accommodation, are available to the successful candidates.
Candidates for each position are requested to submit detailed CVs, with the names and contact details of three international referees, immediately to: Executive Director, ALERT, P.O. Box 165, Addis Ababa, Ethiopia. (Tel + 251 1 71 11 10; Fax + 251 1 71 11 99; Tlx 21821 ALERT ET).
India. 1990-1991 Annual Report of the ICMR Director-General. We are pleased to publish here a section of this 1990-1991 Indian Council of Medical Research annual report dealing with leprosy. -RCH
The National Leprosy Eradication Program has been accorded high priority among the national programs aimed at control of communicable diseases. If dapsone therapy raised hopes of the control of leprosy, multidrug therapy (MDT) not only heralds the possible eradication of leprosy but has indeed rejuvenated the leprosy control program. India continues to harbor the largest number of leprosy patients in the world. It is estimated that nearly 70% of all leprosy patients are currently being given MDT through the National Leprosy Eradication Program (NLEP). Nevertheless, operational, technical and administrative problems in the implementation of MDT continue and coverage of all highly endemic districts with MDT is yet to be achieved.
Clinical trials are being supported to find the optimum combination of drugs for treatment of leprosy and the optimum duration of therapy in multi- and paucibacillary leprosy, methods for improved case detection, case-holding, and psychosocial problems in leprosy. Research aimed at developing newer diagnostic and prognostic indicators, understanding pathogenesis of leprosy and its complications received support during the year. Studies were also continued for elucidating the mechanism of deformities developing in leprosy patients to formulate steps for prevention of deformities and effective surgical techniques for the correction of deformities. These studies are being carried out through the Council's Central JALM A Institute for Leprosy (OIL) at Agra and its field unit at Avadi as also through extramural research projects funded by the Council in different parts of the country.
India has played a leading role in the development and testing of immuno-therapeutic and immuno-prophylactic agents in leprosy. The Council has been funding a trial of ICRC vaccine for immuno-prophylaxis against leprosy in Maharashtra and has recently initiated a comparative evaluation ofantileprosy vaccines in Tamil Nadu.
STUDIES ON MULTIDRUG THERAPY
With the advent of MDT, cure of leprosy has become possible usually within 1 year and invariably by 5 years, in spite of increasing prevalence of dapsone resistance. Several investigations at the CJIL Agra, Central Leprosy Training and Research Institute (CLTRI), Chengleput and the Schieffelin Leprosy Research and Training Centre (SLRTC), Karigiri, have been carried out to find the optimum duration of MD T in multi- and paucibacillary leprosy and to evaluate different combinations of drugs to find the most effective combination with minimal side effects. Parameters used for assessment include clinical response (regression, reactions, relapse rates and neurological problems) and kinetics of decrease in bacterial index over time.
Multibacillary leprosy. The MD T results in rapid reduction in bacterial index and by 1 year less than 10% patients have viable bacilli in the smears. Longterm follow up of the highly bacillated lepromalous leprosy patients who were treated with a modified WHO regimen showed that all patients available for follow up at 6 years achieved smear negativity. None of the patients who achieved smear negativity have relapsed so far. Further follow up of patients who dropped out of treatment has shown that 24-30 months of conventional or slightly modified MD T may be sufficient to treat multibacillary patients. Studies to determine the effect of short-term, fixed duration chemotherapy regimens are continuing. Multibacillary patients who were treated with MD T regimens containing pyrazinamide have completed a follow up of 2 years and the results are being analyzed.
Highly bacillated untreated BL/LL patients are being treated with MDT and MDT plus vaccine (MDT+ BCG or MD T + Mw). These patients have completed 18-24 months of treatment. On preliminary analysis of clinical and bacteriological parameters, it appears that the addition of immunotherapy to chemotherapy can enhance killing and clearance of M. leprae from the lesions. If these findings are confirmed in a larger number of patients followed up over longer periods, this approach may find application in reducing the duration of treatment.
