- Volume 64(4 Suppl 1) , Number 4
- Page: 1–90
International workshop on leprosy research bangkok, thailand 11-13 march 1996
Sponsored by the
Sasakawa Memorial Health Foundation,
Tokyo, Japan
and the
Action Programme for the
Elimination of Leprosy,
World Health Organization,
Geneva, Switzerland
Editor for this Supplement is
Professor Louis Levy,
Department of Dermatology,
Hadassah University Hospital,
Jerusalem, Israel.
OPENING STATEMENT
S.K. Noordeen, Director, Action Programme for the Elimination of Leprosy, World Health Organization, Geneva, Switzerland
It gives me great pleasure to be able to participate in this International Workshop on Leprosy Research, and also to bring greetings from Dr. Hiroshi Nakajima, Director-General of the World Health Organization.
This Workshop is very timely, in view of the new situation in leprosy that is emerging as a consequence of the phenomenal change of the leprosy scene that has taken place in the course of the last two decades, particularly since the introduction in the early 1980s of multidrug therapy (MDT), and the more recent decision to eliminate leprosy as a public health problem-i.e., to reduce prevalence to less than 1:10,000- by the year 2000.
Twenty years ago, the World Health Organization (WHO) initiated, as part of the Special Programme for Research and Training in Tropical Diseases (TDR), a coordinated program of leprosy research consisting of the Scientific Working Groups (SWGs) on Immunology of Leprosy (IMM-LEP) and Chemotherapy of Leprosy (THELEP). At that time, the global situation with respect to leprosy was rather bleak, despite dedicated efforts of several groups in the fight against the disease. Efforts both to treat and to control the disease by treatment of patients with dapsone were successful only under limited circumstances. The difficulties of prolonged treatment, together with the emergence of Mycobacterium leprae resistant to dapsone, then the single, most widely used drug, resulted in serious failures to control the disease. Leprosy workers were frustrated, and only the most determined of them were able to continue the struggle. The establishment of the IMMLEP and THELEP SWGs gave rise to considerable hope for the future, in terms of the possibilities for better tools with which to deal with the disease.
During the early years of the TDR-supported research efforts, there was considerable optimism that an anti-leprosy vaccine would be developed that would completely stop transmission of the causative organism. The impetus for this optimism came from scientific developments in the field of immunology, as well as the availability of large quantities of M. leprae from the newly discovered experimental disease of armadillos. IMMLEP had made important contributions to our understanding of the immunology of leprosy, and intervention by enhancing the immune response to M. leprae appeared possible. In addition, TDR funded very generously the production and banking of large quantities of M. leprae-'m fected armadillo tissue for use by scientists. However, at the same time that IMMLEP and related activities greatly stimulated an increased interest in the problem of leprosy and the need for effective action, and despite enormous progress in several areas of research, the magnitude of the problems involved in the development of a usable antileprosy vaccine was underestimated.
Compared to those for IMMLEP, the initial expectations of THELEP for improved tools for the chemotherapy of leprosy were rather limited, because the necessary research involved tedious and time-consuming work in the laboratory with the mouse foot-pad system, and in the clinic with long-term treatment and even longer-term follow-up of patients. However, THELEP scientists were determined to bring about changes of leprosy treatment. Their interest stimulated the WHO Leprosy Unit to convene, in 1981, a Study Group on the Chemotherapy of Leprosy for Control Programmes, which was to review the serious global situation of leprosy, and to make recommendations with respect to the more effective use of existing drugs. The Study Group, which was composed of a very good balance of scientists, clinicians and leprosy control managers, made recommendations for MDT that were not only scientifically sound but also practicable. In retrospect, the Study Group's recommendations for MDT appear pivotal in the history of leprosy.
The use of MDT has not only enabled the leprosy patients to be cured by treatment of finite duration; it has also made possible improved definition of the disease, simplification of its classification, and standardization of treatment procedures and of leprosy control activities. Thus, it should be recognized that the favorable situation in which leprosy finds itself today has resulted from indirect as well as direct effects of the widespread application of MDT.
The achievements of the past 10-15 years are quite impressive, as demonstrated by the following statistics. The global burden of leprosy today is estimated to be about 1.3 million cases, as opposed to 10-12 million in the early 1980s. The number of registered cases in 1996 is less than 1 million, compared to 5.4 million in 1985. More than 8 million patients have been cured by MDT since 1985. On the-other hand, the decline of the number of new cases detected each year is less impressive-from about 600,000 a few years ago to about 500,000 now. We estimate that MDT, by its concomitant contribution to early case-detection and prompt treatment, has prevented more than 1 million patients from becoming disabled. In addition, MDT has prevented more than half a million relapses, which would have occurred under dapsone monotherapy.
Despite all of the progress made to date, the task of reaching the remaining patients and achieving the elimination of leprosy as a public health problem by the year 2000 is formidable, and requires further intensification of our efforts. Even as we approach the elimination target, it is important to ensure that elimination, once achieved, can be maintained, so that we can move forward to the next target-that of total eradication of the disease, a not impossible goal to be reached early during the next century. For this reason, we cannot afford to slacken our efforts toward coordinated leprosy research aimed at the solution of relevant problems. Because the agenda for research must be carefully considered, this Workshop has been convened. I sincerely hope that the Workshop will make recommendations for such an agenda.
Before concluding, I should like to thank the Thai Ministry of Public Health and the local organizers for making available excellent facilities, and the Sasakawa Memorial Health Foundation for co-sponsoring this Workshop.
WELCOME
E.B. Doberstyn, WHO Representative to Thailand, Bangkok, Thailand
Dr. Janiroon Mikhanon, Dr. Damron Boonyeon, Dr. Yuasa, Dr. Noordeen, distinguished guests, ladies and gentlemen:
On this occasion of the opening of the International Workshop on Leprosy Research, I should like to extend a warm welcome to all of you on behalf of the World Health Organization (WHO) in Thailand and the South-East Asia Regional Office of WHO.
Leprosy has struck fear into the hearts of men for thousands of years, having been well recognized in the oldest civilizations of China, Egypt and India. Since ancient times, leprosy has been regarded as a contagious, mutilating and incurable disease, makingpeople live more in fear of those afflicted with the disease than of the disease itself.
The number of humans, who, in the course of millenia, have suffered its chronic course of disfigurement and disability can never he calculated. In many countries of Asia, Africa and Latin America, there are still significant numbers of patients. As of 1995, about 2.5 billion people-half of the world's population-live in countries in which the prevalence of leprosy is greater than one per 10,000 population. We esti-mate that, in 1995, there were 1-2 million people who have been visibly and irreversibly disabled by past and present leprosy, and who need to be cared for by the communities in which they live.
On the other hand, the social picture of leprosy has changed in the course of the last few decades. As more and more patients are being treated by the general health services, the disease is increasingly being regarded simply as another problem of public health.The out patient clinic has been officially recognized as the base for leprosy treatment by all countries, while stigmatizing leprosaria are being closed. This hopeful approach de-serves strong support from health personnel and others at all levels, in order to guarantee patients adequate treatment and engender increased self-respect and acceptanceby the community.
It is appropriate that this Workshop is being hosted by the Government of Thailand which is one of the increasing number of countries claiming almost IOU per cent coverage by multidrug therapy (MDT). With assistance from the WHO, MDT was introduced in 1984. The Government has focused on the "leprosy-free province", in which rates of prevalence and of annual case-detection were smaller than 1 per10,000. Thailand appears to be well on course to eliminate leprosy as a public health problem by the year 2000.
The International Conference on the Elimination of Leprosy, held in Hanoi in July 1994, was a landmark in the history of the control of this disease. In its wide-ranging discussions, the importance of research was not neglected. In particular, health systems research-especially in building management capacity-was stressed as an important problem-solving tool at both local and national levels. In addition, the International Federation of Anti-Leprosy Associations (ILEP) emphasized the importance of operational research into gender issues, the disabled, and children, as well as into the family, which provides a "safety net" for leprosy patients.
In addition to the issues I have named, we hope that this Workshop will more broadly identify research needs and opportunities, and define global research strategies. The areas to be covered range from the most fundamental, including molecular genetics and biotechnology, to-the most applied and operational, such as techniques for increasing community involvement in support of the individual patients.
The agenda is indeed ambitious, but the goal it is designed to serve-elimination of leprosy as a public health problem-is also ambitious, but also based on a realistic assessment of the problems and the possibilities for their solution.
May I close by welcoming all of you most cordially to Thailand and to this Workshop, and wish you all success in the important discussions in which you will engage ill the course of the next few days.
WELCOME
Damrong Boonyoen, Director General, Department of Communicable Disease Control, Ministry of Public Health, Bangkok, Thailand
I wish first to express our sincere thanks to Dr. Jamroon Mikhanon, Deputy Permanent Secretary, Ministry of Public Health, for agreeing to preside over this opening ceremony of the International Workshop on Leprosy Research.
Leprosy is not a disease of modern civilization and industrialization, but has been well known for thousands of years. The first authentic description of the different types of leprosy, from India, dates back to 600 B.C., nearly 2,600 years ago. In ancient times, leprosy was thought to be an hereditary disease or a punishment by God or a supernatural power. However, after Hansen, a Norwegian physician, discovered the leprosy bacillus 123 years ago, we have learned that leprosy is merely a chronic communicable disease of man. Even though leprosy does not cause the death of its patients, it can cause severe physical, mental and social disabilities to its sufferers and their communities. Based upon the improved understanding of the disease, scientists and health workers around the world have successfully joined together to create sound strategies and effective interventions for the struggle against leprosy, so that its elimination as a public health problem by the year 2000 is no longer an impossible dream.
Thailand exemplifies the current situation. The fight against leprosy in Thailand began in 1908, when His Majesty King Chulalongkorn gave land in Chiang Mai Province to American missionaries on which to establish the first leprosarium in Thailand. Since then, leprosaria and leprosy settlements have been established throughout the country, to provide shelter and treatment to Thai leprosy patients. Subsequently, the first modern treatment for leprosy-dapsone-was made available for mass treatment programs. In 1955, when Thailand lauched its national leprosy control program, prevalence of leprosy in Thailand was estimated to be 5 per 1000; today, after 41 years of a national program of leprosy control, and after 12 years of implementing a program of multidrug therapy (MDT), the prevalence of leprosy in Thailand is estimated to be only 5 per 100,000. The decrease of prevalence has been observed not only in Thailand, but in many parts of the world in which MDT has been implemented.
Even as progress toward the elimination of leprosy is made, we need to be alert to unexpected obstacles on the way to this goal. The obstacles, whether technical or operational, will require research inputs from a variety of scientific disciplines. For this reason, the World Health Organization, together with the Sasakawa Memorial Health Foundation, decided to organize this International Workshop on Leprosy Research, which is intended to identify research needs, review research opportunities, and define research strategies both for the present and for the future. The 25 participants are leading leprologists and scientists working in different disciplines from 14 countries in Asia, the Americas, Australia and Europe, who will participate in the three-day workshop in order to contribute to the final phase of the world's continuing fight against leprosy.
May I, once again, express my deep gratitude to the participants for agreeing to assist us in this Workshop.And may I request of the Deputy Permanent Secretary that he deliver his inaugural address.
INAUGURAL ADDRESS
Jamroon Mikhanon, Deputy Permanent Secretary, Ministry of Public Health, Bangkok, Thailand
Dr. Noordeen, Dr. Yuasa, Dr. Doberstyn, Dr. Damrong, distinguished participants, ladies and gentlemen:
It is my great pleasure to participate in the opening ceremony of the International Workshop on Leprosy Research, which has been organized by the World Health Organization (WHO) and the Sasakawa Memorial Health Foundation (SMHF). On behalf of the Ministry of Public Health of the Royal Thai Government, I wish to welcome the distinguished participants to Thailand. In addition, I wish to express our sincere gratitude for your active roles in the fight against leprosy.
We have been working continuously to solve the problem of leprosy in Thailand, and have had some success. However, as is the case in other parts of the world, suffering from leprosy persists in our country. As long as leprosy has not been eradicated, there is need for more information, in order to make our fight against leprosy intelligent and, ultimately, successful. Evidence indicating the importance of research to the effort to control leprosy is the fact that, in Thailand, 36 years ago, His Majesty King Bhumipol Adulyadej inaugurated the RajPracha-Samasai Institute, which was established to conduct research in leprosy in addition to training the health personnel responsible for treating leprosy patients. We are indeed pleased that this Workshop has been organized this year, in which we celebrate the Golden Jubilee of our beloved King Bhumipol Adulyadej's accession to the throne.
On behalf of the Ministry of Public Health and the leprosy patients of Thailand, permit me once again to thank the WHO, the SMHF, you participants and our Thai colleagues for making this important Workshop possible. I hope that, in the spirit of close cooperation and the firm relationship among leprosy fighters around the world, this Workshop will contribute to a world in which no one suffers from leprosy. Finally, I wish you a pleasant stay in Thailand and a safe trip home.
I now declare the International Workshop on Leprosy Research open.
WELCOME
P.J. Brennan, Workshop Chairman, Department of Microbiology, Colorado State University, Fort Collins, Colorado, U.S.A.
This international Workshop on Leprosy Research has been convened by the World Health Organization and the Sasakawa Memorial Health Foundation to consider the outstanding research needs, as we approach the goal of "elimination of leprosy as a public health problem". We should attempt to achieve a concensus on research directed toward the targets of elimination and eventual eradication. At the same time, we must take into account more fundamental research topics, which may not be directly related to elimination and eradication, such as the genome of Mycobacterium leprae, and the immensely important area of immunology, with emphasis on their applications to tests for subclinical infection, to be used in epidemiological studies, and for studies of protective immunity.
As we approach the elimination of leprosy as a public health problem, we are faced by two related and, perhaps, inevitable phenomena: diminution of the funds available to support research in leprosy; and reduction of the size of the population of leprosy researchers. this latter phenomenon has at least two components: a "brain-drain" of established investigators away from leprosy, and into tuberculosis and other mycobacterial diseases; and the diminished recruitment into the field of new, young investigators.
This workshop may, in fact, represent our last opportunity to define our research goals-especially those important to the realization of elimination, before the "post-elimination" era is upon us.
Discussion of Dr. Brennan's statement
Prof. Britton: To whom are we addressing our questions and thoughts?
Dr Brennan: Primarily to ourselves. Those of us attending this Workshop are a very representative group. We include workersin several age groups, and from a number of disciplines. Only a few researchers in theUS are active in leprosy research today; half of them are here.
Dr: Young: In the process of integrating leprosy research into the broader area of research into tuberculosis and other mycobacterial diseases, we should not lose sight of those problems that are special to leprosy.
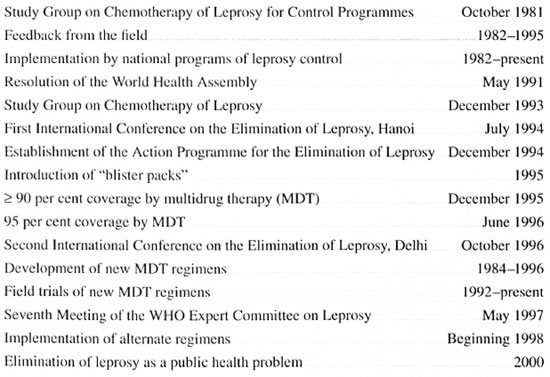
MILESTONES TOWARD THE ELIMINATION OF LEPROSY
V.K. Pannikar, Action Programme for the Elimination of Leprosy, World Health Organization, Geneva, Switzerland
The following is a listing of the milestones on the road to the elimination of leprosy as a public health problem, beginning from the World Health Organization (WHO) Study Group on Chemotherapy of Leprosy for Control Programmes.
Two field trials of new regimens are in progress:
The Ofloxacin Multicenter Trial, now being carried on in 15 treatment centers in 8 countries, is a double-blind trial in both multibacillary (MB) and paucibacillary (PB) leprosy, in which the patients are randomly allocated to regimen. Intake has been completed; 1651 MB patients and
1817 paucibacillary (PB) patients have been recruited. The trial regimens for MB leprosy are: 1) WHO/MD'Nbr 24 months; 2) WHO/MDT for 12 months; 3) WHO/ MD T for 12 months plus daily ofloxacin (OFLO) for the first 4 weeks; and 4) 600 mg rifampicin (RMP) plus 400 mg OFLO daily for 4 weeks. The regimens for PB leprosy are: 1) WHO/MDT for 6 months; and 2) 600 mg RMP plus 400 mg OFLO daily for 4 weeks.
The Single-Lesion Paucibacillary Trial, now in progress in 9 treatment centers in India, is a double-blind trial in PB patients who exhibit only one skin patch, with no nerve trunk involvement, in which the patients are randomly allocated to regimen. Intake was completed in July 1995, by which time 901 adults and 582 child patients had been recruited, and treatment was completed in February 1996. The trial regimens are: 1) 600 mg RMP plus 400 mg OFLO plus 100 mg minocycline (MINO) in a single dose; and 2) WHO/MDT for PB leprosy for 6 months.
In a new trial begun in January 1996 in MINO administered monthly for 12 or 24 Guinea, Myanmar and Senegal, MB padoses to MB patients, and for 3 or 6 doses tients and PB patients with more than one to PB patients; 1500 MB patients and 1800 lesion have been allocated to treatment by PB patients are expected to be recruited by 600 mg RMP plus 400 mg plus 100 mg the end of 1996.
DIAGNOSIS, CLASSIFICATION AND PROGNOSIS
S. Talhari, Department of Dermatology, Institute of Tropical Medicine, University of Amazonas, Manaus, Amazonas, Brazil
According to the World Health Organization (WHO) (4), the diagnosis of leprosy is based on the demonstration of at least two of the following-1) a characteristic skin lesion; 2) sensory loss; 3) thickened nerves-or on the presence of acid-fast bacilli (AFB) in smears of skin-lesions. I believe that, in some patients, the histopathologic features of the skin lesion may also be diagnostic.
Generally speaking, the diagnosis of leprosy is relatively easy. Despite negative skin smears or an inconclusive histopathological picture, in most patients with hypopigmented macules and patches, the diagnosis is confirmed by demonstration of sensory loss, associated with very few other skin diseases. Similarly, with the exception of leprosy, only rarely can AFB be demonstrated in the skin lesions of a patient with disseminated macules, patches, nodules or infiltration. Finally, in difficult cases, an experienced pathologist can often confirm the diagnosis of leprosy.
Diagnosis
The diagnosis of leprosy may occasionally be difficult, as in the following situations:
1) Children or anxious patients may not respond accurately when "characteristic" skin lesions are tested for sensory loss. If AFB cannot be demonstrated, nerves are not thickened, and histopathological examination is inconclusive, no other tool is available by which to establish the diagnosis of leprosy;
2) Hypopigmented lesions of inderminate leprosy may present normal sensation, and other examinations may yield inconclusive results;
3) Normal sensation may be observed in hypopigmented lesions or patches limited to the face. If neither AFB nor thickened nerves can be demonstrated and if the histopathologic examination is inconclusive, the diagnosis will remain uncertain;
4) Ulcers-plantar or leg-or hyperkeratotic lesions may be the only manifestations of leprosy. In such cases, the diagnosis may be difficult;
5) Complaints of localized sensory disturbance without skin lesions or enlarged nerves are relatively frequent. In many such situations, a definitive diagnosis of leprosy cannot be made;
6) Disseminated cutaneous lesions that are atypical for leprosy may be difficult to diagnose if sensory loss is doubtful, no AFB can be demonstrated, and the histopathological examination is inconclusive;
7) The finding of enlarged nerves may suggest the diagnosis of leprosy, but the diagnosis is difficult if sensation and motor function are normal in the areas served by the nerves. In such cases, it is necessary to biopsy a nerve, but it may be difficult to choose a nerve that may be safely biopsied. On occasion, only one nerve, showing many AFB, is involved. Such cases are difficult to classify.
In most of these situations, the diagnosis may be difficult, even for experienced specialists. It is necessary to remember that the results of examinations, particularly the smears and the biopsy, may be erroneous. It has been said (4) that smear microscopy is the weakest link in most leprosy control programmes. And biopsy specimens may not be well preserved, or they may not include the deeper layers of the skin, or the pathologist may not be experienced. In such situations, the paramedical worker needs the assistance of a physician, and the physician working in the field needs the help of a referral center.
Employing the diagnostic tools that are currently available, it is nearly impossible to state with certainty whether or not the patient has leprosy, and one is faced with a difficult choice-to treat for leprosy without a definitive diagnosis, or to follow the course of the patient for a number of months while withholding treatment; neither is a very satisfactory alternative.
Classification
The classification of leprosy has experienced an interesting evolution in the course of the last 150 years-from that of nodular and anesthetic of Danielsen and Boeck (1) to the five-point classification of Ridley and Jopling (3) to the paucibacillary-multibacillary dichotomy of the WHO Study Group (5). More recently, there has appeared a tendency to divide patients between one group having few or only a single lesion and a second group demonstrating many- e.g., five or more-lesions. On the other hand, it may well be that, within a few years, classification will not be important; it will be necessary only to determine whether or not the patient has leprosy.
Prognosis
Since the introduction of MDT, the prognosis of leprosy has changed dramatically. Very few patients have been reported to relapse following completion of the recommended course of MDT (6). However, the recent report of Jamet and his colleagues (2) showed that the risk of relapse may be significantly greater for patients with multibacillary leprosy who begin treatment with a BI of 4 or more.
Although relapse is rare following completion of treatment, reactions-especially type-1 reactions-remain a serious problem that may greatly affect prognosis. In this respect, the advent of MDT appears not to have altered the prognosis of leprosy.
Finally, in some leprosy control programs, too many new patients present with disability. In addition, there is very little information with respect to disability among cured patients, and insufficient emphasis is placed on disability-prevention and -treatment in too many programs. Although the prognosis is good among patients who begin treatment early in the course of their disease, prevention and treatment of disabilities is not nearly as successful as is chemotherapy, and many patients who present today with low-grade disabilities will exhibit more severe disabilities in the future.
Suggested research
1) New methods are required for the training of workers in areas of low endemicity. In such situations, workers must be trained in basic dermatology as well as in leprosy;
2) New tools are required for the diagnosis and classification of primary neuritic leprosy;
3) A promising diagnostic tool may be represented by the PCR technique, and research in this area should be encouraged;
4) Studies should be conducted of the relationship of the number of lesions to the outcome of treatment;
5) The risk of relapse among multibacillary patients who begin treatment with BI > 4 should be further studied;
6) More accurate means of distinguishing between reaction and relapse of paucibacillary leprosy are needed;
7) Additional information with respect to disability, especially in areas of low prevalence, would be helpful.
