- Volume 61 , Number 1
- Page: 107–8
Suppression of human monocyte cytokine release by phenolic Glycolipid-I of Mycobacterium leprae
To the Editor:
It remains unclear why the vast majority of people exposed to Mycobacterium leprae develop no clinical disease and why only a minority who do develop clinical disease become immunologically unresponsive to antigens of the organisms. The presence of large amounts of phenolic glycolipid-I (PGL-I), the unique antigen of M. leprae (4) in tissues infected with M. leprae (3) may be a factor associated with the specific immunologic unresponsiveness seen in lepromatous leprosy patients. PGL-I is capable of inducing suppression of mitogenic responses of leprosy patients' lymphocytes in vitro (5,6), although it was not established whether it acted through T lymphocytes. The role PGL-I may play in modulating monocyte/macrophage function has not been fully explored. It has been suggested that the phthiocerol-containing lipids of M. leprae play a role as protectors of resident M. leprae within phagosomes of the phagocytic cells (1,7). To further study monocyte function, we have measured monocyte activation by cytokine release in response to lipopolysaccharide (LPS) in the presence or absence of PGL-I from M. leprae.
Peripheral blood mononuclear cells (PBMC) from healthy individuals were prepared with Ficoll-Hypaque gradient centrifugation, washed, and resuspended in RPMI 1640 medium supplemented with 100 units of penicillin/ml, 100 µg of streptomycin/ml, 5% heat-inactivated AB human serum, and 20 raM HEPES. The cells (3.0 x 106 in 1.5 ml/well) were incubated in a plastic petri dish (30 mm in diameter) for 2 hr at 37ºC in a humidified atmosphere of 5% CO2 , after which nonadherent cells were removed by washing three times with medium. The adherent cells were subsequently detached with a scraper, washed twice and adjusted to 2 x 106 cells/ml after counting in a Neubauer chamber. Cultures were set up in triplicate in the presence of medium only (unstimulated cultures) or in the presence or absence of PGL-I (PGL-I was kindly supplied by Dr. M. J. Colston, National Institute for Medical Research, London, England) and LPS ( Escherichia coli 026.B6, Sigma; stimulated cultures). After incubation for 24 hr, the culture medium was aspirated and centrifuged at 1000 x g x 10 min, the supernatants were filtered on a 0.22-µm millipore filter, and cytokine concentrations were determined. TNF-alpha, interleukin (IL)-l and IL-6 concentration in the PBMC supernatants were determined by using solid-phase ELISA kits (Quantikine, R& D Systems).
Since PGL-I is insoluble in aqueous medium it was presented to PBMC in the liposomal form (6). Over a concentration range of 0.1 -10 µg/ml PGL-I, no significant stimulation to produce IL-1, IL-6 or TNF was observed in the cultured monocytes when compared to that observed for the monocytes stimulated with LPS. The results were similar to that found for PBMC in the presence of medium alone (The Figure). In contrast, significant (p < 0.005) levels of suppression of cytokine release were observed by the addition of PGL-I (1 Mg/ml) to the LPS-stimulatcd PBMC cultures. This effect has shown to be dose dependent, and PGL-I was not toxic for PBMC as evaluated by MTT assay (data not shown). Thus the percent suppression ranged from 35%-74% for IL-1, 42%-68% for TNF, and 38%-71% for IL-6 release (median of the standard experiment repeated 5 times).
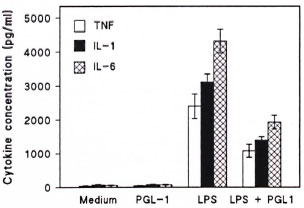
The figure. Cytokine release from human peripheral blood mononuclear cells (PBMC) after stimulation with PGL-I (1 µg/ml), LPS (10 µg/ml) or PGL-I (1 µg/ml) plus LPS (10 µg/ml). As a control, PBM C were cultured in the presence of medium alone.
This study indicates that PGL-I causes a specific inhibition of monocyte cytokine release upon stimulation by LPS. These results might have a profound implication in the host response to M. leprae, a response that requires populations of specifically sensitized T cells and activated macrophages to interact by means of cytokines to give rise to a state of protective immunity to this class of human pathogen. Defective macrophage activation is a prominent feature of lepromatous leprosy that depends on highly localized conditions occurring within macrophage-rich granulomas that contain numerous bacilli (2). It is likely that the specific inhibition of monocyte activation by purified PGL-I in vitro has important parallels in vivo under localized conditions in which lesions contain numerous M. leprae, since PGL-I is an abundant component of these bacilli (4). This also may contribute to the ability of M. leprae to survive as an intracellular pathogen. The mechanism(s) by which PGL-I exerts its effects requires further investigation.
- Celio L. Silva, M.Sc, Ph.D.
Lucia H. Faccioli, B.Sc, Ph.D.
Department of Parasitology, Microbiology and Immunology
-Norma T. Foss, M.D., Ph.D.
Department of Internal Medicine
School of Medicine of Ribeirão Preto
University of São Paulo
14049-900 Ribeirão Preto, SP, Brazil
Acknowledgment. This investigation received financial support from FAPESP and CNPQ. We thank Izaira T. Brandão for her technical assistance.
REFERENCES
1. BRENNAN, P. J. The phthiocerol-containing surface lipids of Mycobacterium leprae-a perspective of past and present work. (Editorial) Int. J. Lepr. 51(1983)387-396.
2. GODAL, T. Immunological aspects of leprosypresent status. Progr. Allergy 25(1978)211-242.
3. HUNTER, S. W. and BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
4. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chcm. 257(1982)15072-15078.
5. MEHRA, V., BRENNAN, P. J., RADA, E., CONVIT, J. and BLOOM, B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308(1984)194-196.
6. PRASAD, H. K., MISHRA, R. S. and NATH, I. Phenolic glycolipid 1 of Mycobacterium leprae induces general suppression of in vitro concanavalin A responses unrelated to leprosy type. J. Exp. Med. 165(1987)239-244.
7. VACHULA, M., HOLZER, T. J. and ANDERSEN, B. R. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.