Paucibacillary leprosy. A 6-year follow up of patients with paucibacillary leprosy who had been treated with MD T and modified MD T showed that the 1-year regimen proposed by CJIL appears to be more effective than the standard WHO regimen for 6 months in terms of clinical subsidence, low relapse rates and few or no late reactions. Enrollment of patients for the trial using prothionamide as part of MD T for patients with paucibacillary leprosy has been completed and most of the patients have been followed up for 1 year.
PREVENTION AND CORRECTION OF DEFORMITIES
Deformities in leprosy due to nerve damage is a major factor responsible for social ostracism and economic problems in leprosy patients. The focus of a leprosy control program is not extermination of M. leprae but to save human beings from being maimed and deformed by this infection and to enable them to lead a normal life. Studies funded by ICMR have led to the formulation of effective methods of prevention and surgical correction of deformities of the hands as well as the feet. Research aimed at evolving improved techniques for accurate assessment of neurological damage and functional status before and after surgery is being continued.
Objective assessment of the sensory impairment in anesthetic hands and feet, using 3.61 and 4.56 monofilament nylon, showed that the patients' reports on loss of pain sensibility is reliable. Studies are underway to evolve objective tests for assessment of pain, including deep pressure pain in leprosy patients.
CLINICAL DIAGNOSIS OF LEPROSY
Clinical studies aimed at improving the understanding of indeterminate leprosy, prognostic markers in paucibacillary neuritic leprosy and evolution of early leprosy have been undertaken by the OIL , Agra. Studies on indeterminates and early "suspicious" cases showed that it is difficult to demonstrate acid-fast bacilli (AFB) in most cases, even histology being nonspecific in them. Studies to assess the possible usefulness of genomic probes and gene amplification techniques (standardized and/or established at OIL , Agra) in characterizing and confirming such cases are underway.
PATHOLOGY AND IMINUNOPATHOLOGY
Single cell suspensions from leprosy lesions are being studied by electron microscopy for cell structure and morphology. Semithin and ultrathin sections of tuberculoid granuloma showed that infiltrating cells were activated T cells, epitheloid cells and activated macrophages. On the other hand, the lepromatous lesions had very few lymphocytes and most of the macrophages had M . leprae or its debris inside them.
Ullracytomchemical studies for characterizing the lysosomal morphology and function showed that the lysosomal morphology is disrupted in reactional states. Sequential biopsies are being carried out to document the effect of various therapeutic measures.
Studies on immune responses to the antigens of M. leprae and related mycobacteria are being carried out to characterize M. leprae-specific and nonspecific antibody responses with a view to developing sérodiagnostic tests for leprosy, to understand the role of antibodies in the immunopathology of the disease, and to characterize the antigens involved in the modulation of cell-mediated immunity. Comparative evaluation of total M . leprae ELISA (ML-ELISA), SACT-ELISA and phenolic glycolipid (PGL) ELISA in active tuberculoid cases showed that the positivity rates were 55% with ML-ELISA, 38% with SACT-ELISA, and 26% with PGL-ELISA. Combined results ofall tests showed a positivity rate of 68%. the overall positivity correlating with the extent of disease.
To identify M. leprae antigens which evoke responses in leprosy patients, sera from patients are being tested for the presence of antibodies to dilferent M. leprae antigens. Immunodominant antigen responsible for the seropositivity in patients of tuberculoid leprosy were observed in the 20-40-kDa range. The study has revealed, for the first time, M. leprae-specific antibodies in the CSF of leprosy patients. The origin of these antibodies is being investigated.
Studies on the analysis of M. leprae antigen(s) in the circulating immune complexes (CICs) of leprosy patients are continuing. There were no differences in the levels of total mycobacterial immunocomplexes and PGL-I immunocomplexes between BL/LL patients with and without erythema nodosum leprosum (ENL) reactions. Experiments are being continued to establish a model system for the study of rcactional states in animals. The lesions produced in mice are being studied by immuno-histochemistry and the interaction of mycobacteria with human complement system is also being studied. To gain an insight into the pathophysiology of the lymphocytes of leprosy patients, a study to characterize various enzymes in the lymphocytes of dilferent types of leprosy cases is being undertaken. Differences in activities of adenosine deaminase (ADA), superoxide dismutase (SOD), peroxidase and RNA synthetases in the lymphocyte extracts have been noted. It is not yet clear whether these alterations are the cause or the effect or just due to casual associations of these patho-physiological events.