REFERENCES
1. DANIELSEN, D. C. and BOECK, C. W. Traile de la spedalskhet on elephantiasis des Grees. J.B. Bailliere, Paris, 1848.
2. JAMET, P., JI, B. and nn; MAKCNOUX CHEMOTHERAPY STUDY GROUP. Relapses alter long-term followup of multibacillary patients treated by the W.H.O. multidrug regimen. Int. J. Lepr. 63(1995)195-201.
3. RIDLEY, D . S. and JOPLING, W. H. Classification of leprosy according to immunity: a live-group system. Int. J. Lepr. 34(1966)255-273.
4. WHO . A guide to leprosy control, second edition, 1988.
5. WH O STUDY GROUP. Chemotherapy of leprosy for control programmes. Tech. Rep. Series No. 675, 1982.
6. WH O STUDY GROUP. Chemotherapy of leprosy. Tech. Rep. Series No. 847, 1994.
Discussion of Dr. Talhari's paper
Prof. Ji: Dr. Talhari mentioned research employing PCR as a diagnostic tool in his presentation. Should we encourage research in this area?
Dr. Klatser: PCR does not represent a diagnostic tool, because it adds nothing to existing diagnostic methods. Although it may be more specific, it is no more sensitive than existing methods. Perhaps it may be useful as an epidemiological tool, because of its great specificity.
Dr. Feenstra: 1 should like to support the need for improved diagnostic tools.
Dr. Waters: Can PCR be made more sensitive? Single-lesion cases are very important. Also, we have a problem distinguishing between relapse of PB leprosy and late reversal reaction. A better diagnostic tool could help us greatly.
Dr. Brennan: In the course of the coming presentations, we'll talk about methods- skin testing, serology, etc.-that may be applicable to the diagnosis of single-lesion leprosy. There is no presentation specifically of PCR. Perhaps Dr. Klatser can speak to this point?
Dr. Klatser. Theoretically, PCR can detect a single bacillus; it cannot be made more sensitive than it is-it cannot detect less than one organism.
Prof. Ji: If we had a test of maximal sensitivity, how would we apply it? The field workers are already working at a maximum. Skin smears are technically more simple than PCR, and we are unable to improve the manner in which smears are made and examined. We can't demand more from the field workers than we already do.
Prof. Cho: I have done some work with PCR in collaboration with the Leonard Wood Memorial in Cebu, which possesses excellent facilities. Of 100 MB cases, 90 were positive by smear; six of the remaining 10 demonstrated AFB in the biopsy specimen. PCR recognized only one additional patient. Perhaps in the field, where BI and biopsy are not so well performed, PCR may have somewhat more to offer.
Prof. Fine: We may be in a "catch-22" situation, with respect to research possibilities. Ethically, we feel required to treat single-lesion patients in whom we suspect leprosy; however, treatment prevents progression of the disease, which would prove the diagnosis. We may need to follow a large number of such patients without treatment in a research environment, in order to sort out this problem.
NEEDED RESEARCH IN CHEMOTHERAPY OF LEPROSY RELATED TO THE INDIVIDUAL PATIENT
R. R. Jacobson, Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A.
The goal of eliminating leprosy as a public health problem- i.e , prevalence < 1:10,000 could certainly not have been proposed if the standard World Health Organization (WHO) chemotherapy regimens (WHO/MDT) had not been successful. It is also clear that, without enhanced control efforts by, and assistance from the WHO, member governments and non-governmental organizations, effective chemotherapy would not, by itself, have been sufficient to achieve this goal. Unfortunately, even in the best programs of leprosy treatment and control, there remain patients who cannot easily be treated by WHO/MDT. Moreover, despite our best efforts, only disappointingly small reductions of incidence have been recorded, and one worries that it will not be possible to reduce the prevalence below the level of 1:10,000 in areas in which the annual incidence approaches this figure. Finally, there remains the question of what will happen when the goal of elimination has been attained. Will control efforts diminish as funding is diverted to the control of other diseases? And will we begin to move backward at that time? It is clear that, although we have experienced considerable success to date, we still have a long road to travel.
What can be done now or in the future to find answers to these questions? How might we reach all patients more quickly and consistently? As was noted in a meeting of the Steering Committee of WHO's Therapy of Mycobacterial Diseases Scientific Working Group (THEMYC) in Madras in December 1993, "Current WHO/MDT is very successful in leprosy control. However, there is a need to continue to search for new drug regimens for future application for the following reasons:
1) It would be helpful if future schemes of therapy could be made simpler than WHO/ MDT and more acceptable for application within the general health services, with the further proviso that they would depend entirely upon once-a-month supervised administration of drugs;
2) If a multibacillary (MB) patient does not accept clofazimine because of skin coloration, he has no access to an easily applicable, safe and cost-effective alternative MDT regimen;
3) A single common regimen suitable for both multibacillary (MB) and paucibacillary (PB) leprosy, but with different duration if necessary, would considerably simplify administration of therapy by the general health services;
4) A fully supervisable regimen with flexible interval between doses could be useful in certain populations or areas in which WHO/MDT may be difficult to apply.
In other words, leprosy control programs and patients could have the opportunity to choose alternative regimens, which are effective and safe, according to the needs of the program or the individual patient." How might chemotherapy enhance future efforts to control leprosy?
Drugs currently available
Dapsone, rifampicin, clofazimine and the thioamides. Dapsone (DDS), rifampicin (RMP), clofazimine (CLO) and the thioamides are the "standard" drugs. The dose of DDS has been standardized at 100 mg daily for adults, and the thioamides are rarely used because of their potential for toxicity. Recent studies (6) have again demonstrated that once-monthly, 600-mg doses of RMP appear to be as effective as the same doses administered daily, although there are those who disagree, and there is some evidence (4) that CLO could also be given once monthly.
The fluoroquinolones. Although a large number of fluoroquinolones have been developed, some, such as ciprofloxacin, are not active against Mycobacterium leprae , and, of those that are, interest has centered on ofloxacin (OFLO). Like all fluoroquinolones, OFLO acts by inhibiting the alpha sub-unit of the enzyme DNA gyrase, thereby interfering with bacterial DNA replication. Clinical trials have demonstrated that a daily dose of 400 mg is bactericidal against M. leprae , although less so than a single dose of RMP, and that 22 daily doses killed 99.99 per cent of the viable organisms. The drug is well absorbed, reaching a peak serum concentration of 2.9 pg per ml after 2 hours, and has a serum halflife of 7 hours. It is excreted mainly unchanged by the kidneys. Side effects include nausea, diarrhea, and other gastrointestinal complaints, and a variety of central nervous system complaints, including insomnia, headache, dizziness, nervousness and hallucinations. Serious problems are infrequent, and only occasionally require discontinuing the drug.
The inacrolicies. Several members of this group, including erythromycin, have been evaluated, but, at this moment, only clarithromycin (CLARI) appears promising. Although studies in the mouse foot pad have demonstrated the potent bactericidal activity of this drug, it is clearly less bactericidal than is RMP. Administered in a dosage of 500 mg daily to leprosy patients, the drug killed 99 per cent of M. leprae by 28 days, and 99.9 per cent by 58 days. CLARI is readily absorbed from the gastrointestinal tract and converted to its active metabolite, 14-OH-CLAR1. Peak serum concentration, approximately 1 µg per ml, is reached 1-4 hours after a 500-mg dose, and its serum half-life averages 6-7 hours. Tissue concentrations are higher than those in the serum. It was reported (8) that the concurrent administration of RMP decreased the serum CLARI concentration by 80 per cent, but the serum concentration of the 14-OH metabolite remained unchanged. The drug acts by linking to the 50S ribosomal sub-unit, thus inhibiting bacterial protein synthesis. As a group, macrolides are relatively non-toxic. Gastrointestinal irritation, nausea, vomiting and diarrhea are the most common symptoms, but they do not usually require discontinuation of the drug.
Minocycline. Minocycline (MINO) is the only tetracycline that demonstrates significant activity against M. leprae, perhaps because its lipophilicity permits it to penetrate the bacterial wall. The standard dose is 100 mg daily, which yields a blood level of 2-4 pg per ml, well above the apparent minimal inhibitory concentration for M. leprae of 0.2 µg per ml. The drug is bactericidal against M. leprae, but less so than is RMP. Minocycline was clinically effective when administered alone to 8 patients, but 2 months of daily treatment were required before the organisms were consistently unable to multiply in the mouse foot pad (1). Like other tetracyclines, MINO binds reversibly at the 30S unit of the ribosome, blocking the binding of aminoacyl transfer RNA to the messenger RNA-ribosomal complex, thereby inhibiting protein synthesis. The drug is well absorbed, with a serum halflife of 1 1-23 hours. Side effects include discoloration of the teeth of infants and children, occasional pigmentation of the skin and mucous membranes, various gastrointestinal complaints, and central nervous system toxicity, including dizziness and unsteadiness. A recent report (4) reviewed 34 cases of hepatitis or systemic lupus that occurred in patients treated by MINO for acne. That prolonged administration of the drug for acne continues indicates, however, that MINO is relatively non-toxic.
Other drugs. With the possible exception of fusidic acid, the other drugs that have been demonstrated to be active against M. leprae are much less potent, or merely bacteriostatic. These drugs include the combination amoxicillin with potassium clavulanate, brodimoprim, thiacetazone, and deoxyfructoserotonin. Considering the large number of much more potent drugs available, that could be included in drug regimens that might be fully active when administered for a shorter period of time than is required by WHO/MDT, there appears to be little reason to use any of these other drugs at this time.
Combinations of the newer anti-leprosy drugs. Ji and his colleagues have demonstrated (5) that single doses of the combination of MINO with CLARI, with or without OFLO, administered once monthly, together with monthly 600-mg doses of RMP, are fully active in the treatment of MB leprosy, and that these combinations, administered daily without added RMP, may represent adecpiate treatment for RMP-resistant MB leprosy. Studies by others have yielded similar results.
Improving MDT
Shortening therapy. The Study Group on Chemotherapy of Leprosy, convened in 1993, suggested (10) that WHO/MDT for PB leprosy continue to be administered for 6 months, whereas that for MB leprosy be limited to 2 years, thus eliminating the proviso that the regimen be continued for "at least 2 years, and be continued, wherever possible, up to smear negativity", as had been recommended in 1981 by the Study Group on the Chemotherapy of Leprosy for Control Programmes (9). Drugs and dosages were left unchanged.
Could WHO/MDT be substantially shortened? At present, there is no good evidence to support such an initiative. Pattyn's experience with shorter regimens suggested to him that excellent results could be obtained for MB disease in as brief a period as 34 weeks only if RMP were administered daily; treatment for shorter periods did not yield satisfactory results (6). His experience suggested to him that the treatment of PB leprosy could be shortened to from 6 days to 3 months. Unfortunately, none of his data included regimens containing drugs other than DDS, RMP, CLO and a thioamide.
On the other hand, adding OFLO, Ml NO or CLARI or some combination of these drugs to WHO/MDT, or substituting one of these drugs or some combination of them for the DDS- or CLO-component of WHO/ MDT, might permit shortening of the treatment, because of their greater bactericidal activity. However, to determine by how much treatment might be shortened would require long-term trials. The cost of the new drugs might also limit their use, although this could be minimized both by monthly administration and by shortening the course of treatment. Finally, only modest shortening of the treatment- e.g., to 3 months for PB and 12 months for MB leprosy-might be only of limited benelit to control programs.
Might treatment be markedly shortened by the administration of very intensive courses of RMP, together with one or more of the new drugs? Little information with respect to such therapy has been published. A trial, now in progress, of the combination of OFLO with RMP administered daily for one month may answer this question. If the results are disappointing, it could be argued that they might have been improved by addition to the regimen of MINO or CLARI or their combination. At the 14th International Leprosy Congress in Orlando in 1993, a one-month trial of RMP combined with MINO was reported (7) to have yielded satisfactory results in a mixed group of 20 PB and MB patients, and no relapses were reported after the first 2 years of follow-up. However, a much larger group of patients must be followed for a much longer period of time, in order to demonstrate that the relapse rate is satisfactorily low. It appears that the potential for significantly shortening the duration of WHO/MDT exists, and, ultimately, the duration of therapy for MB leprosy might be markedly shortened.
Intermittent therapy. Because in any control program some patients are relatively inaccessible, intermittent regimens are much desired, and, given the potent bactericidal drugs now available, even relatively short intermittent regimens appear possible. Ji, et al., had recommended (5) several possible regimens employing the new drugs. Perhaps with these recommendations in mind, the THFMYC subcommittee that met with a number of experts, in Madras in December 1993, developed several protocols employing combinations of the new drugs in fully supervised intermittent regimens. The first of these protocols involves the trial of a single dose of the combination of 600 mg RMP, 100 mg MINO and 400 mg OFLO in the treatment of single-lesion PB patients. The results of this treatment will be compared to those of treatment for 6 months by WHO/MDT for PB leprosy. Single-lesion PB patients constitute a significant proportion of the new cases encountered in some control programs at this time. If the patients are closely followed, the trial appears to involve little risk for them; and should the regimen prove successful, its use could markedly simplify control efforts.
The second series of regimens proposed by the THFMYC subcommittee seeks to demonstrate the efficacy and safety of fully supervised intermittent drug regimens in the treatment ofleprosy. In this trial, PB patients will receive the combination of RMP with OFLO and M1NO just described once a month for 3 or 6 months; for MB patients the drugs will be administered once monthly for 12 or 24 months. The safety of these regimens will be examined by comparing the 6- and 24-month regimens with WHO/MDT.
These regimens may be expected to be very powerful, and the possibility that they will be successful in yielding an acceptably low relapse rate is sufficient to justify these trials. Many of the single-lesion patients may be expected to self-heal, and the single dose of combined therapy might be sufficient to prevent evolution of the disease in the remaining patients. One may wonder, however, if the success of the regimens studied in the other patients would significantly assist control efforts. It would certainly be useful to shorten the treatment of difficult-to-reach patients, and efficacious, directly observed therapy (DOT) for poorly compliant patients would indeed be useful, but the impact of these regimens on control programs appears dubious, unless their duration can be even further shortened.
Other issues. A high rate of relapse after 2 years of WHO/MDT has been reported (3, 6) among MB patients who begin treatment with BI > 4. The authors of this report recommended (3) that the duration of WHO/MDT be increased to 4 years for these patients. If this finding is confirmed by other studies, would addition of one or more of the new drugs to WHO/MDT or use of new intermittent regimens permit them to be treated for only 2 years or even less? This topic requires further study; even if longer therapy were indicated for these patients, can one rely upon the skin smears, as they are performed in many control programs, to select the patients who require prolonged treatment?
Combined immunotherapy and chemotherapy. This is an area of interest, because immunotherapy by such materials as Mycobacterium W might accelerate clearance of the dead organisms. Should this in fact be the case, would it permit marked shortening of the duration of treatment?
Future prospects
A major effort is now in progress in the United States and elsewhere to develop new drugs for tuberculosis. It is likely that several more anti-leprosy drugs will result from this effort. At this moment, however, it is difficult to foresee any immediate benefit from these drugs, unless one was found to be bactericidal for "persisting" M. leprae. Given the current state of the art, ideal chemotherapy- i.e., a single dose of a drug or combination of drugs that will cure all types of leprosy-appears unlikely, but could be achieved some day. However, we are unlikely ever to eradicate leprosy by chemotherapy, even if a single-dose regimen were effective. There may exist important sources oï M. leprae in nature; and unless one could treat entire populations, new cases would continue to arise, even if the sole source of infection was human-to-human transmission.
Sensitive and very specific tests for the early diagnosis of leprosy might permit the use of single-dose therapy in a large proportion of cases, particularly if the trial of such therapy now in progress is successful; however, there is no immediate prospect for such a test. Improved single-dose or multiple-dose prophylaxis administered to family contacts might also be useful; a trial of such prophylaxis is about to be undertaken in the Federated States of Micronesia.
Summary
The chemotherapy of leprosy was rendered markedly more effective by the introduction of WHO/MDT in 1981. The prospects for further improvements, both by shortening duration and by developing fully supervisable intermittent regimens, appear good at this time. These developments should aid the efforts to attain the goal of elimination of leprosy as a public health problem. For the foreseeable future, however, the goal of eradication of leprosy will continue to elude us.
REFERENCES
1. GELBER, K. H., FUKUDA, K., BYRD, S., MURRAY, L. P., Siu, P., TSANG, M. and REA, T. M. A clinical trial of minocycline in lepromatous leprosy. Brit. Med. J. 304(1992)91-92.
2. GOUGH. A. , CHAPMAN, S., WAGSTAFF, K., EMERY, I', and ELIAS, S. Minocycline-induced autoimmune hepatitis and systemic lupus erythematosus-like syndrome. Brit. Med. J. 312(1996)169-172.
3. JAMET, P., JI, B., and THE MARCHOUX CHEMOTHERAPY STUDY GROUP. Relapse after long-term follow-up of multibacillary patients treated by WHO multi-drug regimen. Int. J. Lepr. 63(1995)195-201.
4. JAMET, P., TRAORE, I., HUSSKR, J. A. and Ji, B. Short-term trial of clofazimine in previously untreated lepromatous leprosy. Int. J. Lepr. 60(1992)542-548.
5. Ji, B., PERANl, E. G., PETINON, C. and GROSSET, J. H. Bactericidal activities of single or multiple doses of various combinations of new anti-leprosy drugs and/or rifampin against M. leprae in mice. Int. J. Lepr. 60(1992)556-561.
6. PATTYN, S. R. Search for effective short-course regimens for the treatment of leprosy. Int. J. Lepr. 61(1993)76-81.
7. ROMERO, R. C. Minocycline and rifampicin in leprosy. Abstract CH30, 14th International Leprosy Congress. Int. J. Lepr. 61 Supplement (1993) 8A.
8. WALLACE, R. J., BROWN, B. A., GRIFFITH, D. E., GIRARD, W. and TANAKA, K. Reduced serum levels of clarithromycin in patients treated with multi-drug regimens including rifampin and rifabutin for Mycobacterium avium-intracellulare infection. J. Infect. Dis. 171(1995)747-750.
9. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Tech Rep Series No. 675. WHO, Geneva, 1982.
10. WHO STUDY GROUP. Chemotherapy of leprosy. Tech Rep Series No. 847. WHO, Geneva, 1994.
Discussion of Dr. Jacobson's paper
Prof Ji: Because the two WHO/MDT regimens are so effective, these will continue to be the regimens of choice, even in the postelimination era. It would be helpful if the course of treatment could be significantly shortened. Also, it would be ideal if all of the drugs could be administered once monthly, and, because skin smears are generally so unreliable, if the same regimen could be employed for treatment of both PB and MB leprosy. For those patients who live in inaccessible areas, and who cannot attend monthly treatment, a short, intensive regimen would be desirable. Light-complexioned patients who will not take CLO need an alternative regimen. Finally, patients who do not tolerate RMP, or whose M. leprae are resistant to RMP, also need an alternative regimen. It is for these patients that regimens employing the new drugs might be most useful.
Dr. Gupte: WHO/MDT is a very robust regimen. The special situations that require new regimens do not at all represent fail ures of WHO/MDT. We must make it clear that the proposal of regimens to be studied in clinical or field trial is not a recommendation that the regimens be employed in the treatment of patients.
Dr. Jacobson: I also wish to emphasize the importance of distinguishing between proposal of a regimen for a clinical or a field trial and recommendation of that regimen for treatment of patients.
Dr. Naafs: Because as many as 40 per cent of patients may experience clinical worsening in the course of treatment, we must insure that new regimens deal adequately with reaction.
Dr. Noordeen: With respect to the use in treatment of regimens only proposed for trial, admittedly a serious problem, there is not much we can do. On the other hand, it is perhaps fortunate that > 90 per cent of leprosy patients are treated in the public sector. We should try to convince health planners and administrators not to play with the recomended regimens, but there is not much we can do about private practitioners.
Prof. Fine: With respect to the large trial among patients with single-lesion leprosy, was any thought given to withholding treatment from one group? That so many of these patients self-heal may confound the results.
Prof. Ji: It will be difficult to withhold treatment from patients with leprosy. Perhaps the answer will come from a trial, just begun, of single-dose treatment for single-lesion patients.
Dr. Noordeen: We have defined leprosy as a skin lesion with definite sensory loss. No ethical committee will sanction observation of these patients while withholding treatment.
Dr. Dockrell: As we approach the elimination goal, there may be pressure to underdiagnose single-lesion leprosy. Should this occur, treatment may be withheld from numbers of such patients.
Dr. Feenstra: As long as we express elimination in terms of prevalence, these singlelesion patients who are treated by a singledose regimen will never be added to the register.
TREATMENT OF REACTIONS AND NERVE DAMAGE
B. Naafs, Department of Dermatology and Venereology, University Hospital, Leiden, The Netherlands
Nerve damage leading to permanent disability is the major problem in the course of leprosy. Were it not for this, leprosy would be a rather innocuous skin disease, whereas, even today, it is one of the most feared diseases, often associated with serious social repercussions. Nerve damage may occur in both untreated and treated patients, and even in patients who have completed antimycobacterial therapy. When it occurs during or after treatment, it is especially frustrating to the patient and embarrassing to the physician.
Clinical aspects (l5)
In borderline (BT, BB and BL) leprosy, nerve damage usually develops during the so-called reversal reaction (RR). When this occurs, peripheral nerve trunks become swollen and tender at specific sites, and show deterioration of function, which usually progresses gradually, taking weeks or even months to become irreversible. Occasionally, however, severe damage may take place over-night. Skin involvement frequently accompanies nerve involvement, but may also precede or follow the nerve damage. Clinically, a reaction may be suspected when there is increased inflammation of pre-existing skin lesions. Hypopigmented or slightly erythematous macules may become red and swollen, and, occasionally, may even undergo ulceration. Crops of new lesions may suddenly appear. Sometimes, extensive edema of the hands or face may be present, especially in BL patients. Patients may complain of a burning, stinging sensation in their skin lesions, and complain of aches and pains in the extremities or face and loss of strength or sensory perception. However, they are usually not febrile.