STUDIES ON M. LEPRAE AND RELATED MYCOBACTERIA
Studies are in progress on the metabolism and growth of M. leprae to elucidate the metabolic requirements of M. leprae which may help in its cultivation. Extended studies on energy synthesis have shown that low oxygen tension is an important factor for sustained ATP synthesis by M. leprae. Other significant factors like pH, temperature, etc., have already been identified and studies are in progress to understand more about essential nutrients and factors conducive for better ATP synthesis by M. leprae. The in vitro system for measurement of ATP standardized earlier at the OIL , Agra, has been further improved by using modified biophysical conditions. The current system allows more sustained ATP synthesis by M. leprae which can be maintained for 4 weeks.
Studies on the kinetics of transcription and translation in M. leprae and other mycobacteria showed that there are basic differences in tRNA synthetases and amino transferases between the slow- and rapid-growing mycobacteria. The relationship if any between enzyme levels and growth kinetics of M. leprae and also the drug-sensitive sites is being investigated.
ATP assay which is sensitive enough to detect even a very small number of lepra bacilli present after 2-3 years of treatment has already been standardized at OIL , Agra. The system has been evaluated further to assess the combined effect of immunotherapy and chemotherapy, to diagnose relapses and detect persisters. Results show that the assay standarized at OI L is very sensitive, rapid and economic and can be used for monitoring the effect of treatment, diagnosing relapses and detecting persisters. The drug screening system based on ATP synthesis is being further improved and evaluated.
Characterization of subtypes and strains of M. leprae is important for a belter understanding of the epidemiology of the disease. Several new methods based on analysis of lipids, isoenzymes and immunological relatedness of enzymes have been developed at the CJIL, Agra. Evaluation of protein eleetrophoregrams and zymograms in several mycobacteria, including M, leprae ,has shown that a combination of these two can be used for rapid identification and characterization of various pathogenic mycobacteria. Using these techniques, strain differences among M. leprae isolates have been demonstrated for the first time. Studies are in progress to test their applicability in clinical situations.
DESIGNING AND EVALUATION OF DIAGNOSTIC PROBES FOR MYCOBACTERIA INCLUDING M. LEPRAE
Studies are being undertaken to identify the gene sequence in the variable regions of RNA. Earlier studies have shown the usefulness of restriction fragment length polymorphism (RFLP) analysis of RNA genes of pathogenic mycobacteria. Sequencing of I6S genes and Hanking sequences of 13 species of mycobacteria are Hearing completion. Based on the analysis of these data, a new oligonucleotide probe has been synthesized in the CJIL, Agra, and tested against various mycobacteria, including M . leprae. Prelinary analysis has shown that this is specific for M . leprae. Gene amplification technique like polymerase chain reaction (PCR), using primer based 18-kDa and 36-kI)a antigen genes has been established at CJIL, Agra, and is being compared with a reverse PCR standardized earlier at the Institute.
EXPRESSION OF MYCOBACTERIAL ENZYMES
Macrophages from normal individuals phagocytose M . leprae and kill them through the production of reactive oxygen intermediates such as hydrogen peroxide, superoxide and hydroxyl ions. Macrophages of healthy individuals do not tolerate live M . leprae while the macrophages of lepromatous and tuberculoid leprosy patients do so. Preliminary studies indicate that delipidated cell component of M . leprae is able to activate the leukocytes of leprosy patients and the culture supernatant of such a test system is, in turn, able to activate the macrophages to kill M . leprae through reactive oxygen intermediates. The culture supernatant appeared to modify the surface properties of the macrophages of the leprosy patients so as to normalize the pre-existing defects. Such normalization leads to recognition and killing of M . leprae through reactive oxygen intermediate system.
Studies are in progress to elicit the expression of enzymes which are metabolically or immunologically important. Using polyclonal antibodies raised at the CJIL, Agra, against superoxide dismutases and other enzymes of mycobacteria, gene libraries are being screened to elicit their expression. Studies have been initiated to characterize the DNA-dependent RNA polymerase of M . leprae, to purify these enzymes from different mycobacteria to raise antiscra against them, and screen the gene libraries for their expression.