In lepromatous (BL, LL and LL) leprosy, the damage may take years to develop, or may suddenly increase in severity during a reactional episode termed erythema nodosum leprosum (ENL). In contrast to RR, ENL is a generalized process involving various organ systems concurrently or separately. The patient is often ill, with fever, granulocytosis and albuminuria. The process takes its name from the painful, erythematous nodules of the skin that characterize the process. However, painful enlargement of lymph nodes, liver and spleen may also occur, as well as may episcleritis and iridocyclitis with glaucoma. Lymph-node involvement may lead to edema of the extremities, particularly the legs. This edema should not be confused with that which occurs as a result of nephrotic syndrome. In men, epididymoorchitis may occur. Nerves and joints may become swollen and tender. Periostitis, tendinitis and myositis are sometimes observed. Finally, glomerulonephritis and even peritonitis have been described.
Treatment
Treatment should be based upon an understanding of the immunopathology, and should ideally be tailored to the individual patient. The patient's progress during treatment should be carefully assessed, and treatment should be adapted to changing circumstances, immediately if necessary. Because individual treatment is not possible in the field, however, treatment guidelines and schedules must be designed.
RR. Histopathologically, the lesions show all of the characteristics of a delayed-type hypersensitivity reaction. There is an increase of immune infiltrate, with edema and a change of the composition of the cellular infiltrate, characterized by an influx of lymphocytes, mainly of the CD4 subtype-especially of the Thl-class (16, 17, 34). During the reaction, the peripheral blood lymphocytes demonstrate increased reactivity to Mycobacterium leprae antigens in a lymphocyte transformation test (3). A logical approach to treatment would be to reduce the concentration of M. leprae antigens by means of chemotherapy, while suppressing the damaging cell-mediated immune response (15,16).
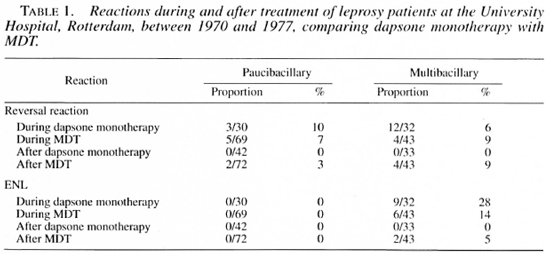
It is important to recognize that dapsone, in a daily dose of 50 mg or greater, markedly suppresses RR (2). Indeed, after the introduction of the multidrug therapy recommended by the World Health Organization in some countries, RR that occurs during treatment decreased in prevalence. However, as shown in Table 1, the prevalence of RR increased after treatment had been completed, thus demonstrating-the immunomodulatory (suppressive) effect of dapsone (l4, 21).
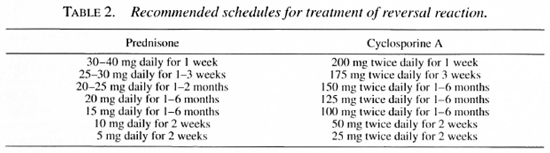
Prednisone remains the drug of choice in the treatment of RR. It reduces the edema virtually over-night (9), exerts an immunosuppressive effect, and decreases postinflammatory scar formation, of great importance for the improvement of nerve function after the reaction (15). Immunosuppressive treatment should be continued throughout the period during which the antigenic load is sufficient to trigger the cell-mediated immune response. Patients with tuberculoid leprosy may require treatment for 3-6 months, mid-borderline patients for 6-9 months, and borderline lepromatous patients for as long as 18-24 months. The dosage schedule recommended for prednisone is shown in Table 2. The initial dosage of prednisone need not exceed 40 mg (0.5-0.6 mg per kg body weight) daily (12). The rate at which the dosage should be reduced may be determined from the results of several tests, the most sensitive being the graded bristle sensory test and the voluntary muscle test (11). The crucial dosage for immunosuppression is about 20 mg (0.3 mg per kg) per day; once the initial high dosage has been reduced, the dosage should be maintained at 20 mg per day for the remainder of the periods just mentioned. After the dosage of prednisone has been reduced to 10 mg per day, it may then be reduced more rapidly, because so small a dosage has little effect on the immune response.
A number of reports support these treatment guidelines. One study demonstrated that more prolonged treatment schedules were more effective than those limited to only 3 months (12), and subsequent studies (6, 26,30, 32) have confirmed the greater effectiveness of the 6-months schedule. A more recent study has shown (29) that not only was there an effect during treatment, but that further improvement occurred after treatment had been completed, confirming the earlier results(12, 30). Two groups of workers have reported (7, 20) less favorable results, most likely because of the smaller dosages and shorter durations of their treatments (29).
More recently, two Dutch medical students were sent to assess a steroid treatment program of short duration. The responsible physician and physiotherapist on the scene had reported that 80 per cent of nerves improved during a 3-month period of treatment. The students found that, after treatment had been discontinued, more than half of the patients deteriorated over the next 6 months to levels of damage not greatly different from those that had been observed before treatment, as shown schematically in Figure 1(18), whereas improvement continuing after completion of treatment occured in only 23 per cent of the patients. In contrast to this demonstration are the reports that fewer than 10 per cent of the patients deteriorate after treatment for 6 months or longer (29, 30), and that more than 60 per cent continue to improve (12).
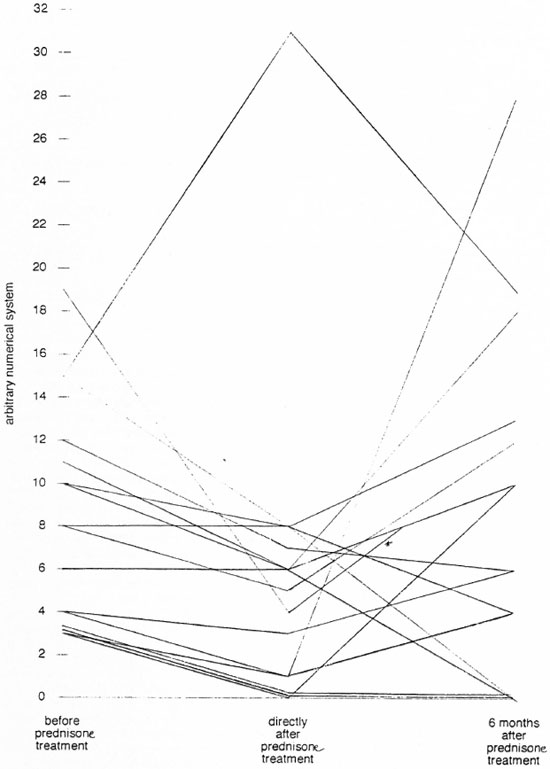
Fig 1. The course of total nerve function during reversal reaction treated by prednisone, measured in arbitrary units. The larger the value, the more damaged the nerve. Each line represents a single patient; * = one patient assessed 3 months directly after completing prednisone treatment.
Within what period after the onset of the RR should immunosuppressive treatment be begun? Ideally, treatment should begin immediately, but this is not always possible. If treatment can be begun within 3 months of onset, the results are usually satisfactory, whereas if it is begun more than 6 months after onset of the reaction, not much improvement may be expected (12, 29, 32), although it may nevertheless be worthwhile to institute treatment as long as one year after the onset of nerve damage.
When prednisone or some other immunosuppressive treatment is employed, it is most important also to treat intercurrent infections-particularly strongyloidiasis, fungal infections, osteomyelitis and tuberculosis, because these may be exacerbated by the immunosuppression. Mal perforans, if present, may also be exacerbated by this treatment.
When prednisone is employed, side-effects are frequently encountered-particularly Cushing's syndrome with weight-gain, moon facies and hump-back, steroid acne and gastritis. The more severe side-effects, such as diabetes and steroid cataract, are not frequently seen in the treatment of RR, because the drug is usually administered in relatively low dosage for a relatively short period of time. Some have questioned whether it might not be better to substitute betamethasone or dexamethasone for prednisone, because the former compounds exert a weaker mineralocorticoid action. However, this topic has not as yet been carefully investigated; considering price and side-effects, one wonders if such a trial would be worth the effort.
For those patients who do not tolerate corticosteroids well, azathioprine might be added; however, this drug acts only slowly, and could replace steroids only partially in a later phase of the treatment, because it has no effect on the intraneural edema, which is so damaging to the nerves (15). The side-effects of azathioprine-intestinal discomfort and anorexia-are usually mild, although a few patients suffer bone marrow depression. In addition, the drug is expensive.
Cyclosporine A, an even more expensive drug, is as effective as steroids in the treatment of RR (4). Theoretically, cyclosporine A is ideal, because it acts primarily to suppress the CD4-Thl helper cell. However, it is not yet certain whether this drug acts as quickly on the intraneural edema as do steroids. Treatment should begin with a dosage of 5-10 mg per kg body weight, and be reduced at the same rate as is the dosage of steroids. At a dosage < 5 mg per kg, side effects are mild; these include hypertension, which usually can be easily controlled, and a decrease of glomerular filtration, which results in an increase of the plasma creatinine concentration that is usually reversible. This drug deserves the attention of researchers in the area of RR.
When, during otherwise effective treatment of RR, one or two nerves do not improve or even sustain more damage, whereas other nerves improve, one should suspect the action of mechanical as well as of immunological mechanisms. In such situations, intraneural edema under high pressure may be present in the nerve, compressing the post-capillary venule that traverses the perineurium obliquely, giving rise to local venostatic edema (10, 15, 16), and surgery to decompress the nerve should be considered. The surgery should be performed, under cover of steroids to suppress cell-mediated immunity, no later than 2-3 months after the onset of nerve damage. The steroids will also prevent postoperatve edema and decrease postoperative adhesions and scarring. Such procedures have been shown to be effective (33). On the other hand, they have become less common as the result of more vigorous immunosuppressive treatment.
ENL (15). Although the immunopathology of ENL is not entirely understood, the following model may be useful (15, 16). Following an unknown trigger, which may be as non-specific as a viral infection, stress, or pregnancy, or more specific like tuberculosis, an influx of CD4 Th2 cells occurs against a background of the histopathological features of lepromatous leprosy. Possibly by production of IL-4, these cells may induce B-cells and plasma cells already present to produce or increase their production of antibodies; these combine in the tissues with preexisting M. leprae antigens to form immune complexes, and give rise to complement activation, evidenced by the spillover into the peripheral blood of the breakdown product, C3d (31). As a consequence of complement activation, the granulocytes, the most prominent cells in a full-blown ENL lesion, break down; the granulocytes, with their enzymes and toxic substances, are responsible for the tissue destruction (Figure 2). TNF-α, a cytokine found in increased concentrations during ENL (19, 22), and a pyrogen, may be responsible for the increase of body temperature during ENL. The nerve damage in ENL may result from an ENL lesion in the nerve, but may also result from venostatic edema, as occurs in RR. Finally, TNF-α has been shown capable of demyelinating nerve fibers in vitro.
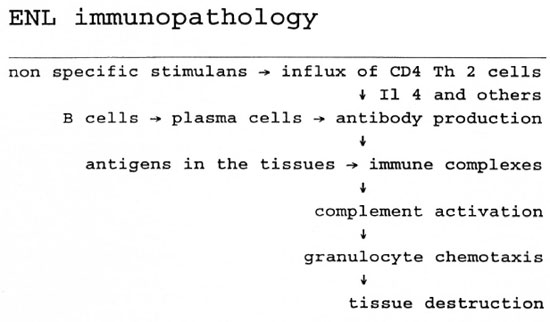
Fig. 2. A schematic presentation of the mechanism of ENL.
Because ENL is an episodic, self-limited process, many drugs have been erroneously reported to be of therapeutic value. Over the years, however, a number of drugs have been shown to be effective, not only in acute episodes, but also in chronic ENL and its complications (8, 15). Mild ENL, with only a few erythematous papules and no involvement of organs other than the skin, is usually not very damaging, and can be easily treated by mild analgesics and nonsteroid anti-inflammatory agents. When the process is a little more severe, and accompanied by fever, leukocytosis and involvement of other organs but not of nerves, eyes or testes, a few days' treatment with antimonials may be used in addition to the prostaglandin inhibitors. Antimonials may interfere with activation of complement (1, 15). Chlorpromazine, which may be helpful, has been shown to inhibit complement-mediated reactions in rabbits, and to prevent tissue injury (1). Promethazine, which inhibits the complement cascade, and interferes with mediators released from mast cells, also increased during ENL, may alleviate the symptoms (15).
When ENL involves nerves, with no obvious deterioration of function, or joints, a combination of non-steroidal anti-inflammatory agents and antimalarials-chloroquine or hydroxychloroquine-appears useful. The antimalarials stabilize the lysosomal membrane, thus preventing tissue destruction, and also inhibit complement activation by antigen-antibody complexes (1) (Table 3).

In severe ENL, with orchitis, iridocyclitis with glaucoma, or neuritis with loss of nerve function, treatment with corticosteroids or thalidomide is indicated. A dose as large as 80-100 mg prednisone may be required, but the dosage can be quickly reduced to half this amount. In ENL, prednisone acts by suppressing cell-mediated immunity, inhibiting antibody synthesis, inhibiting the release of lysosomal enzymes and production of cytokines, decreasing the response of neutrophils to chemotaxis. inhibiting both prostaglandin synthesis and the response to prostaglandins, and decreasing fluid leakage at the site of inflammation(15). Prednisone has been shown to be very effective in severe ENL. although side-effects are frequent because of the large dosages required.
Thalidomide may be the drug of choice in the treatment of severe ENL, although a few patients may not respond. Its teratogenic potential limits use of the drug, and it may cause neuropathy, which can be masked by the leprosy neuropathy. The drug is associated with other side-effects, but these usually do not require that treatment be discontinued. Its mechanism of action remains unclear (8, 13, 15). The drug is effective in adjuvant disease of rats, considered by some a model of ENL(5). In addition, thalidomide inhibits synthesis of IgM de novo, possibly important because IgM and, more specifically. IgM-rheumatoid factor may play a role in perpetuating ENL. The drug also stabilizes lysosomal membranes and inhibits granulocyte chemotaxis. Thalidomide inhibits induction of ENL by causing a significant decrease of the CD4: CD8 ratio, and a change of the mixture of cytokines produced. Recently, thalidomide has been shown to be agonistic to the synthesis of IL-2, and to be both agonistic and antagonistic to the synthesis of TNF-α (25). Treatment is initiated in a dosage of 400-600 mg (10-15 mg per kg body weight) daily, decreasing over a period of 1-2 weeks to a maintenance dose, which may be as small as 25 mg every other day. as shown in Table 4.
Colchicine, which inhibits vascular injury in experimental Arthus reactions by inhibiting chemotaxis of neutrophils, has been shown to be effective in ENL, but not to the degree claimed by the original investigators (24). Cyclosporine A has also been claimed to be effective in severe ENL; however, in our hands, it has been only modestly effective. That its action is directed more against Thl than against Th2 cells that are involved in ENL (4, 16, 34) suggests that the drug could not have been expected to be more effective. An anti-ENL effect has recently been claimed for pentoxifylline; however, this drug has demonstrated little effect in our experience. On the other hand, this drug may exert effects that are additive to those of standard ENL treatment, because it diminishes leukocyte adherence and production of TNF-α.
Clofazimine (CLO) is very important in the maintenance therapy of ENL (Table 5). After the introduction of this drug into routine chemotherapy of leprosy, as a component of WHO/MDT, the prevalence of ENL halved in some control programs (Table 1) (21). Although CLO has been shown to diminish granulocyte chemotaxis, and to stabilize lysosomes, its mechanism of action is unclear. Administered in a dosage of 100-300 mg daily during ENL, it reduces the need for steroids. Its side-effects are minimal-some gastrointestinal discomfort, skin pigmentation and, sometimes, ichthyosis.
Finally, according to some claims, immunotherapy with BCG together with M. leprae, M. vaccae, M. "W" and the ICRC bacillis reduces the severity of ENL (27). More research should be directed to these relatively cheap options.
REFERENCES
1. ASGHAR, S. S., KAMMEYER, A., DlNGEMANS, K. P., FABER, W. R., SlDDIQUI, A. H. and CORMANE, R. H. Pharmacological manipulation of the complement system (abstract). Brit. J. Dermatol. 189(1983)478-479
2. BARNETSON, R. StC, PEARSON, J. M. H. and REES, R. J. W. Evidence for prevention of borderline leprosy reactions by dapsone. Lancet (1976)1171-1172.
3. BJUNE, G., BARNETSON, R. StC., RIDLEY, D. S. and KRONVALL, G. Lymphocyte transformation test in leprosy. Correlation of the response with inflammation of the lesions. Clin. Exp. Immunol. 25(1976)85-94.
4. CHIN-A-LIEN, R. A. M.. FABER, W. R. and NAAFS, B. Cyclosporine A treatment in reversal reaction. Trop. Geogr. Med. 46(1994)123-124.
5. GOIHMAN-YAHR, M., REQUENA, M. A.. VÀLLE-CALLE-SUEGART, E. and CONVIT, J. Autoimmune diseases and thalidomide. II. Adjuvant disease. Experimental allergic encephalitis and experimental allergic neuritis of the rat. Int. J. Lepr. 42(1974)266-275.
6. KIKAN, K. U.. STANLEY. J. N. A. and PEARSON. J. M. H. The outpatient treatment of nerve damage in patients with borderline leprosy using a semistandardi/ed regimen. Lepr. Rev. 56(1985)127-134.
7. LOCKWOOD, D. N. J.. VlNAYAKUMAR, S., STANLEY, J. N. A., McADAM, K. P. W. L. and COLSTON, M. J. Clinical features and outcome of reversal (type I)reactions in Hyderabad, India. Int. J. Lepr. 61(1993)8-15.
8. MEYERSON. M. S. Erythema nodosum leprosum. Int. J. Dermatol. 35(1996)389-392.
9. Naafs, b.. Pearson, J. M. H. and Baar, a. J. M. A follow-up study of nerve lesions in leprosy dur ing and after reaction using motor nerve conduc tion velocity. Int. J. Lepr. 44(1976)188-197.
10. NAAFS, B. and VAN DROOGENBROECK, J. B. A. Decompression des névrites reactionelles dans la lepre. Justification physiopathologique et méthode objectives pour en apprécier les résultats. Med. Trop. 37(1977)771-776.
11. NAAFS, B. and TAMRU, D. Sensory testing. A sensitive method in the follow-up of nerve involvement. Int. J. Lepr. 45(1977)364-368.
12. NAAFS, B., PEARSON, J. M. H. and WHEATE. H. W. Reversal reaction: the prevention of permanent nerve damage. Comparison of short-and longterm steroid treatment. Int. J. Lepr. 47(1979)7-12.
13. NAAFS, B. and HaSPER, M. E. Thalidomide: nieuwe ontwikkelingen. Pharm. Weekblad 119(1984)1286-1192.
14. NAAFS, B., LYONS, N. E, MADOMBI, L., MATK-MERA, B. O. and lulls. B. P. B. Short-term WHOadvised multiple drug treatment of paucibacillaryleprosy patients. Indian J. Lepr. 58(1986)348-353.
15. NAAFS, B. Reaction in leprosy. In: Biology of the Mycobacteria, vol 3 (Ratledge. G., Stanford. J. L. and Grange, J.M., eds) Academic Press 359-403(1989).
16. NAAFS, B. Reactions: new knowledge. Trop. Geogr. Med. 46(1994) 80-84.
17. OTTENHOF, T. H. M. Immunology of leprosy: new developments. Trop. Geogr. Med. 46(1994)72-80.
18. OTTERS, J. B. M. and GIETELING, M. J. A followup study of 21 outpatients treated with a shortterm steroid regime. Scription, University of Rotterdam, 1995.
19. PARIDA, S. K., GRAU, G. E., ZAHEER, S. A. and MUKHERJEE, R. Serum tumor necrosis factor and interleukin in leprosy and during lepra reactions. Clin. Immunol. Immunopath. 63(1992)23-27.
20. PFALTZGRAFF, R. E. Clinical notes: the management of reactions in leprosy. Int. J. Lepr. 57(1989) 103-109.
21. POST, E., CHIN-A-LIEN, R. A. M., BOUMAN, C, NAAFS, B. and FABER, W. R. Lepra in Nederland in de periode 1970-1991. Ned. Tijdsch. Geneesk 138(1994)1960-1963.
22. SARNO, E. N., GRAU, G. E., VIERRA, L. M. M. and NERY, J. A. C. Serum levels of tumor necrosis factor-alpha and interleukine 1 beta during leprosy reactional states. Clin. Exp. Immunol. 84(1991)103-108.
23. SARNO, E. N., NERY, J. A. C, GARCIA, C. C and SAMPAIO, E. P. IS pentoxifylline a viable alternative in the treatment of ENL? Int. J. Lepr. 63(1995)570-571.
24. SAROJINI, P. A. and MSHANA, R. N. Use of colchicine in the management of ENL. Lepr. Rev. 54(1983)151-153.
25. SHANNON, E. J. and SANDOVAL, F. Thalidomide is agonistic to the synthesis of IL-2 and it can be agonistic or antagonistic to the synthesis of TNF- alpha. Int. J. Lepr. 63(1995) 654-656.
26. SRINIVASAN, H., RAO, K. S. and SHANMUGAM, N. Steroid therapy in recent "quiet nerve paralysis" in leprosy. Lepr. India 54(1982)412-419.
27. STANFORD, J. L. The history and future of vaccination and immunotherapy for leprosy. Trop. Geogr. Med. 46(1994)93-107.
28. STRIETER, R. M., REMICK, D. G., WARD, P. A., SPENGLER, R. N., LYNCH, J. P., LARRICK, J. and KUNKLL, S. L. Cellular and molecular regulation of tumor necrosis factor-alpha production by pentoxifylline. Biochem. Biophys. Res. Comm. 155(1988)1230-1236.
29. SUGUMARAN, S. T. and PALANDE, D. D. Personal communication. (1994).
30. TOUW-LANGENDUK, E. M. J., BRANDSMA, J. W. and ANDERSEN, J. G. Treatment of ulnar and median nerve function loss in borderline leprosy. Lepr. Rev. 55(1984)41-46.
31. VALENTUN, R. M., FABER, W. R., LAI-A-FAT, R. F. M., CHAN PIN JIE, J. C, DAHA, M. R. and VAN ES, L. A. Immune complexes in leprosy patients from endemic and non-endemic areas and a longitudinal study of the relationship between complement breakdown products and the clinical activity of erythema nodosum leprosum. Clin. Immunol. Immunopath. 22(1982)194-202.
32. VAN BRAKEL, W. H. and KHAWAS, I. B. Nerve function impairment in leprosy: an epidemiological and clinical study. Part 2: results of various treatment regimens. Lepr. Rev. 66(1995).
33. VAN DROOGENBROEK, J. B. A. and NAAES, B. Etude comperative d'une serie de nerfs lepreux decomprimes chirurgicalement par rapport aux nerfs contralatereaux non-operes. Med. Trop. 37(1977)771-776.
34. YAMAMURA, A., WANG, X.-H., OHMEN, J. D., UYE-MURA, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149(1992)1470-1475.
RESEARCH NEEDS RELATED TO EPIDEMIOLOGY AND CONTROL: SUBCLINICAL INFECTION
M.D. Gupte, CJIL Field Unit, Avadi, Madras, India
In his review of the immunological aspects of leprosy, Godal defined subclinical infection as follows: "In most, if not all, infectious diseases, apparently only a proportion of those who become exposed to the germ will develop the disease, while the rest will combat the infectious agent by developing effective immunity before it has time, either directly or indirectly, to cause overt disease. Such individuals are said to pass through the stage of subclinical infection" (19). The Figure, which attempts to portray this situation, is based on the simplified model suggested by Godal.
Godal also cited, as methods for identifying the subclinically infected: 1) detection of the infectious agent; 2) detection of an immune response to the agent; 3) detection of minor pathological changes in the target organ. All of these methods have been tried at one time or another in the attempt to detect subclinical infection by Mycobacterium leprae, including, specifically, the reaction to lepromin, both early and late, skin tests employing soluble antigens, lymphocyte transformation tests, sero-epidemiological studies based on the "FLA-ABS" test, radioimmunoassay, tests based on monoclonal antibodies or heat-shock proteins, nerve damage, and, most recently, PCR based upon detection of either DNA or RNA.
By means of a single test, it may not be possible to distinguish among the stages of preclinical infection, early leprosy, and subclinical infection following exposure to M. leprae. However, once valid and reproducible tests for detecting subclinical infection become available, it might then be possible to understand the nature of the disease process. Although M. leprae is one of the first organisms to be identified as a human pathogen, we still do not fully understand the process by which the organism is transmitted, nor the natural history of the infection.
One suspects that subclinical infection is far more common than the clinical disease, and this hypothesis is supported by several studies. However, our observations, based on regular surveys of a population in which leprosy is endemic, carried out every 2.5 years, indicate that the life-time risk of developing clinical disease lies between 20 and 60 per cent. It is impossible to determine whether many but not all of the inhabitants become infected by M. leprae, with a high potential for disease among those infected, or whether nearly everyone is infected, but only those who are "super"infected become ill.
We are indeed fortunate that effective antimicrobial therapy is available, so that one may undertake programs of leprosy control without being forced to await a complete understanding of the epidemiology of the disease. However, only after a test for subclinical infection becomes available can we undertake interventions such as immunoand chemoprophylaxis on a sound, scientific basis. In fact, although development of a test for subclinical infection continues to represent a challenging problem for scientists engaged in leprosy research, it appears likely that leprosy will have been eliminated as a public health problem before such a test becomes available.
Statistical and epidemiological requirements of a test for subclinical infection
The prevalence of clinical leprosy is always extremely low in the statistical sense. Even in so-called highly endemic villages, the prevalence rarely exceeds 5 per cent. And in most endemic areas, the prevalence, by definition, is greater than 1:10,000 population, but rarely exceeds 100:10,000 (1 per cent). We expect the prevalence of subclinical infection to be much greater than that of clinical leprosy, although it might be rather low, compared to other infections. Therefore, a test capable of detecting subclinical infection must be highly sensitive as well as specific and reproducible. This issue may be clarified by the three examples, described in Table 1, which demonstrate that the higher the prevalence of infection, the less the sensitivity required of the test, so long as specificity remains high. The converse is also true- i.e., the lower the prevalence, the greater must be the sensitivity.
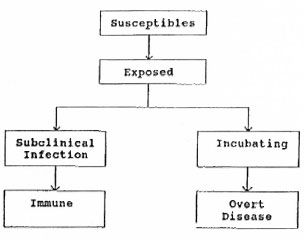
Fig. 1. Model of subclinical infection with Mycobacterium leprae, proposed by Godal (19).
Skin tests: lepromin a test of infection?
In the field of leprosy, lepromin has been employed for a variety of purposes, both clinical and epidemiological, for many years. The "Fernandez" (early) and "Mitsuda" (late) reactions were observed to be well correlated in a large set of observations on leprosy patients (43). However, dissociation of the two reactions has also been reported (33). Newell considered (30) the early reaction difficult to interpret epidemiologically, whereas the Mitsuda reaction has generally been considered a valid indicator for both clinical and epidemiological purposes.
Diagnosis of "infection ". Early and late "lepromin-positivity" has been observed in populations in which leprosy is not endemic, as well as in populations in which the disease is endemic, and in both sorts of populations, an influence of age on the reaction has been reported, exemplified by the data from Myanmar (5). Considering a 2+ Mitsuda reaction positive, almost 50 per cent of those aged 0-14 years were positive; so large an "infection prevalence" appears improbable.
An increase of the size of the lepromin reaction with age also suggests that the population has been exposed to specific or nonspecific stimuli. Evidence of this are the data suggesting that vaccination with BCG converts lepromin "negatives" to lepromin reactors, and increase of the size of the reaction after BCG is well documented (5). Beigulman suggested that the Mitsuda reaction depended mainly upon environmental factors (6). In fact, lepromin appears to act as a vaccine (40); lepromin reactivity has been observed to be present in virtually all those vaccinated with BCG, and 74 per cent of Burmese patients with scars after lepromin-testing were found subsequently to be lepromin positive (39).
Thus, lepromin appears to be neither a sensitive nor a specific indicator of M. leprae infection. The Myanmar data (5) do not support the hypothesis that leprosy is more likely to occur among those who fail to react to lepromin, nor do they support the alternative hypothesis that leprosy is more likely to occur among reactors, as might be the case if lepromin reactivity indicated infection by M. leprae.
Soluble antigens of M. leprae
In a study in South India (20), we compared the soluble antigens prepared from M. leprae grown in the armadillo by both Convit and Rees (41) with tuberculin. Almost twice as much variation between observers and from time to time by the same observer was encountered in reading the reactions to the soluble materials as was observed in interpreting the reactions to tuberculin. Moreover, significant batch-to-batch variation was noted among the preparations of Convit antigen employed. Both materials have been extensively used (41). Zuniga and Convit found in Venezuela that the Convit antigen was useful for epidemiological purposes. However, our studies, in a population in South India in which leprosy was endemic, revealed very similar distributions of the size of the reactions to both the Convit and the Rees antigens among leprosy patients, their household contacts and the general population (21). Therefore, it appears that the present generation of soluble antigens does not meet the requirements of a good test of subclinical infection by M. leprae.
Seroepidemiology
Abe and his coworkers, who developed the fluorescent leprosy antibody absorption (FLA-ABS) test for early serodiagnosis of leprosy (1), reported that both sensitivity and specificity approached 100 per cent. Proposing that this test was capable of detecting subclinical infection by M. leprae, the authors concluded from their observations that there were 200 subclinical infections for every leprosy patient (2). Subsequent work reported by Ji, et al ., (26), AmezcLia and his coworkers (3), and Bharadwaj and Katoch (7), supported the conclusions of Abe. Buchanan and his colleagues advocated (10) use of the FLA-ABS test together with an ELISA for the phenolic glycolipid, PGL-I. On the other hand, Melsom, who extensively reviewed the topic of serodiagnosis of leprosy in 1983, from a complement-fixation test developed in 1906 to solid-phase radioimmunoassay described in 1982, concluded (29) that neither the FLA-ABS test nor the radioimmunoassay met the requirements of a test that detected specific anti- M. leprae antibodies.
The FLA-ABS test and some other sérodiagnostic techniques were evaluated by an international team (24). The sera of 14 of 17 patients with paucibacillary leprosy and 127 of 180 patients with multibacillary leprosy were found to react in the FLA-ABS test, as did 23 of 135 control sera, that had been obtained from patients with tuberculosis or another disease, who were believed not to have leprosy. The team concluded that the FLA-ABS test demonstrated sensitivity of 70.6-82.4% and specificity of 83%. Although the FLA-ABS test represents a milestone in the development of a sérodiagnostic test for leprosy, it can have only limited usefulness in detecting subclinical infection with M. leprae at the community level.
Nerve antigens and antineural antibodies
Of all tissues, nerve has been thought to provide the most nearly optimal conditions for growth of M. leprae in man. Chacko argued (12) that the process in nerves is the primary disease, and that primary neuritic leprosy should be considered an evolution ary form of the disease following infection. Others have hypothesized an autoimmune mechanism to explain nerve damage (8). Thomas and Mukherjee suggested that there was cross-reactivity between components of nerve and antigens of M. leprae (36). Alternatively, they suggested a role of cytokines in tissue damage, followed by the release of nerve components into the circulation, leading to the antineural antibodies described in TT leprosy, and claimed that they could discriminate between leprosy patients and controls on the basis of the presence of antineural antibodies. Chujor, et al., who reviewed various studies of antineural antibodies, found (16) that comparisons among the studies were difficult, because of the variety of methods employed, but concluded that the results were in conflict. These workers found the antibodies to be present only infrequently in the sera of leprosy patients and their contacts, and were unable to detect a relationship between presence of the antibodies on the one hand, and the bacterial index or the presence of neuropathy on the other. Park, et al., also found (31) that the presence of antineural antibodies was only poorly correlated with disease, and hypothesized that the antibodies would be elicited in the course of leprosy, and might be proportional to the extent of nerve damage. At this time, serodiagnosis based on the demonstration of antineural antibodies appears to be only in an exploratory stage. A useful test based on this approach might help us to understand better the pathogenesis of nerve damage in leprosy.
Monoclonal antibodies (mAb)
Sengupta has reviewed the various assays that employ mAb (34). A test based on mAb ML04, which identifies an epitope on the 35 kDa antigen of M. leprae, detected almost 40 per cent of tuberculois patients, whereas about 10 per cent of healthy contacts were seropositive. MAbs directed against the 65 kDa antigen have also been produced. However, Sengupta concluded that none of the assays present available meets the requirements of serodiagnosis.
Anti-PGL-I antibodies
Since Brennan's characterization of PGL-I, a unique glycolipid that is secreted by M.leprae (9, 22, 23) various tests have been developed for detection of antibodies, principally of the IgM class, that react with native PGL-I or synthetic, analogous compounds. Ulrich and her coworkers reviewed (37) some of the studies that have employed these tests; the results are summarized in Table 2.
These data indicate some limitations of these tests. They appear, in general, to detect only a fraction of the patients with PB leprosy. Moreover, there is evidence that treatment may reduce the level of PGL-I antibodies. On the other hand, the specificity of the test is of the order of 95-97 per cent. The higher levels of antibodies in contacts than in the controls suggests that these tests may be useful for detecting subclinical M. leprae infection.
Fine and his colleagues, who studied 6,002 samples collected between 1980 and 1984 in the Karonga district of Malawi, noted (18) that antibodies to PGL-I were detected with maximal frequency in the age group 20-30 years, and were detected more frequently in women than in men. The test employed did not distinguish between contacts and non-contacts, and demonstrated only weak correlation of seropositivity with the local prevalence of leprosy. The sensitivity of their test in patients with PB leprosy was poor; depending upon the criterion of positivity, antibodies were detected only in 5 or 25 per cent of sera, with specificity of 100 or 61 per cent, respectively. These data suggested that a serological test based upon detection of anti-PGL-I antibodies could play only a very limited role in detecting subclinical infection.
Despite the lack of sensitivity of such tests, they have been employed in efforts to elucidate the epidemiology of leprosy. Cho and his coworkers, as the result of their studies in Korea and the Philippines, suggested that these tests might be useful in assessing active transmission of M. leprae (15). Krishnamurthy, et al., who carried out a commuity-based study in South India, reported (28) results similar to those of Fine and his colleagues. Soebono and Klatser, who worked in Indonesia, found that the sensitivity of an ELISA-based assay of anti-PGL-I antibodies was 98% for MB patients and 57% for PB patients, with specificity of 91% (35). Cellona, et al., who worked in Cebu, the Philippines, observed seropositivity in 6.5 and 7 per cent of contacts of MB and PB patients, respectively, and in 1.7 per cent of the general population (11). In a 10-year prospective study carried out among family contacts in French Polynesia, Chanteau, et al., found (13) a low predictive value of PGL-I serology in early leprosy. On the other hand, Ulrich and her coworkers observed (37) a strong association between the level of anti-PGL-I antibodies and the risk of leprosy. Although these investigators observed higher antibody levels among contacts and in areas in which leprosy was endemic, they concluded that the assay of anti-PGL-I antibodies did not represent a sensitive and specific test of subclinical infection with M. leprae, whereas it might be useful in monitoring trends during or after the application of some control strategy. Employing such an approach in a 5-year study in Papua New Guinea, Baumgart, et al., reported (4) that, after the introduction of MDT, there was marked reduction of seropositivity among children, and an increase of median age among those found to be seropositive.
Detection of M. leprae or its components
The presence of acid-fast bacilli (AFB) in skin smears made from clinically healthy people has been documented in at least three studies (14, 17, 27), and there will always remain questions with respect to the identity of the AFB. The relevance of this question has been sharpened by WHO's recommendation that any individual with a positive skin smear be considered a patient with leprosy (42). Tests have been developed to identify M. leprae antigens in the tissues, including tests based on the polymerase chain reaction (PCR).
PCR
PCR is an extremely sensitive and specific method by which to detect nucleic acids. Various techniques have already been developed for application to field research, particularly in the areas of diagnosis and epidemiological studies. Jamil, et al., have described several tests based upon PCR which use single pairs of primers for amplification of sequences within the genes encoding the 18 kDa, 36 kDa, and 65 kDa antigens and rRNA (25). These investigators have developed a one-tube nested PCR method for diagnosis of leprosy that employs the repetitive RLEP sequence as target, with a claimed sensitivity of 1 fg purified genomic M. leprae DNA.
In Anjouan, Comores, Pattyn and his coworkers employed two PCR techniques to study swabs of nasal secretions obtained from MB patients, PB patients, and contacts of the patients of both groups (32). Of the 23 swabs from 8 MB patients, 3 were positive; of 236 swabs from their contacts, 13 were positive. Some of the swabs obtained from contacts of treated and cured MB patients were positive. Of 11 swabs from 4 PB patients and 77 swabs from their household contacts, one sample from a contact was positive. The authors concluded that, in areas in which leprosy is endemic, most infections are "community acquired".
Beers, et al., have reported (38) an interesting epidemiological study from Indonesia, in which both serological and PCR techniques were used. These workers found no relationship between the results yielded by the two techniques. PCR was positive in 7.7% of 965 nasal swabs obtained from seronegative individuals. The authors concluded that M. leprae was widespread in the population, which included nasal carriers who demonstrated no evidence of leprosy. These observations have raised questions that are troublesome to the epidemiologist. Does the presence of M. leprae DNA in a nasal swab necessarily mean infection by M. leprae, or might these results be explained by methodological errors?
What may be expected from tests for subclinical infection?
The dynamics of infection and the natural history of several communicable diseases are well understood. In acute infections, the entire process may be observed in a very short time. But the process requires a long time to unfold in the course of a chronic infection like leprosy. It is unreaslistic, therefore, to expect a single test to be useful throughout the entire spectrum from the susceptible individual to the disabled patient who has had symptoms for many years. A major proportion of leprosy patients develop lesions that come and go without a trace; even the patients themselves may be unaware that they have had leprosy. Hence, for a study of subclinical infection, it is essential to have available a well-characterized population "laboratory"- i.e., a sufficiently large population that has been kept under surveillance by periodic examinations according to standard protocols. Facilities of this kind provide an ideal setting in which to validate the newer tests that may emerge.
On the other hand, the experience gained from several investigations indicates that the epidemiology of most health-related problems are not very complex. Therefore, the requirements for the tools needed for epidemiological studies are not very demanding. In fact, much has been learned of the epidemiology of leprosy by using clinical disease as a measure of outcome or the end-point observation. Detection of subclinical infection may require only the combination of one good skin test and one good serological test. Developing a test by which to detect subclinical M. leprae infection- e.g., the equivalent of M. tuberculosis PPD-has been identified as an important research priority by the IMMYC and THE-MYC Steering Committees. Careful clinical cohort observations combined with these good tests would help us to unravel the epidemiology of leprosy.
REFERENCES
1. ABE, M., IZUMI, S., SAITO, T. and MATHUR, S. K. Early serodiagnosis of leprosy by indirect immunofluorescence. Lepr. India 48(1976)272-276.
2. ABE, M. Fluorescent antibody absorption (FLA- ABS) test for detecting sub-clinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
3. AMEZCUA, M. E., ESCOBAR-GUTIERREZ, A., MAYEN, E. and CAZARES, J. V. Sensitivity and specificity of the FLA-ABS test for leprosy in Mexican populations. Int. J. Lepr. 55(1987)286-292.
4. BAUMGART, K. W., BRITTON, W. J., MULLINS, R. J., BASTEN, A. and BARNETSON. R. StC. Subclinical infection with Mycobacterium leprae -a problem for leprosy control strategies. Trans. Roy. Soc. Trop. Med. Hyg. 87(1993)412-415.
5. BECHELLI, L. M., GALLEGO G ARBAJOSA, P., UEMURA, K., ENGEER, V., MARTINEZ DOMINGUEZ, V., PAREDES, L., SUNDARESAN, T., KOCH, G. and MATEJKA, M. BCG vaccination of children against leprosy. Preliminary findings of the WHO controlled trial in Burma. Bull. WHO 42(1970)235-281.
6. BEIGUEMAN, B. Genetics in leprosy. Window on leprosy. Gandhi Memorial Leprosy Foundation Silver Jubilee Commemorative Volume, 1978, pp 71-81.
7. BHARADWAJ, V. P. and KATOCH, K. Detection of subclinical infection in leprosy. An 8 years followup study. Indian J. Lepr. 61(1989)495-502.
8. BODDINCJIUS, J. Mechanisms of" nerve damage in leprosy. In: "Immunological aspects of leprosy, tuberculosis and leishmaniasis". (Humber, D.P., ed). Oxford:Excerpta Medica, 1981, pp 64-73.
9. BRENNAN, P. J. and BARROW, W. W. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int. J. Lepr. 48(1980)382-387.
10. BUCHANAN, T. M., YOUNG, D. B., MILLER, R. A. and KHANOLKAR, S. R. Serodiagnosis of infection with Mycobacterium leprae. Int. J. Lepr. 51(1983)524-530.
11. CELLONA, R. V., WALSH, G. P., FAJARDO, T. T., AHALOS, R. M., DELA CRUZ, E. C, GUIDO-VILLA-HERMOSA, F.-B., STEENBERGEN, G. J. and DOUGLAS J. T. Cross-sectional assessment of ELISA reactivity in leprosy patients, contacts and normal population using the semi-synthetic antigen natural disaceharide octyl bovine serum allbumin (ND-o-BSA) in Cebu, the Philippines. Int. J. Lepr. 61(1993)192-198.
12. CHACKO, C. J. G. Pathology of leprosy. In: "Leprosy: etiobiology of manifestations, treatment and control". (Chatterjee, B.R., ed). 1993, pp 264-269.
13. CHANTLAU, S., GLAZIOU, P., PLICHART, C, LUQUIAUD, P., PLICHART, R., FAUCHER, J. F. and CARTEL, J. L. Low predictive value of PGL-I serology for the early diagnosis of leprosy in family contacts: results of a 10-year prospective study in French Polynesia. Int. J. Lepr. 61(1993)533-541.
14. CHATTERJEE, B. R. IS early diagnosis of leprosy in an Indian village not possible? Lepr. India 45(1972)225.
15. CHO, S.-N., KIM, S. H., CELLONA, R. V., CHAN, G. P., FAJARDO, T. T., WALSH, G. P. and KIM, J. D. Prevalence of IgM antibodies to phenolic glycolipid-I among household contacts and controls in Korea and the Philippines. Lepr. Rev. 63(1992)12-20.
16. CHUJOR, C. S. N., REGELE, H., BERNHEIMER, H., MEEKER, H. C, LEVIS, W. R. and SCHWERER, B. Serum antibodies against peripheral nervous system antigens in leprosy. Int. J. Lepr. 59(1991)590-597.
17. FIGUEREDO, N. and DESAI, S. D. Positive bacillary findings in the skin of contacts of leprosy patients. Indian J. Med. Sci. 3(1949)253-265.
18. FINE, P. E. M., PONNIGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. C Seroepidemiological studies of leprosy in Northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
19. GODAL, T. Immunological aspects of leprosy- present status. Prog. Allergy 25(1978)211-242.
20. GUPTE, M. D. and ANANTHARAMAN, D. S. Use of soluble antigens in leprosy epidemiology. Lepr. Rev. 59(1988)329-335.
21. GUPTE, M. D.. ANANTHARAMAM, D. S., NAGARAJU, B., KANNAN, S. and VALLISHAYEE, R. S. Experiences with Mycobacterium leprae soluble antigens in a leprosy endemic population. Lepr. Rev. 61(1990)132-144.
22. HUNTER, S. W. and BRENNAN, P. J. A novel phenolic glycolipid of Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
23. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 258(1982)15072-15078.
24. INTERNATIONAL COOPERATIVE TEAM FOR EVALUATING SEROLOGICAL TESTS IN LEPROSY. A trial to compare sérodiagnostic tests for leprosy. Sasakawa Memorial Health Foundation, 1988.
25. JAMIL, S., WILSON, S. M., HACKET, M., HUSSAIN, R. and STOKER, N. G. A colorimetric PCR method for the detection of M. leprae in skin biopsies from leprosy patients. Int. J. Lepr. 62(1994)512-520.
26. Ji, B., TANG, Q., LI, Y, CHEN, J., ZHANG, J., DONG, L., WANG, C, MA, J. and YE, D. The sensitivity and specificity of fluorescent leprosy antibody absorption (FLA-ABS) test for delecting subclinical infection with Mycobacterium leprae. Lepr. Rev. 55(1984)327-335.