IMMUNOPROPHYLAXIS AND IMMUNOTHERAPY FOR LEPROSY
India has played a leading role in developing and testing immunotherapeutic and immunoprophylactic agents for leprosy. During the last 5 years, the Council has been supporting a trial of the ICRC vaccine for immunoprophylaxis against leprosy, to assess the immunoprophylactic efficacy of the two vaccines containing (i) ICRC bacillus and (ii) BCG, by measuring the incidence of both multi- and paucibacillary forms of leprosy in ICRC vaccinated as compared to the BCG groups. The randomized, controlled trial involves healthy subjects of either sex. aged between 1 to 65 years, who are household contacts of leprosy patients. The study is also aimed at preparing the characterizing subunit vaccine and studies on immunobiology of T cells before and after vaccination.
Vaccination of healthy household contacts has been completed. The reported compliance rate was 95% to 98%. Studies on the effect of vaccination of LL patients, clinically nonresponsive to multidrug regimen showed a significant drop in the bacillary index after vaccination, concomitant with an increase in lymphocyte proliferation response to both ICRC/M. leprae antigens. A marked increase in the frequency of antigen reactive T cells was also evident. Cytokine production such as interferon (IFN-γ) was increased in some of the vaccinated patients.
The CJIL Field Unit at Avadi (Tamil Nadu) has completed the preparatory phase for initiating leprosy vaccine trials. Baseline surveys, immunological characterization of the population, and methodological studies have been carried out earlier. Phase-II studies with BCG in combination with killed M. leprae were also carried out during the year. It was observed that the sensitizing effect of this combination was persistent and was dose related. Suppurative adenitis seen in a few cases could be brought down by reducing the dose of BCG. Comparative trials of leprosy vaccines have been initiated recently to study the prophylactic efficacy of ICRC, Mw and BCG + killed M . leprae.
Electron microscopic studies on various leprosy lesions are in progress at the Council's Institute of Pathology (IOP) at New Delhi.
In studies carried out at the Council's Institute of Pathology (IOP) at New Delhi, histopathological monitoring of phase II and III clinical trials with a candidate vaccine based on Mycobacterium W . indicated that there is definite evidence of either granuloma clearance or immunological upgradation in the test group receiving vaccine and MD T as compared to the control group which received MD T alone.
The Netherlands. INFOLEP: Leprosy Information Services. INFOLEP Leprosy Information Services is a project of the Netherlands Leprosy Relief Association (NSL) in Amsterdam, The Netherlands. INFOLEP's services are , to a great extent, aimed at the leprosy field projects in countries supported by the NSL. INFOLEP also functions as a library and documentation center offering its services to all those who need information on leprosy, in all its various aspects.
The objective of INFOLEP is to set up and maintain a vivid information network in the field of leprosy primarily for the NSLsupported leprosy projects and for those studying or preparing to work in this field. On a pro-active basis, INFOLEP supplies information to all the NSL-supported leprosy field projects. This occurs through regular mailings, consisting of publications, articles, journals and other materials deemed relevant. Local initiatives, in endemic countries, to produce teaching and learning materials in leprosy control can receive technical and financial support through INFOLEP.
INFOLEP has an extensive collection of materials on leprosy and leprosy control, consisting of books, journals, brochures, articles, reports, slides, (video) films and others, which can be consulted and/or borrowed. The greater part of the collection deals with scientific medical aspects; whereas the smaller, but steadily increasing part, focuses on teaching and learning materials on leprosy. The collection is accessible through different catalogues. Since January 1990, retrospective storage and retrieval takes place by a computerized system (CDS/ ISIS). This of Ters the possibility of free text searching, which enables you to find your information on title, author, subject or keyword.
INFOLEP has subscribed to two CD-ROM databases, i.e., MEDLINE from 1985 and HealthPLAN from 1981 onwards. Both databases are frequently updated and complement each other. MEDLINE covers the biomedical, clinical aspects of medicine; whereas HealthPLAN gives information on the nonclinical aspects of health care, like health education, management, administration, social aspects, etc. Both are bibliographic files with informative abstracts giving you an idea of the contents of an article.