27. KHANOLKAR, V. R. Studies in the histology of early lesions in leprosy. ICMR Special Rep Ser 19(1951).
28. KRISHNAMURTHY, P., RAO, P. S., REDDY, B. N., SUHRAMANIAN, M., DHANDAYUDAPANI, S., BHATIA, V. N., NEELAN, P. N. and DUTTA, A. Seroepidemiological study in leprosy in highly endemic population of South India, based on an ELISA using synthetic PGL-I. Int. J. Lepr. 59( 1991 )426-431.
29. MELSOM, R. Serodiagnosis of leprosy. The past, the present and some prospects for the future. Int. J. Lepr. 51(1983)235-252.
30. NEWELL, K. W. An epidemiologist's view of leprosy. Bull. WHO 34(1966)827-857.
31. PARK, J. Y , CHO, S.-N., YOUN, J. K., KIM, D. I., CELLONA, R. V., FAJARIX), T. T., WALSH, G. P. and KIM, J. D. Detection of antibodies to human nerve antigens in sera from leprosy patients by ELISA. Clin. Exp. Immunol. 87(1992)368-372.
32. PATTYN, S. R., URSI, D.. IEVEN, M., GRILLONE, S. and RAES, V. Detection of Mycobacterium leprae by polymerase chain reaction in nasal swabs of leprosy patients and their contacts. Int. J. Lepr. 61(1993)389-393.
33. RAMU, G. Lepromin studies in India. Paper presented at the Joint IMMLEP-Indian Scientists meeting, SEARO, WHO, New Delhi, 14-16 February 1983.
34. SENGUPTA, U. Mycobacterium leprae antigens and their utility in serodiagnosis of leprosy. Trop. Med. Parasitol. 40(1990)361-362.
35. SOEBONO, H. and KLATSER, P. R. A seroepidemiological study of leprosy in high and low endemic Indonesian villages. Int. J. Lepr. 59( 1991 )416-525.
36. THOMAS, B. and MUKHERJl, R. AntineuraJ antibodies in sera of leprosy patients. Clin. Immunol. Immunopathol. 57(1990)420-429.
37. ULRICH, M., SMITH, P. G., S AMPSON, C, ZUNIGA, M., C ENTENO, M., GARCIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to na tive phenolic glycolipid-I in contacts of leprosy patients in Venezuela: epidemiological observa tions and a prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-415.
38. VAN BEERS, S. M., IZUMI, S., MADJID, B., MAEDA, Y., DAY, R. and KLATSER, P.R. An epidemiological study of leprosy infection by serology and polymerase chain reaction. Int. J. Lepr. 62(1994)1-9.
39. WALTER, J., TAMONDONG, C. T., GALLEGO GARBA-JOSA, P., BECHELLI, L. M., SANSARRICQ, H., KYAW LWIN and GYI, M.M. Note on some observations about the post-lepromin scar. Lepr. Rev. 48(1977)169-174.
40. WHO/TDR/IMMLEP/SC/Test/81.1. Testing of purified armadillo-derived M. leprae in man. Document finalized by the IMMLEP Steering Committee at its meeting 10-12 June 1981.
41. WHOATDR/IMMLEP/EPD/85.3:7-8. Vaccination trials against leprosy. A meeting of the Epidemiology Sub-Group of the Scientific Working Group on the Immunology of Leprosy, Geneva, 11-13 February 1985.
42. WHO/LEP/95.1 A guide to eliminating leprosy as a public health problem. Action programme for the elimination of leprosy. 15( 1995).
43. YANAGISAWA, K., A SAMI, N., MAEDA, M., et al. Studies on the lepromin test. (The second report). La Lepro 24(1955)34-45.
Discussion of Dr. Gupte's paper
Dr. Pannikar. How should one deal with an individual whose test for subclinical M. leprae infection is positive. And, should he demand to be rendered test-negative, what does one do?
Dr. Gupte: Considering the putative tests of infection currently available, none is sufficiently reliable to require action in the case that you describe.
Dr. Naafs: In the West, one can plan to observe the patient carefully, without active intervention.
Dr. Feenstra: Could one think of two tests-one that detects (early) disease that requires treatment, and another that identifies only (past) infection.
Dr. Pannikar. Assuming that we have a good test that identifies infection, what do we tell the individual?
Dr. Gupte: In India, in the case of a singlelesion patient in whose lesion sensory loss is not definite, the patient is not considered a patient with leprosy, but is kept under observation. With respect to the individual who is test-positive, but who has no lesion, one might wish to keep him under observation. It may not be appropriate to treat any patient who is simply test-positive.
Prof. Britton: Just as one did not consider as a patient with tuberculosis every individual with a positive PPD, one should not consider as a leprosy patient every one who reacts in a test for subclinical M. leprae infection.
On the subject of tests for subclinical infection, we know that leprosy patients exhibit a variety of immune responses to M. leprae antigens. If we have a combination of tests that detect different immune responses, the cumulative total of infected individuals will be greater. This strengthens the case for having both a skin test and a serological test. If one could analyse the combined results in different age-cohorts, one might gain information with respect to changes of infection with time.
Dr. Waters: We don't know enough about the outcome among individuals who react to this or that test to be able to recommend treatment of asymptomatic reactors.
Dr. Brennan: We don't yet have a good skin test or a good serological test; both of these are required.
Prof. Fine: I'm surprised by Dr. Pannikar's question, which implies that a test for subclinical infection might cause problems. In Malawi, where we've been using SDA and MLSA, it appears that reactors are less likely to develop leprosy than are non-reactors, perhaps because prior infection with M. leprae has been successfully dealt with, or because of prior exposure to other Mycobacteria. Certainly, our work has suggested that these antigens are not specific, and a reaction to them may well reflect prior BCG vaccination or exposure to environmental Mycobacteria. We should not now discourage work on tests for subclinical infection.
I'm not certain that we are ethically compelled to reveal to the individual that he harbors anti-PGL-I antibodies; and if we are, we can also point out that the test is new, and we don't know how to interpret the results. We are certainly a long way from a test that is a very strong predictor of leprosy; only when we have a good test for subclinical infection need we deal with the question of what to do with healthy reactors. It may be that we will feel obligated to treat such individuals in certain contexts. But in no case should we permit such concerns to inhibit continuing work to develop a test.
Prof. Lechaf. It is not sufficient to know the specificity and sensitivity of a test; it is also important to know how to respond to the results of the test. It will be necessary to study cohorts of individuals, in order to learn their outcomes with respect to the development of leprosy, so that we can plan what to do as a consequence of the test results.
Dr. Smith: In addition to encouraging development of a test, we must decide what to do with it. How will it be used? And how useful will it be? Development of a test for subclinical infection will be carried out at the expense of some other research, and we will wish to be certain that we have calculated our priorities correctly.
Prof. Britton: A test of subclinical infection, particularly in an area in which there have been dramatic changes of prevalence, could be useful to planners, who must determine the level of services to be provided in the future.
PREDICTING TRENDS
M.F. Lechat, Department of Epidemiology, Catholic University of Louvain, Brussels, Belgium
Prediction needs purpose. What to predict? And what to do with the prediction? In the broad context of the leprosy elimination program, three indicators should be considered: prevalence, incidence, and case-detection rate.
Prevalence
The prevalence rate has been selected as the target of the leprosy elimination program. It is defined as point prevalence-the number of cases registered for chemotherapy at a given moment (end of the year), divided by the population in which the cases have occurred. It is to be reduced to less than 1 per 10,000 by the year 2000. The handling of prevalence rates raises a number of problems, such as case-definition and choice of the denominator. These problems will not be reviewed here.
Why has prevalence, and not incidence, been recommended to monitor elimination? Because the ultimate objective of leprosy control is interruption of transmission of the disease, incidence, which measures the occurrence of secondary cases, is ideally the appropriate epidemiological indicator, as stated in 1983 by the WHO Working Group on Epidemiology of Leprosy in Relation to Control (5). The reason for selecting prevalence rather than incidence is that incidence has many drawbacks. It is, so to speak, a "downstream" indicator, a window on the past. Although incidence provides direct evidence regarding transmission, this is post-facto evidence, because of the long latency of leprosy. If the rates are declining, they signal a happy ending to a success story played long before-hand. If rates fail to decline, it is too late to change course.
By contrast, prevalence is a draft on the future, a warrant. The basic argument justifying the use of prevalence to measure progress toward elimination runs as follows: because leprosy patients are assumed to be the sole source of infection, and because multidrug therapy (MDT) has proved hightly effective in rendering patients noninfective, treatment of the reservoir of patients should gradually reduce and ultimately stop transmission. Prevalence is thus an upstream indicator. It measures the potential for infection, and therefore, provided the assumptions are correct, it is a proxy for prediction of the later occurrence of new cases. It should again be emphasized that prevalence does not in any way constitute an evaluator of current strategy. It is strictly an indicator for monitoring the implementation of a strategy, which is assumed to be correct. Only incidence may, in the long term, verify that the assumptions underlying the strategy are correct*. Worldwide, the prevalence of leprosy has declined drastically since MDT was launched in the early 1980s. According to WHO's statistics, the number of estimated cases has been reduced from 10-12 million in 1985 to 1.8 million in 1995, a reduction of more than 80 per cent. The number of registered cases has been reduced by more than 75 per cent-from 5.4 million to 1.3 million cases. The accumulated number of patients who have been cured by MDT since 1985 is nearly 6.7 million, and an additional one million patients are currently undergoing treatment with MDT (4).
It is tempting to extrapolate these observations, using appropriate denominators, to predict future trends of prevalence, in order to answer the pivotal questions: will the elimination target be attained? and when? However, such an extrapolation would represent an oversimplification, for a number of reasons.
The decline of prevalence observed following initiation of MDT resulted from cleaning of the leprosy registers. Inactive patients who had been kept under surveillance were removed from the registers, and patients who had been bound for life to dapsone monotherapy were treated with MDT and released. These are both administrative procedures that do not reflect changes of the capacity for transmission. They are artefacts, and call for readjustment of the prevalence trends. The contribution of this procedure to the initial decline of prevalence cannot be measured, because the necessary records appear not to have been maintained.
At the same time, a decline of prevalence has occurred with shortening of treatment; from 6 months to 2 years, or to, at most, 3 years of MDT have been substituted for prolonged, and even life-long dapsone monotherapy. One might expect a further sudden, step-wise decline of registered prevalence, should the use of new drugs permit further shortening of treatment in the future.
Could prevalence lend itself to extrapolation if adjustments could be made for the declines that have resulted from culling inactive patients from the registers and from shortening the duration of treatment? Prevalence is a composite indicator, reflecting the epidemiological dynamics of the disease (past incidence), operational performance of the program (case-finding), duration of treatment, cure-rates in previous years, death rates and the proportion of those under treatment who default. To simplify the matter, the changes of prevalence that occur from year to year depend upon the input-the number of newly detected cases, and the output-the number of cases cured or dying. Assuming that all of the patients were known at the beginning of a treatment cycle-2 years for multibacillary cases, the prevalence should shrink to the number of new cases detected during the cycle by the end of the cycle.
The output component between successive point prevalences can be simulated. Disregarding deaths and defaulters, it depends only upon the performance of the control program. The input component (new cases detected) depends not only upon the efficiency of case-finding, but also upon the size of the pool of patients awaiting detection (the hidden prevalence), either cases of recent onset, or unregistered cases of long standing, which, for whatever reason, have escaped detection (backlog, or residual prevalence). Thus, future prevalence trends depend largely upon future detection rates.
Case-detection
In 1995, WHO reported (7) that there were 1.3 million registered patients and 1.8 million estimated patients with leprosy in the world- i.e., that there were an additional 500,000 patients who were awaiting detection. The numbers of cases newly detected in 1993 and 1994 were, respectively, 591,000 and 561,000. The cases to be detected in future years will consist of: 1) the pool of patients still unregistered (hidden prevalence); 2) secondary cases infected before MDT had been implemented; and 3) secondary cases who were infected after MDT had been implemented, by patients who had not yet been treated- i.e., they were infected by the patients comprising the hidden prevalence. What trends might be anticipated in the detection of the patients of these three categories?
Hidden Prevalence. In many countries, case-detection rates are much higher than the expected incidence. One may wonder, therefore, if, in these countries, there is not a large reservoir of cases, who were not detected before MDT was implemented. The most comfortable explanation of this phenomenon is that geographical coverage by MDT is not yet complete. The problem is then reduced to the consistency between the numerator and the denominator from which the rates are calculated- i.e., the problem is one of definition of the population, and a disproportionately large fraction of the new cases would come from the areas in which MDT had been implemented most recently.
In countries in which geographical coverage by MDT is complete, the explanation of an unexpectedly high case-detection rate might be that the MDT itself has been so well accepted by the patients and the community that it has stimulated detection of patients who otherwise would have remained unregistered. If this were the case, one may wonder how it came about that, in some countries, incidence decreased, sometimes in a drastic way, before MDT was implemented, if so many patients were hiding.
Provided case-finding activities are sustained and reinforced wherever necessary, the existing backlog of old, undetected cases should be progressively reduced. It is widely accepted that MDT, with its supportive environment of training, patient-education and community participation, stimulates the detection of cases that had previously been ignored. The elimination program is also thought to promote the early diagnosis of cases of recent onset. The rates at which the hidden prevalence cases are detected will thus depend upon how effective case-finding is, and how large is the size of the reservoir of undetected cases. The size of this reservoir is an important parameter to consider. If it is very large, the number of newly detected patients may exceed the number of patients cured, with the paradoxical effect of an apparent increase of prevalence. The larger the reservoir of undetected cases, the less reliable the prediction.
There are no established procedures by which to estimate the hidden prevalence. WHO has proposed several ad hoc methods, which have the advantage that they do not pretend to be anything other than guesses, while affording apparently reasonable working approximations (6). In 1995. the ratio of the estimated to the registered number of patients in 19 major endemic countries ranged from 1.1 to 9.1 (The Table). It is obvious that extreme values such as the latter might twist forecasts and deceive expectations.
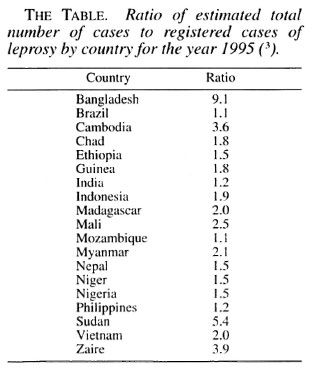
Accurate predictions of trends, be it casedetection or prevalence, are not feasible as long as the size of the hidden prevalence remains unknown. A way to circumvent this difficulty is by simulating various estimates of the backlog, which could then be tested, applying case-detection rates consistent with the local context, in order to calculate the resulting prevalence trends and see how they fit the target of elimination.
Secondary Cases Infected Prior to Implementation of MDT. The latency period of clinical leprosy is long, extending over years. Therefore, new cases will continue to appear even in a paradigmatic population in which all leprosy patients have been registered and treated with MDT. The distribution of the latency period is not known; there is good evidence that, for most patients, the time between infection and appearance of clinical disease lies in the range 2-5 years, and does not frequently exceed 10 years. Therefore, the number of new cases who had been infected before implementation of MDT-the pre-MDT incidence component of the hidden prevalence-should diminish steadily.
Incidence from Hidden Prevalence. As long as coverage by MDT is incomplete, and unregistered, infective patients are present in the community, new infections will occur, which will later develop into new cases. This so-to-speak self-supporting incidence will decrease as MDT coverage is expanded, case-detection is intensified, and the hidden prevalence is reduced.
In recent years, in most of the highprevalence countries monitored by WHO, case-detection rates have not only not decreased; they have even increased in Bangladesh. Brazil, India, Indonesia, Mozambique, Myanmar, Nepal and Vietnam. This, however, is no reason for panic. These trends result from a combination of the factors already described. High case-detection rates do not indicate an increase of incidence, but rather improvement of case-finding. It is also possible that case-detection rates have been inflated as the result of a change of the diagnostic criteria of leprosy. As a result of stimulation of case-detection by the elimination program, the diagnosis might become less specific, with skin lesions of unclear etiology being labeled as leprosy more often than had been the case before MDT; although this phenomenon is not known to have occurred, it should be kept in mind.
Will high case-detection rates persist in the future? Is it possible to make predictions? It has been asserted (1) that, by the year 2000, as many as 400,000-500,000 new cases will be detected annually. The basis of this assertion is not clear. Should it prove correct, the number of newly detected cases would approach 3 million by the end of the decade, twice the number of registered patients today. These figures give rise to the uncomfortable notion that a prevalence of 1 per 10,000 is an unrealistic target, and that the elimination program simply produces new cases as rapidly as it cures old patients. A more sober view is warranted. As mentioned, future case-detection trends depend upon a number of factors-backlog prevalence, incidence before implementation of MDT, distribution of the latency period, the time elapsed since MDT was implemented, the pace at which MDT coverage expanded, and the efficiency of case-finding activities. In addition, the ratio of multbacillary to paucibacillary cases should be taken into account. Some of these factors are unknown, others cannot be measured, and for still others, estimates are highly unreliable.
Under these conditions, it is not possible to make valid predictions, and no predictions should be made in terms of numbers. What is certain is that, provided our assumptions with respect to the natural history of leprosy are correct, MDT continues to be effectove, and that as long as case-detection activities remain intense, detection rates must decline. We do not know precisely the mechanism of the decline, but we know how to bring it about-this is the only valid prediction that can be made.
Incidence
Incidence data are difficult to collect, being included in case-detection rates, and are most generally unreliable, at least on a large scale. Because the ultimate goal of the elimination program is the decline to zero incidence, a goal that has already been achieved in some countries or areas of the world, it would be interesting to predict the trends.
Twenty-five years ago, an epidemiometric model was built from data collected in South India for the purpose of predicting incidence of leprosy as a function of prevalence (2). A set of equations was derived from actual observations recorded over a 7 year period or estimated by a statistical approach. Is it possible to construct a similar model for the elimination program?
I fear not. The South Indian model assumed, supported by rather strong evidence, that the registered prevalence was the total prevalence, and that case-detection corresponded to incidence, with only a minimal delay. The country data recorded in the global elimination program do not justify such an assumption. In addition, the South Indian model made provision for different categories of patients, according to type of leprosy and degree of compliance with treatment. These data are not included among the indicators recommended by WHO, and are generally not available.
In the South Indian model, the mathematical relation between prevalence and incidence was, as mentioned, derived from local observations. However, there is much more to the transmission of leprosy than a donor-the infective patient-and a recipient-the infected contact. Demographic characteristics, genetic susceptibility, socioeconomic conditions, life-style, immunological status, including BCG vaccination-all are factors that can modulate the dynamics of the disease. What is valid in South India may not be valid elsewhere. As a consequence, the parameters employed in the South Indian model cannot be simply transposed to other ares, each of which has its own epidemiological context (3).
Conclusions
Accurate predictions of trends, be they of prevalence, case-detection or incidence rates, do not appear to be feasible, as long as the size of the pool of undetected patients remains unknown. Simulations on the basis of country or area could, however, be performed, assuming different values of hidden prevalence, and a range of case-detection rates. Such relatively simple simulations represent a priority, and will be helpful in adjusting the intensity of case-detection activities. In the meantime, where case-detection rates have been increasing, one should be on the watch for signs of their decline. Unexpected trends could provide valuable insight into the local epidemiological situation, calling attention to undetected foci, or calling for new hypotheses to account for persistence of transmission. It will become essential to monitor incidence rates as the backlog prevalence diminishes and registered prevalence reaches the elimination target. At this stage, prevalence should stabilize at the level of incidence.
REFERENCES
1. FEENSTRA, P. Interpretation of the elimination goal. Need for a realistic approach. ILA Forum 1(1994)1-5.
2. LECHAT, M. F, MISSON, J. Y., VELLUT, C. M., MIS-SON, C. B. and BOUCKAERT, A. Un modele epidemiometrique de la lepre. Bull. WHO 51(1974)361-373.
3. LECHAT, M. F. Epidemiometric modeling based on Indian data. Lepr. Rev. 63 (Suppl.) (1992)319-191.
4. N OORDEEN, S. K. Eliminating leprosy as a public health problem: why the optimism is justified. Int. J. Lepr. 63(1995)559-566.
5. WHO STUDY GROUP. Epidemiology of leprosy in relation to control. Tech Rep Series No. 716.-Geneva, 1985.
6. WHO. Global strategy for the elimination of leprosy as a public health problem. WHO publication WHO/CTD/LEP/94.2, Geneva, 1994.
7. WHO. Weekly Epidemiological Record 25(1995)177-182.
Discussion of Prof. Lechat's paper
Dr. Smith: You said that prevalence provides a useful measure of how well we are doing with chemotherapy, but, apart from this, prevalence rates don't have much value for what we are attempting to do. For us, the key measures are incidence and disability. The problem with incidence is that we are dependent upon case-detection as a proxy for incidence; yet, we know that minor changes of program organization can have major effects on case-detection. You have considered the global situation. If, however, we consider the situation country by country, we see that there are two groups of countries. One group of countries is characterized by declining case-detection rates over a long time; I believe that these declines, which, in many cases, began as early as the 1950s, are genuine. However, there is another group of countries, in which leprosy is endemic, and in which case detection rates are not declining and may even be increasing. That case-detection rates remain high and even increase represents a summation of the effects of two phenomena: 1) the discovery of "backlog" patients, as control efforts are extended into areas not previously covered; and 2) more intensive case-finding activity on the part of leprosy workers, who don't have as much to do as in the past. It will be most important to monitor very long-term trends by sentinel systems in some of the countries comprising this latter group, employing standardized measures of incidence.
Prof. Lechat: I agree entirely. However, if the incidence, and, even more so, the prevalence decreased in a number of countries before implementation of MDT, where was the hidden prevalence? The problem appears to have been solved by dapsone monotherapy, as if there had been no backlog. This appears paradoxical.
Dr. Gupte: You referred to Dr. Desikan's paper, presented at a Workshop conducted by the Indian Association of Leprologists, and a paper that I published subsequently. In district after district in India, we saw an unchanging new-case detection rate over 7-8 years. In addition, a number of sample surveys revealed a prevalence 4-5 times that recorded. As many as 60-70 per cent of the cases detected in sample surveys are single-lesion patients. Finally, if one conducts an intensive campaign of case-finding, after which efforts are diminished, one does not encounter a resurgence of leprosy. Most of the important cases-smear-positive and disabled patients-are detected in the course of the intensive campaign, and the cases that are detected thereafter are generally of the more inconsequential type. In other words, the situation does not return to that which existed prior to the intensive campaign.