Other services include: a) photocopy service (for a fee); b) inter-library loans, including photocopy service (for a fee); c) international bibliography on teaching and learning materials on leprosy; d) audiovisual equipment for the use of video-films and slides.
For further information write to: INFOLEP: Leprosy Information Services, Netherlands Leprosy Relief Association (NSL), Wibautstraat 135 II, NL-1097 DN Amsterdam, The Netherlands.
Switzerland. TDR needs health education materials. TDR's communications unit is about to start a pilot project in communications for development. The project will concentrate on health education for the TDR target diseases (malaria, schistosomiasis, lymphatic filariasis, onchocerciasis, African trypanosomiasis, Chagas disease, leishmaniasis and leprosy) and will be carried out in consultation with TDR's social and economic research steering committee and WHO's Divisions of Control of Tropical Diseases (CTD) and Health Education (HED).
As part of this project, TDR has hired a consultant to collect health education materials on the TDR diseases, as well as reports of studies relating to health education in these diseases. TDR would greatly appreciate help in putting this collection together. If you have copies of such materials or can indicate where they can be obtained or can give references to or send copies of relevant study results, please address your materials and information to: Ms Anc Haaland, TDR Communications Consultant, Skogbrynct 9, 1458 Fjellstrand, Norway [tel: (47-9) 918419; fax: (47-9) 918713].-TDR news 40(1992)10
TDR regional linkage grants for research training. TDR began its Research Capability Strengthening activities in 1977. Since then it has awarded 185 grants to strengthen institutions in developing countries and 1250 grants to scientists wishing to be trained in research on the tropical diseases with which TDR is concerned -malaria, schistosomiasis, lymphatic filariasis, onchocerciasis, African trypanosomiasis, Chagas disease, leishmaniasis and leprosy. These grants have done much to create a body of expertise on these diseases in the countries where they are endemic. TDR wishes to take advantage of this expertise to create a network of research centers that could provide training-through so-called linkage grants -for young researchers in developing countries. These grants would enable young researchers to be trained in research skills in countries affected by the TDR target diseases and in an academic and social environment not very different from that of their home countries.
TDR linkage grants are intended for two or more research groups -applications from single research groups will not be considered-from countries where the TDR "target" diseases are endemic. The groups must have demonstrated research expertise and be currently conducting research in fields relevant to these diseases, such as parasitology, entomology, epidemiology, immunology, molecular and cell biology, clinical pharmacology and social sciences (including economics). It is immaterial whether or not the groups have received TDR grants in the past. One essential condition is that each of the groups applying for the linkage grants be prepared to collaborate with similar groups possessing complementary skills, in the same or a neighboring country but within the same region. Another condition is that, together, the collaborating research groups have the basic facilities and can obtain the necessary accreditation from their local universities or research centers to provide junior researchers with opportunities for training in field or laboratory research at graduate (M.Sc. and/or Ph.D.) and postdoctoral levels.
The grants may also be used to support research training links between groups from developing and developed countries: such support, however, will be considered on a case-by-case basis and will cover only the travel expenses of visiting experts from developed countries.
The linkage grants will provide each research group (within a single application) with a minimum "core" support of about US$30,000 a year for 3 years, renewable for a further 2 years. These funds can be used to cover the cost of supplies, communication, visiting experts and relevant literature. For each junior researcher accepted for training by the recipient groups TDR could, on request, provide additional funding to cover research expenses for graduate or postdoctoral studies or for the acquisition of specific skills over a shorter period: the topics of this training must relate to a TDR target disease. Funding of group learning activities, including short-term training courses, can also be provided.
Interested research groups should send a joint application, providing the following information, to the address below (no application forms are required):
1. Full name (in capital letters), address, telephone, telex and fax numbers of each of the collaborating research groups.