In one district, in which we were examining methods for rapid assessment of the situation with respect to leprosy, we found that 7-8 per cent of the patients, who were not known to the leprosy control program, had grade 2 disability. Although the disabilities were not pronounced, this was a worrying finding. Should we expect resurgence of cases of consquence, once intensive campaigns have been completed, not perhaps on the scale of what has occurred in malaria, but nevertheless important? I am not now discussing the hidden prevalence-that backlog of patients who preexisted MDT; however, I wonder if we may generate a new hidden prevalence-that involving patients whose disease develops after the campaign. This is a question to be addressed by research.
Dr. Noordeen: With respect to hidden prevalence and case detection, we may be facing another problem. As Dr. Gupte mentioned, the lifetime risk of leprosy in some areas of high endemicity approaches 60 per cent in some areas. Thus, if an active program of case-finding is undertaken, a large pool of individuals will be encountered who demonstrate evidence of healed leprosy- either self-healed or healed as the result of treatment unknown to the control service. The leprosy worker should be able to distinguish between healed and active leprosy; but if he is correct only 80 per cent of the time, a large number of "false-positives" will result, which will greatly inflate the case-detection rate. The likelihood that this will occur is directly proportional to the vigor with which the case-finding activities are carried out.
Prof. Britton: With respect to a sentinel system, what are the requirements? Will the system be dependent upon the routine health services for information? Or do you envision a separate unit responsible for collecting data and determining prevalence and incidence rates in a given community?
Prof. Lechat: The sentinel or surveillance system must be based on the population of a defined area; one cannot maintain surveillance of an entire country. In addition, it will be necessary to discriminate between old and new cases of leprosy. Thus, the requirements are that the system be population-based, with complete coverage of the population, and a standard definition of a case of leprosy. Many cancer registries are built in just this way, as are sentinel systems for many other diseases. When the the source of information is notification by the physicians in the community, the results are often disappointing; ascertainment of cases must be carried out actively.
PRIMARY PREVENTION OF LEPROSY
P.E.M. Fine, London School of Hygiene and Tropical Medicine, London, U.K.
Primary prevention inplies interventions to reduce disease incidence. In the context of leprosy, one might consider two basic ways to achieve this: by prevention of infection with Mycobacterium leprae; or by prevention of disease, once infection has occurred. However, given that we are unable to identify those infected with M. leprae, and given that we are not certain of the mechanism of action of some of our interventions, this perspective is not entirely satisfactory. Thus, I will consider here three approaches to primary prevention: reduction of infection (transmission of M. leprae ), chemoprophylaxis, and immunoprophylaxis.
Prevention of Infection
It is generally believed that the most important sources of leprosy bacilli in endemic areas are patients with multibacillary (MB) leprosy. There is evidence that several animal species may be infected naturally, and that, in particular, the nine-banded armadillo may represent a non-human source in the southern United States. Whereas these animal sources may be responsible for a few human cases, they do not appear to contribute importantly to the incidence of the disease on a global scale. There have also been repeated suggestions that M. leprae may exist in an environmental reservoir in soil or moss (11), but these claims have not been confirmed, and are not widely accepted. The inability to culture M. leprae makes studies of non-human, and especially of environmental, sources very difficult. In terms of practical consequences, according to the available data, these potential non-human sources do not pose a major threat to leprosy control efforts, although they could ultimately prove an obstacle to eradication of the disease, if that were ever to be contemplated seriously.
Prevention of Transmission from Human Sources. Prevention of transmission of M. leprae from human sources can in theory be achieved by identification of patients and by rendering them non-infectious by effective chemotherapy. Indeed, this is the logic upon which the program to eliminate leprosy as a public health problem is based. We have good evidence that currently employed multidrug therapy (MDT) sharply reduces the numbers of viable bacilli within several days of beginning treatment. However, although there have been repeated claims that case-finding and MDT should reduce transmission, this has proved very difficult to demonstrate. There are at least three reasons for this: 1) we lack a test of subclinical infection, and therefore cannot study directly trends of infection in human communities; 2) the long incubation period of clinical leprosy means that any reduction of infection will become manifest as a reduction of disease incidence only after many years; 3) the incidence of leprosy has been declining in many-perhaps in most-populations for many years, and it is difficult to demonstrate changes of the established trends, especially in the absence of appropriate untreated control populations.
Early attempts to control leprosy based upon segregation of patients in leprosaria, as was widely practised in some countries and persisting today in a few areas, should also have reduced transmission. However, because it increases the fear of the disease, and may actually discourage patients from presenting for diagnosis and treatment, this is not a viable option, even were it feasible.
If either treatment or segregation of the known cases is to reduce transmission of the infecting organism, case-finding must be efficient enough to identify the patients before they have already infected most of their contacts. Given the slow and insidious onset of MB leprosy, this may represent the most serious challenge to our strategy, and a major reason for our failure to see any obvious impact of case-finding and treatment upon the incidence of the disease.
Environmental Approaches to Prevention of Infection. Treatment or segregation of patients provides a means to reduce infectious sources. In addition, there are, or should be, means to prevent transmission by protecting potential recipients against exposure or infection. This approach generally takes the form of environmental or behavioral interventions. An obvious example is health education to prevent exposure to infectious armadillo material, by advising people in endemic areas to take precautions when they are engaged in activities that involve contact with armadillos.
Other approaches of this sort that are less obvious may be effective in reducing transmission of M. leprae. One of the very striking features of the epidemiology of leprosy is its strong association with poverty and the poverty complex (18). Which components of the poverty complex-poor nutrition, close physical contact, lack of personal cleanliness, poor ventilation, bedbugs, intercurrent infection-accounts for this association is not known, but it is at least highly likely that "hygiene" in its broadest sense is somehow important in the epidemiology of the infection. As a consequence, it is highly likely that the general trend toward education and improved general health and hygiene practices, evident in many societies of the world today, plays a role in the primary prevention of leprosy, as well as that of many other infectious diseases**.
Chemoprophylaxis ("Preventive Therapy ")
Administration of appropriate antimycobacterial drugs to individuals at risk represents another approach to primary prevention. This is widely practised in tuberculosis control in developed countries, as well as for prevention of many other infectious diseases such as malaria and meningococcal meningitis. Whether the effect of the drug is to prevent infection entirely, suppress it or cure it may be immaterial. More important is the fact that chemoprophylactic medication will only remain active for a brief period of time, although this may be measured in weeks in the case of some depot preparations, and thus this approach is aimed at protecting against past or current, but not against future, exposure to infection.
Tuberculosis provides an analogy for chemoprophylaxis against leprosy (14). However, in the case of tuberculosis, preventive therapy is generally aimed at individuals who have been identified as being at risk, either because of a large tuberculin reaction, or because of a positive HIV antibody test. There has been discussion of a similar approach to leprosy chemoprophylaxis, based upon identification of infected individuals, employing PGL-I or some alternative immunological screen. Although this approach has been applied in Cuba and evaluated in India (6), it is unlikely to be cost-effective (7), and has not been adopted widely.
In the absence of specific (immunological) indicators of risk, the main consideration of preventive therapy in leprosy has been with reference to populations or subpopulations, such as household contacts, thought to be at high risk. Thus, population treatment with depot dapsone injections was explored in the 1960s, and although this was shown to provide some protection (13), it has not been widely employed. There are three reasons for this: cost, concern for safety, and concern that this approach might encourage the appearance of drug-resistance. These concerns persist today.
More recently, this approach has been resurrected; a single dose of multi-drug treatment, consisting of rifampicin, ofloxacin and minocycline, is being administered to a small, highly endemic population in the South Pacific. This chemoprophylaxis was organized to deal with a special situation, as this population exhibits the highest case-detection rate in the world, despite apparently good coverage by MDT. Nevertheless, it is unlikely that chemoprophylaxis will play a major role in the prevention of leprosy, given the high cost of preventing even a single case, and the risk of side-effects of the medication.
Immunoprophylaxis
Protective vaccination is widely considered the approach par excellence to primary prevention of infectious diseases. The story of vaccines against leprosy is unusual for its irony; although some claim that there is no anti-leprosy vaccine (16), more people alive today have received an anti-leprosy vaccine than have received any other vaccine; in fact, more than 100 million infants and children received it in 1995. The vaccine is BCG, and the irony arises because the vaccine is so widely considered to be directed against tuberculosis that many have forgotten its important implications for leprosy. Figure 1, in which are summarized published estimates of the effect of BCG in preventing leprosy, reveals a range of efficacy, from approximately 20 per cent to 80 per cent in different populations.
Variable Effectiveness of BCG Against Leprosy and Tuberculosis. The variations of efficacy evident in The Figure are reminiscent of variations observed of the effect of BCG against tuberculosis (9). In fact, the variation of effectiveness against tuberculosis is even greater than that against leprosy, with BCG providing no protection at all, at least against pulmonary disease, in several studies. Many explanations of this variation have been advanced, including differences among strains of BCG and of the genetic background of human populations. It is likely that the variation is attributable in part to immunological effects of exposure to various environmental Mycobacteria other than BCG and the pathogenic species,
M. leprae and M. tuberculosis (8). What is particularly important is that, as far as available data are concerned, BCG is at least as effective in preventing leprosy as it is in preventing tuberculosis, and the only three studies, in which the activity of the same BCG in preventing the two diseases has been studied in the same populations, have actually demonstrated BCG to be more effective against leprosy than against tuberculosis [54% vs 11% in Malawi (17); 81% vs 22% in Kenya (15); and 20% vs 0% in South India (22)]. This has important practical implications as well as implications for research. In practice, it means that there is in progress at this moment a massive anti-leprosy vaccination program, which is undoubtedly playing an important role in the worldwide reductions of the incidence of leprosy. Secondly, the relationship between the actions of BCG in leprosy and in tuberculosis may provide a clue to the very important questions of protective immunity and correlates of protection against tuberculosis as well as leprosy (8).
BCG and MB Leprosy. It has been suggested that BCG prevents only paucibacillary (PB) leprosy (16), but The Figure indicates that it has generally proved effective in preventing MB leprosy as well. This has important practical and theoretical implications. In practice, it indicates that BCG prevents MB leprosy in endemic areas-an effect that increases its value in primary prevention, because it not only prevents cases by directly protecting the vaccinees, but it also reduces the number of source cases in the community, thereby reducing transmission of the organism. Thus, it is a powerful tool for primary prevention.
Other Anti-Leprosy Vaccines. Partly because of the failure or reluctance to acknowledge the effectiveness of BCG in preventing leprosy, and partly because of the variability observed in the protection conferred by BCG against leprosy, there has been interest in finding alternatives to BCG. Most attention has been focused upon BCG combined with heat-killed M. leprae vaccines, which were first employed by Convit and his colleagues for immunotherapy, and later evaluated in randomized, blinded controlled prophylactic trials in Venezuela, Malawi and South India. The available results indicate that, although the addition of killed M. leprae may enhance slightly the effectiveness of BCG alone (4), the enhancement is not sufficient to warrant production of such vaccines; production on a scale sufficient to supply the needs of a major program of immunoprophylaxis would be difficult indeed, because the supply of M. leprae is limited, and there are no current plans to expand armadillo colonies.
Other potential anti-leprosy vaccines include strains of M. avium-intracellulare known as the ICRC bacillus and as Mycobacterium "w", both of which are currently being evaluated in a large controlled trial in Avadi, Tamil Nadu, South India. These vaccines have an advantage, in that they employ a cultivable bacillus, but whether they prove effective, and, if so, whether they will ever be produced and extensively employed remains to be seen.
Discussion and conclusions
There is ample evidence that incidence rates of leprosy are declining in most, if not all, leprosy-endemic populations. This in itself implies that effective primary prevention is occurring, even though we are unable to identify precisely the mechanisms responsible for the decline. Given the massive use of BCG vaccines around the world in the course of the past three decades, we may be confident that BCG vaccination must be an important contributor to the decline of the incidence of leprosy, particularly as evidence indicates that the vaccine protects against MB disease and, therefore, must be reducing transmission of M. leprae as well as protecting otherwise exposed individuals. In addition, although it is difficult to demonstrate, extensive case-finding and treatment activities of leprosy control programs throughout the leprosy-endemic world must be reducing transmission to some extent, thereby reducing the risk of new infections and disease. Beyond this, various aspects and correlates of socio-economic development must be contributing to leprosy control, as they obviously have in the past. It is salutary to contemplate these various aspects of primary prevention, and to recognize that the major contributors are likely to be socio-economic development and BCG vaccination, neither of which is generally included as a formal activity of leprosy control programs.
The figure. Published estimates of the observed protection of BCG vaccines against leprosy by area, disease classification and type of study (T = trial; CC = case-control study; COH = cohort study). Protection is ex-pressed as a relative risk (RR = odds ratio) of leprosy among vaccinees compared to non-vaccinees (note that,traditionally, vaccine efficacy is expressed as VE = 1-RR). Thus, RR = O implies 100% protection, and RR = 1 implies no protection. The horizontal lines indicate 95% confidence intervals. Data sources are: Venezuela (5), Brazil (19), Uganda (20), Malawi/Karonga (17), Malawi/ Balaka (2), Kenya (15), South India/Chingleput (22) South India/Vellore (12), Burma (10) Vietnam (21), Indonesia (3).
Among the major research issues relevant to primary prevention are questions of the evaluation of leprosy incidence trends, recognizing that confirmation of declines of incidence is confirmation of effective primary prevention. Beyond the confirmation of declining trends, identification of the factors responsible for these trends is a major epidemiological challenge. Disentangling socio-economic factors, BCG, and the influences of improved case-finding and treatment activities is likely to be extremely difficult, given that these factors are likely to be confounded in most populations, and it is difficult to identify appropriate control groups.
Of particular importance is the issue of the variability of the effectiveness of BCG as a leprosy preventive, and its implications for different contributions to control in different populations. It may be that BCG is less effective against leprosy in India than in some other areas (The Figure). If this be so, this might in turn be related to the fact that there is, at this moment, less evidence for a decline of leprosy incidence in India than in most other endemic areas.
Another fruitful area for research in primary prevention of leprosy will be the interface between leprosy and tuberculosis, with particular reference to vaccines. Because of the increasing burden of tuberculosis in the world (in 1993, WHO declared tuberculosis to be a global emergency), much more attention is now being directed toward tuberculosis than toward leprosy vaccines. However, progress has been slowed by the absence of any clear and measurable immunological correlate of protective immunity, whether naturally acquired or vaccineinduced. Given the observed differential in protection by BCG against leprosy and tuberculosis, the most efficient approach to solving the problem of anti-tuberculosis immunity may be to examine both diseases in parallel.
REFERENCES
1. BAGSHAWE, A., SCOTT, G. C., RUSSELL, D. A., WIGLEY, S. C., MERIANOS, A. and BERRY, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea, 1963-1979. Bull. WH O 67(1989)389-399.
2. BAKER, D. M., NGUYEN VAN THAM, J. S. and SMITH, S. J. Protective efficacy of BCG vaccine against leprosy in southern Malawi. Epidemiol. Infect. 111(1993)21-25.
3. BOELENS, J. J., KROES, R., VAN BEERS, S. and LEVER, P. Protective effect of BCG against leprosy in South Sulawesi, Indonesia. Int. J. Lepr. 63(1995)456-457.
4. CONVIT, J., SAMSON, C, ZUNIGA, M., SMITH, P. G., PLATA, J., SILVA, J., MOLINA, J., PINARDI, M. E., BLOOM, B. R. and SALGADO, A. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet 339(1992)446-450.
5. CONVIT, J., SMITH, P. G., ZUNIGA, M., SAMSON, C, ULRICH, M., PLATA, J. A., SILVA, J., MOLINA, J. and SALGADO, A. BCG vaccination protects against leprosy in Venezuela: a case control study. Int. J. Lepr. 61(1993)185-191.
6. DAYAL, R. and BHARADWAJ, V. P. Prevention and early detection of leprosy in children. J. Trop. Ped. 41(1995)132-138.
7. FINE, P. E. M. Immunological tools in leprosy control. Int. J. Lepr. 57(1989)671-686.
8. FINE, P. E. M. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346(1995)1339-1345.
9. FINE, P. E. M. and RODRIGUES, L. C. Modern vaccines: mycobacterial diseases. Lancet 335(1990)1016-1020.
10. LWIN, K., SUNDARESAN.T., GYI, M. M., BECHELLI, L. M., TAMONDONG, C, GALLEGO GAKISAJOSA, P., SANSARRICQ, H. and NOORDEEN, S. K. BCG vaccination against leprosy: fourteen-year findings of the trial in Burma. Bull. WHO 63(1985)1069-1078.
11. MOSTAFA, H. M., KAZDA, J., IROENS, L. M. and LUESSE;, H. G. Acid-fast bacilli from former leprosy regions in coastal Norway showing PCR positivity for Mycobacterium leprae. Int. J. Lepr. 63(1995)97-99.
12. MULIYIL, J., NELSON, K. E. and DIAMOND, E. L. Effect of BCG on the risk of leprosy in an endemic area: a case control study. Int. J. Lepr. 59(1991)229-236.
13. NOORDEEN, S. K. Chemoprophylaxis. Quademi di cooperazione sanitaria 1(1982)173-180.
14. O'BRIEN, R. J. Preventitive therapy for tuberculosis. In: "Tuberculosis: back to the future". (Porter, J. D. H. and McAdam, K. P. W. J., eds). John Wiley, Chichester. 1994, pp 151-169.
15. OREGE, P. A., FINE-, P. E. M., LUCAS, S. B., OHURA, M, OKELO, C. and OKUKU, P. Case control study of BCG vaccination as a risk factor for leprosy and tuberculosis in western Kenya. Int. J. Lepr. 61(1993)542-549.
16. PARKASH, O. Antileprosy vaccine: an apprehension. Int. J. Lepr. 63(1995) 572-573.
17. PONNIGHAUS, J. M., FINE, P. E. M., STERNE, J. A. C , Buss, L., WILSON, R. J., MALEMA, S. S. and KILETA, S. Incidence rates of leprosy in Karonga District, northern Malawi: patterns by age, sex, BCG status and classification. Int. J. Lepr. 62(1994)10-23.
18. PONNIGHAUS, J. M., FINE, P. E. M., STERNE, J. A. C, MALEMA, S. S., BUSS, L. and WILSON, R. J. Extended schooling and good housing conditions are associated with reduced risk of leprosy in rural Malawi. Int. J. Lepr. 62(1994)345-352.
19. RODRIGUES, M. L. O., SILVA, S. A., Nino, J. A. C, DeANDRADE, A. L. S. S., MARTELLI, C. M. T. and ZICKER, F. Protective effect of intradermal BCG against leprosy: a case-control study in central Brazil. Int. J. Lepr. 60(1992)335-339.
20. STANLEY, S. J., HOWLAND, C, STONE, M. M. and SUTHERLAND, L BCG vaccination of children against leprosy in Uganda: final results. J. Hyg. 87(1981)235-248.
21. THUC, N. V., ABEL, L., LAP, V. D., OBERTI, J. and LAGRANGE, P. Protective effect of BCG against leprosy and its subtypes: a case control study in southern Vietnam. Int. J. Lepr. 62(1994)532-538.
22. TRIPATHY, S. P. The case for BCG. Ann. Nat. Acad. Med. Sci. 19(1983) 11-21.
Discussion of Prof. Fine's paper
Prof. Ji: Although BCG may be active in preventing leprosy, this vaccine is not a target of the 1MMYC program. How do you assess the prospects for development of another vaccine to be used in leprosy? And do you believe that we need a vaccine?
Prof. Fine: BCG should certainly be considered a vaccine for use in preventing leprosy. In addition, it may well be that BCG vaccination could be rendered more effective by use of a more immunogenic strain, by administration of more than one dose, or by engineering the BCG bacillus. Other vaccines are currently being developed; let's see what we will have to work with before we decide how we should apply the vaccine.
Dr. Gupte: BCG appears certainly to be effective in preventing leprosy.
Dr. Cole: How many of the BCG trials were designed to look at leprosy?
Prof. Fine: Not all of the data were derived from trials; the trials in Myanmar, Papua-New Guinea, Uganda and South India were designed to look at the effect of BCG in preventing leprosy. Most of the data were obtained from national BCG vaccination programs, which were intended to prevent tuberculosis.
Dr. Feenstra: Even if we should have a vaccine that is more active than BCG, imagine the problems facing national health planners in deciding how to use the vaccine. Not only is the disease disappearing; it can be easily diagnosed, and can be treated cheaply, safely and effectively. Therefore, I don't believe that a new vaccine will play a role in leprosy control activities.
Prof. Fine: Repeat BCG vaccination may, in fact, be very useful in high-risk groups, and is very much on the table.
RESEARCH PRIORITIES IN LEPROSY: AN OPERATIONAL PERSPECTIVE
P. Feenstra, Leprosy Unit, Royal Tropical Institute, Amsterdam, The Netherlands
Research is relevant only when it contributes to the achievement of the goals of leprosy control, which are: 1) to cure the patients; 2) to prevent leprosy-related disabilities; and 3) to interrupt transmission of Mycobacterium leprae, thereby reducing the incidence of both infection and disease. At present, we possess the knowledge and tools required to achieve the first two goals: early diagnosis, multidrug therapy (MDT), and early recognition and appropriate treatment of functional impairment of nerves. Moreover, the timely and consistent use of these tools may, in addition to the impact of BCG vaccination and other factors related to socio-economic progress, also' reduce transmission of the organism.
Unfortunately, many health services are not able to use these tools in an effective way. As a result, many cases are diagnosed only after irreversible impairment of nerve function has already occurred. Fully 25 per cent of the registered cases are not treated by MDT, and too many patients who begin MDT do not complete a full course of treatment. And finally, even among registered cases who receive a full course of MDT, impairment of nerve function, resulting in new disabilities and exacerbation of preexisting disability, occurs with distressing frequency. These failures may, to a great extent, be attributed to lack of proper organization and management of leprosy control programs. In addition to improvement of management and organization, by training, more intensive supervision, etc., health systems research (HSR) represents a tool by which this situation may be improved.
HSR is action-oriented, operational research, in which the questions studied are linked to decision-making. HSR studies specific field problems in order to find practical solutions that are feasible under the prevailing local conditions. Health services staff always participate in the studies. HSR can be carried out in a reasonably short time-less than one year, at moderate cost. Major issues to be investigated by HSR are how to achieve effective coverage of patients in difficult-to-reach populations, and how to sustain cost-effective leprosy services under conditions of low prevalence.
The studies may be very pragmatic, focused and action-oriented, as has been demonstrated in some Special Action Programme for the Elimination of Leprosy (SAPEL) projects. A problem is defined (in SAPEL, it may be the accessibility of patients to MDT), a pragmatic solution is identified, its feasibility is tested, and, after it has been shown that the intervention effectively solves the problem, the intervention is applied by the program on a broader scale.
Improvement of management, and operational research are not the only answers to the problem of inadequate leprosy control. There is also a need for new, more effective tools and for simplification of the currently available tools. Because of the severe limitations of research capacity and resources, especially in developing countries, research should focus on problems of high priority, that have been identified in the field. Placing myself in the situation of a health services manager, responsible for leprosy control, I consider the following issues to represent research priorities ***:
Reactions and recent nerve damage: 1) development of tests to identify patients at risk of reactions and nerve damage (laboratory/fundamental); 2) development of tools by which to prevent reactions and nerve damage (laboratory/ fundamental and operational/epidemiological); 3) development of tests for early detection of reactions and nerve damage (laboratory/ fundamental and operational/epidemiological); 4) development of more effective treatment for reactions and nerve damage (laboratory/fundamental).
Prevention of disabilities (POD): 1) development of simple and practical measures of impairment that are responsive to change over time (operational/epidemiological); 2) development of indicators for the process and outcomes of POD activities (operational/ epidemiological); 3) development of more effective and efficient POD in primary health care and community-based rehabilitation (CBR) settings (operational/ epidemiological); 4) development of more effective self-care and footwear for patients with impairments (operational/epidemiological).
Chemotherapy: 1) development of methods to improve coverage by MDT in inaccessible areas, and to improve MDT completion rates (laboratory/fundamental and operational/ epidemiological); 2) development of strategies to implement MDT effectively through general health services and primary health care in low-endemic settings (operational/epidemiological); 3) development of shorter and cheaper MDT (laboratory/fundamental).
Rehabilitation: 1) Development of methods to assess the need for rehabilitation (operational/epidemiological); 2) development of effective rehabilitation for leprosy patients within general rehabilitation programs, including CBR (operational/epidemiological).
Early diagnosis, incidence and transmission: 1) development of a simple test for early diagnosis of leprosy (laboratory/fundamental); 2) development of methods by which to assess the incidence of leprosy (laboratory/ fundamental and operational/ epidemiological); 3) development of methods by which to study the transmission of leprosy (laboratory/fundamental and operational/epidemiological); 4) development of methds for predicting future trends of incidence and prevalence (operational/epidemiological).
Although development of the tools may require sophisticated technologies, the tools themselves must be appropriate for routine use under field conditions in leprosy-endemic countries, and should not require complicated or expensive equipment. "Dipsticks" for analysis of samples of urine or saliva are examples of appropriate tools.
Discussion of Dr. Feenstra's paper
Dr. Naafs: I'm delighted to hear that your first priority is to cure the patient, and that stopping transmission of M. leprae is only your third priority. Only a few years ago, interruption of transmission was the first priority.
Prof. Ji: MDT must remain the first priority. And because MDT has been so effective, we are now able to attempt other activities, such as prevention of disability.
Prof. Britton: How confident can we be that the annual case-detection rate will be reduced from the current level of 500,000? Won't the answer to this question help determine priorities?
Dr. Feenstra: Although I don't believe that incidence will decrease as rapidly as some are predicting, there can be no doubt that it is dropping, despite confusing case-detection rates. What has happened is a change of case-definition. In India, for example, the proportion of single-lesion cases among the new cases reported is very large; if these are subtracted, the case-detection rates become very much smaller.
Dr. Noordeen: Much of what has been called operational research is better called management exercise, and should become a part of the management process. Research requires protocols, controls, etc., which greatly limit productivity. Also, funding is much more readily obtained when the activity is carried out as part of the manager's training.
Prof. Fine: I disagree. Reliable information is derived from research, and cannot be simply left to the managers.
RESEARCH NEEDS RELATED TO DISABILITIES AND REHABILITATION
W.C.S. Smith, University of Aberdeen, Scotland, and W.H. van Brakel, INFLP, Pokhara, Nepal
Progressive disability resulting from nerve damage is what separates leprosy from other diseases, and is responsible for the stigmatization of those affected. Nerve damage, the pathological process that leads to the deformities typical of leprosy, occurs either gradually or acutely as part of a reactional state (7). Early detection and specific treatment with corticosteroids during the acute phase of nerve damage can reverse the damage in a large proportion of cases(6) It is generally considered that nerve damage of more than 6 months' duration is irreversible, and patients presenting with nerve damage of long standing are not actively treated with steroids. Few other treatments are available; one of these is surgical decompression of the affected nerve, which provides only inconsistent benefit, and is not generally practised.
Early diagnosis of leprosy and treatment with multidrug therapy (MDT) is the most effective means of preventing disability in leprosy. Acute nerve damage can occur before diagnosis, during MDT, or after completion of chemotherapy; however, most frequently it occurs either just before diagnosis or during the first 6 months of treatment (1). Acute nerve damage and reactional states appear to have become more frequent, particularly in patients with multibacillary (MB) leprosy, since the introduction of MDT. However, methods of casedetection and of diagnosing acute nerve damage have changed, so that it is not possible to compare the present situation with that during the era of dapsone monotherapy. Reversal reactions (RR) vary in frequency among the different types of leprosy, and in different parts of the world (5). On average, RR occurs after commencing MDT in as many as 15 per cent of patients with paucibacillary (PB) leprosy, and in more than 30 per cent of MB patients. Steroids suppress the immune response that is responsible for the acute nerve damage in the course of RR, and are therefore used not only to treat reactions, but also to suppress them in patients at risk.
Definition of terms
During the last few years, leprosy workers have been encouraged by some to adopt the International Classification of Impairments, Disabilities and Handicaps (ICIDH) (3), whereas, at the same time, challenges to the ICIDH have been heard. Impairment is defined as any loss or abnormality of psychological, physiological or anatomical structure or function; disability is defined as any restriction or lack, resulting from impairment, of ability to perform an activity in the manner or within the range considered normal for a human being; and handicap is defined as a disadvantage for a given individual, resulting from an impairment or disability, that limits or prevents fulfillment of a role, depending upon the age, sex, and social and cultural characteristics of the individual. In general, leprosy workers use the term "disability" to mean "impairment"; this distinction is less important when dealing with the physical aspects of leprosy, but is important when discussing the social, cultural and vocational impact of the impairments and the topic of rehabilitation.
Size of the problem
Assessing the size of the problem of impairments and disabilities caused by leprosy in the world is difficult. The only data routinely collected are the proportions of newly detected patients who present with WHO grade 2 disability. A number of attempts have been made to calculate the global burden from these data, using age at diagnosis and life-expectancy, but these calculations yield only rough estimates at best. A number of cross-sectional surveys have been conducted in various places to estimate the size of the problem.
Perhaps the more important statistic is the number of people requiring rehabilitation. This is more difficult to assess, and is influenced by social, religious and cultural factors. The number requiring rehabilitation is probably a small proportion of all those who have impairments as a result of leprosy. Some of the factors affecting the distribution and determinants of disability in leprosy have been described (8, 10). Now, as a result of the successful implementation of MDT, we have a unique opportunity to implement specific disability-prevention activities (9).
Identifying research needs
At the basic level, research is needed to understand the pathogenesis of nerve damage. At the clinical level, research is needed to test the effectiveness of interventions, using the methods of randomized controlled clinical trials. At the operational level, research is needed to optimize the process of early diagnosis of leprosy and of nerve damage caused by leprosy, and of treating this damage, as well as of improving methods of rehabilitation (4. 11, 12) .
In London in 1995, the Medical Commision of the International Federation of Anti-Leprosy Associations (ILEP) convened a workshop on preventing disability in leprosy (2), the objectives of which were to produce guidelines on simple and effective means of preventing disability and to identify research needs. The workshop process included reviewing the results of an international survey of prevention-of-disability (POD) activities, presentation of position papers by experts in the field, and group discussion. The international survey showed that, although POD activities were now being implemented widely, more was needed to improve the quality of the work. The workshop produced a list of research priorities in the areas of POD and rehabilitation, as follows.
Recommendations for further research of the ILEP expert committee on prevention of disability
The proposed studies have been grouped into the broad areas of monitoring and evaluation, primary prevention of impairment and disability (POID), secondary prevention of impairment and disability, and community-based rehabilitation.
Monitoring and Evaluation: 1) studies to evaluate the validity, reliability and responsiveness to change over time of simple methods of scoring impairment and disability in leprosy patients, with the aim of identifying the system most suitable for monitoring POID activities. Such a scoring system could be used globally to monitor and evaluate POID activities in leprosy work; 2) studies to develop and pilot the routine collection of POID information for all ILEPsupported projects, to identify the most efficient means of collecting such data; 3) conduct of trials to assess the cost-effectiveness and, possibly in the future, also the costbenefit of different components of POID, in order to determine the relative cost-effectiveness of the different components of a comprehensive program of prevention of impairments and disabilities.
Primary Prevention of Impairment and Disability: 1) development of valid, reliable and feasible methods of assessing nerve function, and a series of studies replicated in different environments, including at the field level, to identify the best methods of identifying early impairment of nerve function appropriate to a variety of situations; 2) trials to identify the optimal corticosteroid regimens for treating recent impairment of nerve function, with the aim of identifying the optimal dosages and durations of the treatment; 3) a randomized, double-blind trial of steroid prophylaxis, to prevent acute impairment of nerve function in patients at risk, to determine if the administration of prednisolone in a small daily dose during the first 6 months of MDT will prevent acute impairment of nerve function; 4) a trial to compare the prognostic value of nerve function assessment by Semmes-Weinstein monofilaments and ball-point pen in detecting nerve function impairment, to learn if the impairment detected by nylon filaments is of greater prognostic value than that detected by ball-point pen; 5) a trial of patient education emphasizing self-reporting of impairment, to determine if patients, who have been educated specifically with respect to the early signs and symptoms of impairment and its prevention, are more likely to self-report impairment than are patients who have not been so educated.
Community-Based Rehabilitation (CBR): 1) surveys to assess to what extent, if any, leprosy patients are included in existing programs of CBR, in order to determine to what extent the needs of leprosy patients are being met in various countries; 2) studies employing a modified CBR approach among those locally responsible for the care of leprosy patients with chronic impairment and disability, to determine if volunteers from among family members, neighbors or prominent members of the community could be used in POID activities, thus reducing the need for referral and hospitaladmission for treatment of ulcers and wounds.
REFERENCES
1. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy. Int. J. Lepr. 60(1992)173-184.
2. ILEP MEDICAL COMMISSION. Prevention of disability in leprosy-ILEP Medical Bulletin. Lepr. Rev. 67(1996)68-72.
3. INTERNATIONAL CLASSIFICATION OF IMPAIRMENTS, DISABILITIES AND HANDICAPS. WHO, Geneva, 1980.
4. JAKEMAN, P. and SMITH, W. C. S. Evaluation of a multidrug therapy programme of leprosy control. Lepr. Rev. 65(1994)289-296.
5. LIENHARDT, C. and FINE, P. E. M. Type I reactions, neuritis and disability in leprosy- what is the current epidemiological situation. Lepr. Rev. 65(1994)9-33.
6. PEARSON, J. M. H. The use of corticosteroids in leprosy. Lepr. Rev. 52( 1981 )293-298.
7. RIDLEY, D. S. Neuropathy in leprosy. In: "Pathogenesis of leprosy and related disease", Wright, London, 1988.
8. SMITH, W. C. S. Epidemiology of disability in leprosy including risk factors. Lepr. Rev. 63 Suppl. (1992)22-30.
9. SMITH, W. C. S. and JESUDASAN, K. The elimination of leprosy and prospects for rehabilitation. Lancet 341(1993)89-90.
10. SMITH, W. C. S. and PARKHE, S. M. Disability assessment as a measure of progress in leprosy control. Lepr. Rev. 57(1986)251-259.
11. SMITH, W. C. S., ZHANG, G. C. ZHENG, T. S., WATSON. J. M., LEHMAN, L. F. and LEVER, P. Prevention of impairment in leprosy: results from a collaborative project in China. Int. J. Lepr. 63(1995)507-517.
12. VAN BRAKEL, W. 11. Peripheral neuropathy in leprosy-the continuing challenge. Utrecht University Thesis, 1994.
Discussion of Dr. Smith's paper
Dr. Waters: I have been impressed by the high prevalence of impairments related to the eye. You didn't mention the eye in your listing of priorities with respect to research into the prevention of disabilities.
Dr. Smith: You are correct, in that I didn't mention the eye specifically. Of course, we are concerned about preventing disabilities of eyes, hands and feet. I'd like to hear comments with regard to combining steroids with MDT in a trial intended to examine the possibility of preventing reactions.
Dr. Naafs: We discussed the possibility of such a trial in ALERT more than 20 years ago. At the time, we were concerned about the dosage of DDS-whether it was necessary to use high doses, or could small doses be used. Barnetson conducted a trial of 5 mg DDS daily vs 50 mg DDS daily; it appeared that reactions were milder among those patients treated by the larger dose. Subsequently, we added an initial course of steroids, in a dosage of 20 mg daily, to the regimen of those patients who we felt were more likely to have reactions. These patients appeared to do well. The majority of patients, in whom we did not expect reactions, were not given steroids; some of them subsequently had reactions. We were concerned that it might be dangerous to administer steroids to large numbers of outpatients, living in an environment characterized by the ubiquity of intestinal parasites and similar problems. As a consequence, we abandoned the trial. I think that it will be important, in any trial of steroids combined with MDT, to define carefully those patients at risk of reactions, who should be treated by steroids, and I'm afraid that we are not yet able to do this.
Dr. Gupte: We have used steroids in the attempt to prevent quiet nerve paralysis, without evidence of efficacy. I believe that it will be difficult to administer steroids to large numbers of patients in the field. Administration of steroids cannot be supervised, and self-administration is certain to be unreliable. In addition, steroids will be contraindicated in significant numbers of patients. Finally, it may be necessary to continue steroids after the course of MDT has been completed.
Prof. Ji: Have we any information with respect to the influence of steroids on the efficacy of MDT? And will administration of steroids under field conditions be feasible?
Dr. Jacobson: I worry that we may be playing with fire if we propose to administer large doses of steroids daily over long periods of time. However, if a trial were to be organized, it would be interesting to compare daily dosage with every-other-day dosage. Side-effects appear to be much less common with every-other-day administration. Placing the steroids within the blisterpack might minimize the problem of poor compliance. In our experience, the co-administration of steroids has not adversely affected MDT, and the new guidelines recommend that clofazimine be administered when steroids must be given to a patient who has completed MDT
On a related topic, I believe that it would be useful to attempt to confirm in a clinical trial that the administration of steroids beginning more than 6 months after the onset of the impairment really produces some amelioration of the impairment; our experience has not been encouraging.
Dr. Talhari: In Brazil, we recommend the use of steroids in some situations, but we lack important knowledge. This is an excellent area for research.
Dr. Pannikar: We are often accused of not paying enough attention to the area of prevention of impairment. I believe that our neglect is only apparent; simply, we don't know what we should do. A second matter on which I wish to comment is that we are constantly being urged to develop "simplified" methods for use in the field. However, I believe that we need a definition of what is simple. What may be simple in the context of a vertical program may, in fact, be difficult in the context of an integrated program. Third, can you comment on the use of "POD kits? And, finally, I wish to point to the problem of underutilization of services. So often, particularly in vertical programs, the problem is not that services are not available, but rather that the patients don't avail themselves of the services offered.
Dr. Feenstra: One of the deficiencies of vertical programs is that accessibility to them may be only periodic. This is so particularly in those situations in which the leprosy control team visits the patient's locality only on a monthly basis. Dr. Smith is entirely correct in stressing the need to integrate rehabilitation services for leprosy patients into the general health services, which are accessible on a daily basis.
Dr. Lambert: You mentioned that only a "small investment" need be made in a research program designed to improve disability-prevention and rehabilitation activities. As a research manager, I should like to know how you define a small investment. It will finally be necessary to deal with numbers, when we think of genome sequencing, for example.
Dr. Smith: I believe that an investment in this area of only a few hundred thousand U.S. dollars would pay large dividends.
Dr. Noordeen: NGOs are investing heavily in socio-economic rehabilitation, a subject that Dr. Smith did not cover. In my opinion, much of this investment is misdirected. Rather than depending entirely upon solutions to socio-economic problems that have been imported, we need to know more about the coping mechanisms indigenous to the society. I believe this to be an important area for research.
Dr. Feenstra: Leprosy patients must have access to the same services available to other disabled patients in the community, but they should not be privileged; they should not be offered services that are not generally available. If NGOs interested in leprosy wish to invest in rehabilitation, they must be careful not to make available to the leprosy patients services that are not available to other patients with disabilities.
RESEARCH NEEDS OF THE POST-ELIMINATION PHASE
Charoon Pirayavaraporn, Ministry of Public Health, Bangkok, Thailand
Before focusing on the research needs of the post-elimination phase, employing the situation of leprosy in Thailand as an example, I wish to share my feeling, as a program manager, that we risk living in a dream world, if the recommendations for research priorities are impractical, or if they will lead to research outcomes that can never be employed to develop simplified tools or to solve real problems. In addition, it should be noted that some research questions have been studied for many years with only little progress.
During the period 1980-1994, when leprosy control activities in Thailand were performed by the local health staff, under the supervision of Leprosy Division staff, it was possible to collect data for both operational and epidemiological assessments, including analysis of trends. At the end of fiscal year 1995, there were 3,015 Thai patients registered for treatment, yielding a prevalence rate of 0.51 per 10,000 population, and 1,297 new cases had been detected, yielding a new case-detection rate of 2.2 cases per 100,000 population. Analysis of the situation by province revealed that in only seven provinces was the leprosy prevalence greater than 1 per 10,000, whereas the prevalence was below 1 per 10,000 in the remaining 69 provinces.
The problems of disability resulting from leprosy and the requirements for main-tenance and improvement of the quality of the leprosy control services, especially for prevention of disability and rehabilitative care, are felt to represent major concerns, together with the needs to update information, and to develop an effective surveillance system. These are particular concerns, because, as a result of the decline of prevalence, a large number of experienced staff have been transferred to other duties. Collection of data and analysis of the situation on a current basis, as has been the practice in the past, will no longer be possible. Moreover, as many as 14 per cent of the newly detected patients are still diagnosed only after irreversible impairment of nerve function, and as many as 30 per cent of patients develop new disabilities or experience exacerbation of existing disabilities during and after completion of treatment. These data indicate the importance of developing new tests and tools with which to prevent reaction and nerve damage, as well as practical and simple means of measuring nerve function impairment.
Thus, Thailand now exemplifies the problems inherent in leprosy control in the situation of low endemicity. In addition to the needs to maintain and improve the quality of the service, the needs remain to verify the extent of the leprosy problem, and to monitor the existing situation closely. Some questions remain to be answered; for example, how can we be certain that leprosy prevalence, incidence or transmission of infection are really low in a particular area? What do good information systems and an effective system of leprosy surveillance look like in the situation of low endemicity? How can we be alerted, should our current control measures-chiefly multidrug therapy (MDT)-become not as effective as they have been in the past?
What options do we have if MDT fails to cure leprosy because of drug-resistance or for some other reason? What kind of organization do we need to keep an eye on the leprosy problem in a country in which leprosy has become a rare disease, and how do we equip that organization with sufficient, sustainable expertise to deal with those problems that may occur? These may appear to be simple questions, but they must be answered clearly and reliably, not by intuition but by research, including health systems, biomedical, operational and socioeconomic research.
Finally, in the post-elimination phase, we shall wish to deal with the more challenging question of how to eradicate leprosy globally. It is, perhaps, premature to address this question now, but certainly we must begin to address the research needs of the postelimination phase from today.
Discussion of Dr. Charoon's paper
Dr. Feenstra: 1 notice that your data on case-detection suggest that the rate has been stable for the last few years, and that it may even have increased during the last year. Do you have an explanation for this phenomenon? Has case-finding been more active?
Dr. Charoon: Yes, we have recently intensified case-finding activities.
Dr. Talhari: You stated that you had achieved 100 per cent coverage with MDT. What is the regularity of attendance for MDT?
Dr. Charoon: The regularity of attendance is 83 per cent, on the average.
Dr. Pannikar: I believe that, in situations of low endemicity, we should deal with absolute numbers, as rates may be misleading.
Prof. Ji: What is the sum available annually in Thailand for leprosy control, including contributions from NGOs?
Dr. Charoon: Including salaries, the national budget was 160 million Baht****, including 42 million Baht for payments to patients. In addition, the Sasakawa Memorial Health Foundation contributes funds primarily for support of the Sasakawa Research Building. The NSLA and the GLRA each contribute approximately 300,000 Baht, primarily to support training activities. Finally, the national leprosy relief association also contributes.
Prof. Lechat: I wonder if some of the research outlined by Dr. Charoon has not already been carried out for other diseases, and if the results of this research might not be applcable to leprosy control in Thailand and in developing countries in general. You spoke, for example, of the need to develop surveillance systems applicable to the situation of low endemicity. Perhaps we need additional links with similar programs that deal with the same issues applied to other diseases.
Dr. Noordeen: As leprosy becomes a relatively less common disease, we face serious problems with respect to surveillance. As we move from vertical to integrated programs, we become increasingly dependent upon the routine surveillance systems for communicable diseases. We will need to strengthen the routine surveillance systems so that they can deal more competently with leprosy surveillance. This is work that needs to be done.
Dr. Feenstra: The WHO Task Force for Health Systems in Leprosy has funded studies in Thailand of the feasibility of combining leprosy and tuberculosis control activities. Can you comment on this work?
Dr. Charoon: Such studies have been undertaken in North-East Thailand; to my knowledge, they have been restricted to case-finding methods. The results are now being prepared for publication.
Dr. Smith: With respect to case detection after elimination, we expect that the prevalence of leprosy among children will decrease, and that the proportion of MB cases will increase. However, we might see the same changes as case detection becomes poorer in quality and awareness of leprosy decreases. Therefore, we must be careful to discriminate between those changes that result from decreased transmission of M. leprae and those that would result, should the quality of case-detection activities decrease.