2. Brief curricula vitae of the principal investigators (group leaders) of the research groups and all the members of the groups who will be directly involved in the training of junior scientists. Each curriculum vitac should give name, date of birth and nationality as well as educational qualifications, with dates of degrees, diplomas, etc., current professional position and research interests, and lists of papers published in peer-reviewed journals and of research grants received from national and international bodies. If any member of a collaborating group applying has participated in a project funded by TDR, provide the name of the principal investigator, the title of the project and its TDR ID number.
3. Regarding the institutions of each of the collaborating research groups:
a) Current requirements for enrollment in the graduate program
b) Annual tuition fees and other costs
c) Available laboratory and office space (in square meters), with number of rooms for each
d) Number and type of microcomputers and software packages, including statistical programs, in current use
e) Field station and transport facilities
f) Names and addresses of postgraduates trained during the past 5 years, giving project titles, a list of publications and current positions
g) Brief description of health literature available, including access to international databases or electronic networks
4. Outline the main research strengths of each collaborating group (a single typed page per group), stating how they complement each other and mentioning specific areas where each group could provide opportunities for training in research. Specify if the members of the applicant research groups have in the past worked together on collaborative research projects.
5. Annual budget, for a 3-year period, for the following items (provide full justification for each): personnel, equipment, supplies, animals, local transportation, international travel (for visits to collaborating research groups only), communication and health literature.
6. If support is requested for one or more junior researchers already registered or accepted for training, provide a brief curriculum vitae of each trainee researcher, the proposed research to be conducted under the linkage grant and a budget for this research itemizing stipends (only for trainees from outside the country), tuition fees, laboratory expenses and local travel.
Applications should be addressed to: Dr. J. A. Hashmi, TDR, World Health Organization, 1211 Geneva 27, Switzerland [tel: 41-22/7913805; fax: 41-22/7910746; telegrams: UNISANTE GENEVA; telex: 415416 OMS] and should be sent in time to reach TDR no later than 31 March 1993 (applications received after this date will not be considered). Applicants will be informed of the outcome of their applications in June 1993.-TDR news 40(1992)7, 8
TDR Steering Committee deadlines.
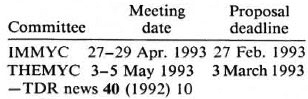
TDR's Leprosy Steering Committees (IMMLEP and THELEP) have been reorganized to form two new joint leprosy-tuberculosis (i.e., mycobacterial disease) steering committees in collaboration with WHO programs concerned with tuberculosis: one committee (IMMYC) will be concerned with basic research and immunology; the other (THEMYC), with chemotherapy of the two diseases. Meeting dates of these committees and proposal deadlines are:
U.K. London School of Hygiene & Tropical Medicine to hold Third Annual Public Health Forum 1993. On 18-21 April 1933 the London School of Hygiene & Tropical Medicine will hold its Third Annual Public Health Forum: Tuberculosis-Back to the Future. The meeting is intended for health care professionals and research scientists working on the control of tuberculosis, particularly in developing countries. The specific objectives for the Forum are : a) To bring together both scientists working on different aspects of tuberculosis research and those involved with planning and implementing control of strategies, b) To describe the global burden of tuberculosis and predict the likely trends over the next decade with special attention to the impact of the HIV epidemic, e) To identify and prioritize major research issues relating to: pathogenesis, diagnosis, clinical management, epidemiology, molecular biology, economics and control of tuberculosis, d) To address specific questions relating to: diagnosis and clinical management, immunology and vaccines, chemoprophylaxis and drug therapy, and control strategies and resource allocation.
For further information, including the program and registration form, contact: Conference Secretary, London School of Hygiene & Tropical Medicine, Keppel Street, London WC1E 7HT, U.K. (tel +44 (0)71 927 2314; fax +44 (0)71 436 5389; telex 8953474).
Paul Brand new President ofTLM International. Dr. Paul Brand, C.B.E., F.R.C.S., F.A.C.S., formerly a TLM surgeon and medical educator at Christian Medical College in Vellore, India, a leading specialist in leprosy surgery, a pioneer in leprosy rehabilitation known worldwide, and for many years Chief of the Rehabilitation Branch of the GWL Hansen's Disease Center at Carville, Louisiana, U.S.A., has become the new President of The Leprosy Mission International. We extend our congratulations and wish him well in his new position. - RCH
Robert Cochrane Fund for Leprosy. The Fund, in memory of the great leprologist Robert Cochrane, is administered by the Royal Society ofTropical Medicine and Hygiene. It is to be used to finance up to three travel fellowships each year, to a maximum value of £1500 each.
The Fund will support travel for: a) leprosy workers who need to obtain practical training in field work or in research; b) experienced leprologists to provide practical training in a developing country. There is no restriction on the country of origin or destination providing the above requirements are fulfilled.
Application forms are available from the Society and completed forms must be received by the Society at least 6 months ahead of the proposed visit. All applications must be sponsored by a suitable representative of the applicant's employer or study center, and agreed by the host organization. A twopage report on the travel/study should be submitted to the Society within 1 month of the recipient's return. For further details write: Royal Society of Tropical Medicine and Hvgiene, Manson House, 26 Portland Place, London WI N 4EY, U.K.
U.S.A. Frist elected President of ILEP. Thomas Ferran Frist, President of ALM International (American Leprosy Missions), has been voted President-elect of the International Federation of World Leprosy Organizations (ILEP). Frist will serve on ILEP's standing committee until his 2-year term of office begins in 1994. The selection of Frist was made during ILEP's June 1992 conference in Montreal, Canada.
ALM International, headquartered in Greenville, South Carolina, is the U.S.'s oldest and largest nonprofit organization engaged in the fight against Hansen's disease (HD). As a member agency of ILEP, ALM has assumed leadership of many areas in the world-wide campaign to eradicate HD. ALM Medical Director, Dr. W. Fclton Ross, is head of ILEP's Medical Commission. President Frist currently heads its Social Aspects Commission.
ILEP was founded in 1966 and consists of 21 member agencies from Europe, North America, Asia, and Africa. In 1990 members committed themselves to the global goal of "multidrug therapy for all leprosy patients by the year 2000" [following World Health Organization drug trials in the early 1980s, multidrug therapy (MDT) became the recommended regimen for treatment and cure of HD. In the last twenty years the number of HD sufferers in the world has dropped from 20 million to under 10 million.] Currently, ILEP agencies contribute about $80,000,000 yearly to treat and rehabilitate HD patients.
According to Frist, "ALM International and other ILEP agencies face four special challenges during the 90s: to reduce the pool of infection by treating and monitoring approximately five and a half million 'active' cases; to begin multidrug therapy (MDT) for the two to three million of these infected individuals not yet on treatment; to continue to 'integrate' HD treatment with other health and social services in order to reduce the stigma associated with this disease, and to rehabilitate another two to three million people for whom treatment came too late to prevent disabilities."
Frist sees ILEP as a unique organization, "one of the most successful models in the world of international cooperation and coordination. The Federation helps members ensure that funds they receive from donors are used in the best possible way, without duplication of funding or program activities. Because of its coordinated activities, ILEP agencies are able to extend treatment to nearly 100 countries where HD remains a problem." -From ALM news release
Jacobson new Carville Director. Effective 1 July 1992 Robert R. Jacobson, M.D., Ph.D., officially became Director of the U.S. Public Health Services' Division of National Hansen's Disease Programs and Director of the Gillis W. Long Hansen's Disease Center at Carville, Louisiana.
Jacobson, a native of Austin, Minnesota, began his career at Carville in 1966 as Chief of Medicine becoming Chief of the Clinical Branch in 1978. In addition to his various other duties, lie is in charge of all investigational drug studies conducted at Carville.

An internationally known consultant on HD, Dr. Jacobson has shared his expertise for the World Health Organization and others in Japan, South Korea, People's Republic of China, WHO -Geneva, Venezuela, Fiji, India, and elsewhere throughout the world.
He is the recipient of several awards for his work at Carville, including the U.S. Public Health Service Distinguished Service Medal, the P.H.S. Meritorious Service Medal, and the P.H.S. Commendation Medal. He holds membership in the Society of Sigma Xi and the International Leprosy Association.
We extend our congratulations to Dr. Jacobson for this highly deserved appointment. - RCH/DDG