Another emerging problem appears to have arisen in some Western Pacific countries, that are in a situation similar to that of Thailand. A substantial proportion of the new cases appears to have occurred as relapses of leprosy among patients who, in the past, had been treated only with DDS monotherapy. Have you recognized a similar phenomenon in Thailand?
Dr. Charoon: Beginning several years ago, we retreated with MDT all of those patients whose leprosy had been cured with DDS monotherapy, so that we have encountered only a rare relapse of the kind you describe.
IMMUNODIAGNOSTICS, INCLUDING SKIN TESTS
P.J. Brennan1; S.-N. Cho2; PR. Klatser3
1. Colorado State University, Fort Collins, Colorado, U.S.A..
2. Yonsei University, Seoul, Korea.
3. Royal Tropical Institute, Amsterdam, The Netherlands
The most recent information with respect to the prevalence of leprosy demonstrates(9,12,13) a greater than 80 per cent reduction since 1985, from an estimated 10-12 million to 1.8 million patients in 1995. Both directly, and indirectly as a result of enthusiasm generated by the elimination program, the World Health Organization/Multidrug-Regimen (WHO/MDT) is central to this success. Yet, new case detection remains at a high level; 414,894 new patients were detected in India alone during 1994 (9,12,13).All agree that the most important contributions by current research efforts will be tools to identify those subclinically infected by Mycobacterium leprae, of sufficient sensitivity and specificity to facilitate epidemiological monitoring of the disease in the community (8). The application of skin testing and serological studies to this need is addressed below.
A new generation of skin-test reagents (PJB)
One hope for a method of identiliying those subclinically infected by M. leprae rests in a new generation of skin-test antigens. The earlier products-lepromin H (2,7), Dharmendra lepromin (1), and lepromin A(5,6)-and those produced more recently from fractionated M. leprae -Convit's SDA and Rees' MLSA (11)-have a place in leprosy control, but fail as universally applicable diagnostic and epidemiological tools; SDA and MLSA meet the requirement for potency, but are lacking in terms of sensitivity and specificity. Our approach toward more useful reagents lies in further fractionation and identification of appropriate antigens of M. leprae, and testing these products and other antigens already identified first in guinea pigs and then in man, both in vitro by assaying, particularly for interferon-gamma (IFN-γ), and in vivo by skin testing. This approach is directed to application to man, and must, therefore, involve manufacture under the conditions of Good Laboratory Practices (GLPVPilot Plant), as well as applications for Investigational New Drug Exemptions (IND) to the U.S. Food and Drug Administration (FDA) in the case of the U.S., or to the corresponding regulatory agencies in other countries.
GLP/Pilot Plant. We are in the process of setting up a GLP facility in our own department for the purpose of producing skin-test antigens. In order to comply with GLP conditions, the first essential ingredient is a documentation system. A network of standard operating procedures (SOPs) is used to validate every piece of equipment, and to control every step of the processing, to insure that performance is consistent, and that the antigens satisfy exacting standards of quality control. The other aspect of GLP is employment of "clean room conditions", to insure safety of the products. An environmentally controlled workspace suitable for aseptic processing of the skin-test antigens has been installed; this consists of a suitably constructed, properly functioning and regularly certified Laminar Airflow Workbench (LAFW), which sweeps the workspace with HEPA-filtered air at a velocity of 90 ± 20% feet per minute. The air-quality within the LAFW adjacent to "critical sites" must meet a Class 100 (MCB-1) clean room specification during normal work activity. However, the air entering the critical site must meet the requirements of a Class 100,000 clean room, and must undergo ten air-changes per hour. Validation of the "Clean Room" begins with monitoring the air; this is accomplished qualitatively by settling plates, and quantitatively by use of a Personnel Sampling Pump and Cyclone Filter Assembly, which measures the number of particulates in the air of the Skin Test Antigen Manufacturing Facility. To date, wipe tests have not yielded a single colonyforming unit.
Manufacture of the First Generation of Skin-Test Antigens. We have already produced two new fractions, modified versions of earlier antigens, for the purpose of getting products through the system, establishing baseline data, and, we hope, increasing sensitivity and specificity over those of the earlier products. First, we modified MLSA to produce MLSA-LAM by removing immunosuppressive cross-reactive components (LAM, LM and PIMs, and other lipids). Second, together with B.R. Bloom, V. Mehra and R.L. Modlin, we demonstrated by extensive immunological study and skin-testing in guinea pigs that the cell wall proteins of M. leprae, which had been discarded in preparing SDA and MLSA, were powerful immunogens (3, 4). Based on this work, we produced MLCwA, a fraction that contains these cell wall-derived antigens. Both MLSA-LAM and MLCwA are awaiting approval by the FDA.
Manufacture of these skin-test antigens begins with purification of M. leprae from infected armadillo tissues, using Draper's 3/77 protocol (10). This protocol involves homogenization, using dipotassium EDTA as a metal chelator, followed by alkali treatment to remove pigment, and over-night collagenase treatment to degrade the tissue. The partially purified organisms are then applied to a two-phase separation system involving polyethylene glycol (PEG) and Dcxtran T500. The purified M. leprae are examined microscopically for residual tissue and pigment, and brain-heart infusion broth, blood-agar plates and Lowenstein-Jensen slants are inoculated. The concentration of the product is established by measuring absorption at 540 nm.
Preparation of MLSA-LAM begins with the Rees protocol. M. leprae are sonicated in PBS and centrifuged twice at 4"C. The supernate is centrifuged at 105,000 x g, to produce MLSA, which is adjusted to a concentration of 1 mg per ml, after which precondensed Triton X-l 14 is added to a final concentration of 4%. This mixture is subjected to slow-speed centrifugation to form two phases; lipoglycans and other hydrophobic molecules partition to the detergent layer. Residual detergent is removed from the aqueous phase by means of an Extractigel D column, yielding the final product.
Formulation of MLCwA begins from the 27,000 x g pellet, which is extracted with 2% SDS in PBS while stirring at 56"C for 1 hour. The suspension is centrifuged, applied to an Extracti-gel D column to remove SDS, and extracted with Triton X-l 14 as was the MLSA-LAM.
Both antigens are sterilized for 20 mill at 12I"C, and subjected to a variety of qualitycontrol assays, including: 1) protein profiles by electrophoresis on SDS-PAGE and staining by silver nitrate; 2) Western blotting with several monoclonal antibodies; 3) determination of protein concentration; 4) assay for endotoxin; and 5) culturing.
Studies of Skin-'Test Antigens in Vitro. We have conducted studies of these new skintest antigens in vitro, together with H.M. Dockrell, R.E. Wier and K. Britton of the Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, U. K., R. Hussain of the Department of Microbiology, Aga Khan University, Karachi, Pakistan, and W.J. Britton of the Department of Clinical Immunology and P. Roche of the Centenary Institute of Cancer Medicine and Cell Biology of the University of Sydney, Australia. Peripheral blood mononuclear cells obtained from patients with tuberculoid leprosy, patients with lepromatous leprosy who were unresponsive to M. leprae sonicate by lymphocyte proliferation, treated lepromatous patients who reacted to M. leprae sonicate by lymphocyte proliferation, and controls from both endemic and non-endemic areas.
MLSA and MLCwA induced strong proliferation in the majority of the M. leprae responders, LAM induced minimal proliferative responses, and MLSA-LAM induced responses stronger than those to MLSA. The same order of response was observed when IFN-γ was measured in a whole-blood assay. None of the antigens induced detectable secretion of IL-4 or IL-5. Untreated lepromatous patients were anergic to M. leprae, although they often responded with IFN-γ secretion to stimulation by M. hovis BCG or PPD. After prolonged treatment, many of the lepromatous patients responded to MLSA-LAM and MLCwA with a proliferative response and IFN-γ secretion. Thus, both MLSA-LAM and MLCwA share the ability to stimulate a Thl Tcell response, and both antigens are suitable for human application, from this perspective.
Skin Testing in the Guinea Pig. The potency of these antigens was also tested on outbred guinea pigs of the Hartley strain, that had been sensitized by injection with 200-500 pg autoclaved M. leprae suspended in Freund's incomplete adjuvant administered at the base of the neck. After 6-8 weeks, dorsal skin-test sites were injected, and two diagonals of the areas of induration were measured by calipers after 24 and 48 hours. The results demonstrated that MLSA and MLSA-LAM in concentrations of 0.1 pg per ml elicited equal delayed-type hypersensitivity (DTH) responses. ML-CwA elicited a response at a concentration of 0.04 pg per ml, whereas neither MLSA nor MLSA-LAM elicited responses at this concentration. The antigens must stimulate a DTH response when administered in a concentration of 0.1 pg per ml, in order to pass quality control.
The stability of the antigens was also studied. Each antigen was diluted with PBS to a concentration of 100 µg protein per ml diluent, filtered and sterilized. Each preparation was stored at -70" (the controls), 4°, 37º and 56"C. Forty-five days after manufacture, the preparations were analysed for potency in sensitized guinea pigs as described, in concentrations of 0.1 and 1.0 pg protein per ml. The results were very encouraging, in that no loss of potency was observed; thus, it should be easy to work with these materials in the field.
Phase I Trials. Once these antigens have been approved by the FDA for use in man, we will proceed to Phase I clinical testing, to be conducted under the direction of G. P. Walsh at the Leonard Wood Memorial Leprosy Research Center, Cebu, the Philippines, an area endemic for leprosy, and in Santander, the Philippines, a non-endemic area, according to protocols that have already been approved by the responsible human research committees. Phase I testing will also be carried out in Colorado.
Phase II and Phase III Trials. Assuming a satisfactory outcome from the Phase I trials, we are planning Phase II and Phase III trials to be carried out by M.C.V. Pessolani of the Oswaldo Cruz Foundation in Rio de Janeiro, Brazil, by W. Britton, P. Roche, R. Weir, R. Hussain and H. Dockrell in Anandaban, Nepal, by M.D. Gupte in Madras, India, and by P. Fine in Malawi.
Toward More Specific, More Sensitive Skin-Test Antigens. We are not yet confident that the reductionist approach is the best way to achieve a more sensitive and more specific antigen. Certainly, a remarkable number of individual proteins of M. leprae, that have been resolved, characterized and expressed in recombinant form, are available for skin testing.
Prevalence of Antibodies to Phenolic
Glycolipid I Among School Children in
Populations in which Leprosy is
Endemic (PRK)
A study was conducted in three Indonesian school districts (Manado, Maros and Muna) with differing prevalence rates to determine whether the prevalence of anti-PGL-I antibodies, which are specific for M. leprae, measured by the MLPA test in school children 10-12 years of age, is an index of the the prevalence of leprosy in the community. A total of 1996 fifth-grade pupils were examined clinically, and serum was collected from 1726 of them.
As shown in The Table, prevalence rates of leprosy in 1994 were 7:10,000 and 5.7:10,000 in Manado and 0.46 Maros districts, respectively. Anti-PGL-I antibodies were detected in 26.3 per cent of the 498 children examined in 31 schools in Manado district, and in 27.9 per cent of 649 students from 40 schools in Maros district, rates that were not significantly different (p = 0.55). The distribution of seropositivity was not homogeneous in these two districts, but varied from village to village (p < 0.05); however, no relation was detected between the rate of seropositivity among the villages and the cumulative leprosy prevalence of the last five years. Leprosy prevalence in Muna district was only 0.46:10,000 in 1994; anti-PGL-I antibodies were detected in only 7.1 per cent of 579 school children examined in 37 schools in this district, a rate significantly lower (p < 0.001) than those in the first two districts.
Thus, the prevalence of anti-PGL-I antibodies among school children may be a useful index of the prevalence of leprosy at the district level.
Serological tests (SNC)
Studies have been carried out of serological tests based on antigens of M. leprae and of host nerves.
Identification of Individuals with a High Leprosy. Even after the goal of eliminating leprosy has been achieved, pockets characterized by a high prevalence of leprosy may be expected to remain. In such situations, serological tests could be employed to identify those among household and community contacts who are at high risk of leprosy. Particularly, longitudinal studies employing repeated testing by antigens specific for M. leprae, such as PGL-1 and protein antigens, should identify those at risk. In a case-control study, household contacts of leprosy patients-mostly multibacillary-were examined clinically and for anti-PGL-I antibodies every six months for four years. Of the 29 contacts who developed leprosy in the course of the study, 14 developed indeterminate (1), BL or LL leprosy, whereas 15 developed TT or BT leprosy. All of the patients who developed I, BL or LL leprosy demonstrated an increase of the titer of anti-PGL-I antibodies in the course of the study, whereas only 5 of the 15 TT or BT patients demonstrated an increase; two showed a constant low concentration of antibodies, and the remaining 8 were seronegative throughout the study (p < 0.001). Thus, in hyperendemic areas, longitudinal serological monitoring of household and community contacts may assist in the early diagnosis of patients with multibacillary leprosy, the major source of M. leprae in the cimmunity. Of 29 contacts who did not develop leprosy in the course of the study, 6 demonstrated an increase of antibody titer. Because the incubation period of leprosy is so long, clinical leprosy may yet develop among these last 29 contacts.
Other Applications of Serological Testing. Because the presence of M. leprae specific antibodies indicates infection by the organism, the prevalence of antibodies, particularly among the younger members of the community, may reflect the extent of active transmission of M. leprae in the community. If this is so, it may be possible to measure the effectiveness of a leprosy control program by monitoring the prevalence among school children of antibodies directed against PGL-I, for example.
Serological tests, based on both antigenand antibody-detection, may also be useful in monitoring the effectiveness of chemotherapy, as a alternative to the skin smear, which may be unreliable. Studies have indicated that the titer of circulating PGL-I antigen declines rapidly during MDT; titers of anti-PGL-I antibody also decline, but at a slower rate. In addition, serological monitoring may be useful in early detection of relapse among those released from treatment.
Finally, although evidence of an association between anti-neural antibodies and nerve damage has been found only in experimental leprosy in the mangabey monkey, serological tests based on neural antigens should be explored for their potential to predict nerve damage, thus providing the opportunity to intervene to prevent further damage.
REFERENCES
1. DHARMENDRA. The immunological skin tests in leprosy: the isolation of a protein antigen of Mycobacterium leprae. Indian J. Med. Res. 30(1942)1-7.
2. FERNANDEZ, J. M. M. The early reaction induced by lepromin. Int. J. Lepr. 8(1940)1-14.
3. HUNTER, W. W., MCNEIL, M., MODLIN, R. L., MEHRA, V., BLOOM, B. R. AND BRENNAN, P. J. Isolation and characterization of the highly immunogenic cell wall-associated protein of Mycobacterium leprae. J. Immunol. 142(1989)2864-2872.
4. MELANCON-KAPLAN, J., HUNTER, S. W., MCNEIL, M., STEWART, C, MODLIN, R. L., REA, T. H., CONVIT, J., SALGAME, P., MEHRA, V., BLOOM, B. R. and BRENNAN, P. J. Immunological significance of Mycobacterium leprae cell walls. Proc. Natl. Acad. Sci. USA 85(1988)1917-1921.
5. MEYERS, W. M., KVERNES, S. and BINFORD, C. H. Comparison of reaction to human and armadillo lepromin in leprosy. Int. J. Lepr. 43(1975)218-225.
6. MILLAR, J. W. Comparison in leprosy patients of Fernandez and Mitsuda reactions using human and armadillo antigens. A double-blind study. Int. J. Lepr. 43(1975) 226-233.
7. MITSUDA, K. On the value of a skin reaction to a suspension of leprosy nodules. Jap. J. Dermatol. Urol. 19(1919)697-708.
8. NOORDEEN, S. K. Elimination of leprosy as a public health problem. Int. J. Lepr. 62(1994)278-283.
9. NOORDEEN, S. K. Elimination of leprosy as a public health problem; why the optimism is justified. Int. J. Lepr. 63(1995)559-566.
10. WORLD HEALTH ORGANIZATION. Report of the third meeting of the Scientific Working Group on the Immunology of Leprosy (IMMLEP). Protocol 3/77. WHO DOCUMENT TDR/SWG/IMMLEP (3)/77, p. 20.
11. WORLD HEALTH ORGANIZATION. Testing of purified armadillo-derived M. leprae in man. Document finalized by the IMMLEP Steering Committee at its meeting 10-12 June 1981. WHO DOCUMENT TDR/IMMLEP/SC/TEST/81.1.
12. WORLD HEALTH ORGANIZATION. Weekly Epidemiological Record No. 25(1995)177-182.
13. WORLD HEALTH ORGANIZATION. Weekly Epidemiological Record No. 26(1995)185-188.
THE CONTRIBUTION OF SEROLOGICAL TESTS TO LEPROSY CONTROL
P.R. Klatser1; S.-N. Cho2; Patrick J. Brennan3
1. Royal Tropical Institute, Amsterdam, The Netherlands.
2. Yonsei University, Seoul, Korea.
3. Colorado State, University, Fort Collins, Colorado, U.S.A.
Mycobacterium leprae is the causative agent of leprosy, a disease that at present affects 1.3 million people in the world, who are currently undergoing treatment; the estimated number of cases in the world is 1.8 million (21). The disease can cause permanent and extensive deformities of the skin and peripheral nerves, resulting in a variety of physical impairments. Because of the severe disabilities that may result, and because of the social stigmatization and economic losses resulting from the disabilities, the problem of leprosy is greater than the number of patients might suggest.
The goal of the World Health Organization (WHO) to eliminate leprosy as a public health problem- i.e., to reduce prevalence of the disease to no greater than 1 per 10,000 in any area-by the year 2000 may be achieved (21). However, this will not signify the end of the problem; because of its long incubation period, many more new patients may be expected to emerge after the year 2000 in those areas in which leprosy has been endemic. It is in this situation that serological tests for leprosy may contribute to control of the disease, providing these tests are rapid, simple and cost-effective.
In principle, serological tests for detecting antibodies to M. leprae would be useful in making an early diagnosis, in monitoring the effectiveness of chemotherapy, in detecting relapse early, and in identifying patients at high risk of developing reactions during therapy. In addition, assuming that the prevalence of scropositivity in a population reflects infection rates, the effects of control measures might be evaluated by repeated serological screening.
Provided that detection of species-specific antibodies indicates infection with M. leprae, serological assays would greatly advance our understanding of the epidemiology of leprosy. Identification of those subclinically infected who are prone to develop the disease and become themselves infective would open the possibility of preventive treatment during the incubation period, perhaps another route toward improved control of leprosy.
Serological tests
Tests have been described that detect antibodies to a species-specific determinant on phenolic glycolipid-1 (PGL-I) (5), and the 18kDa (2), 35 kDa (17) and the 36 kDa(15) protein antigens. Because the tests that detect antibodies directed against PGL-I have been evaluated most extensively, these are described here in greater detail. The tests are based upon detection of antibodies of the IgM class against the immunodominant 3,6-di-O-methyl-glucopyranosyl residue of the trisaccharide component of PGL-I. Most test systems use semi-synthetic analogs, consisting of the terminal and penultimate sugar residues of PGL-I coupled to bovine serum albumin as carrier protein (5, 18). The analog is used most often in an enzyme-linked immunosorbent assay (ELISA), in which binding of antibodies to the antigen, which has been fixed onto a polystyrene microtiter plate is revealed by adding first an enzyme-conjugate and then a substrate. The substrate is converted by the enzyme to a colored product. A commercially produced particle-agglutination test is available (14); this consists of agglutination of gelatin beads coated with a layer of synthetic trisaccharide by antibodies directed against PGL-1 in the serum. This test is simpler to perform than ELISA, and suitable for less well equipped laboratories. More recently, a simple "dipstick" for detecting antibodies to PGL-I has been developed (P.R.K., unpublished results); this test, which requires no equipment, and employs highly stable reagents that do not require refrigeration, is simple and rapid, and would be the procedure of choice in the field.
Early diagnosis
In general, the specificity and sensitivity of these tests is determined by comparing the results from patients' sera with those from healthy controls. In this way, a specificity of approximately 98% was found, with sensitivity ranging from 80 to 100% for detection of patients with multibacillary (MB) leprosy, and from 30 to 60% for detection of patients with paucibacillary (PB) leprosy. The differences between the rates of seropositivity in sera from non-endemic and from endemic areas may reflect subclinical infection (7). The variation of sensitivity from report to report may best be attributed to differences among the groups under study, and to differences of clinical and therapeutic status of the patients. Thus, serological assays do not reflect all clinical infections. Household contacts and family members of patients can also give positive results in serological tests, probably the result of subclinical infection with M. leprae, further complicating the diagnostic value of the serological tests. Detection of antibodies indicates present or past infection with M. leprae, whether or not there are clinical signs. It should be clear that serological testing is only useful for diagnosis when the results are considered together with other diagnostic information (20).
Several studies have shown that serological tests can be employed to identify individuals who have a high risk of developing clinical (notably MB) leprosy among household and community contacts (7, 10). Particularly, repeated testing over time increases markedly the specificity for early detection of people who develop lepromatous leprosy. A recent prospective study, in which about 600 household contacts were monitored for 5-7 years demonstrated that the contacts who were seropositive to PGL-I had an odds ratio of 65.4 of developing MB leprosy compared to the seronegative contacts (9).
Prognosis
During treatment of patients with MB leprosy, the antibody titers generally decrease, in parallel with the decrease of the bacterial load, measured by the bacterial index (BI) (11, 16). A significant correlation between the serological values and the BI has been demonstrated (11, 16). Nonetheless, some patients with a low BI have a high serological value and vice versa. The BI, measured in skin smears or biopsy specimens, gives an indication of the number of M. leprae in the skin; it is assumed that antibody titers give a better indication of the total number of organisms borne by the patient than does the BI (16). Therefore, serological tests can provide a useful additional quantitative measure of the effect of therapy in patients with MB leprosy.
An increasing titer of antibodies directed against PGL-I precedes relapse in a number of treated patients (4), and the changes of antibody titer can apparently serve to predict relapse. The formation of antibodies can, however, be suppressed in patients treated with immunosuppressive drugs for reversal reaction (4). Changes of antibody titers may also be useful in predicting reactions in patients undergoing chemotherapy. There is evidence that the titer of anti-PGL-I antibodies decreases significantly before episodes of ENL (1) and reversal reaction (6) become manifest. The PGL-I-based serological tests will thus make it possible to intervene with appropriate drugs in order to prevent relapse or leprosy reactions.
Epidemiology
The employment of serological assays based upon detection of antibodies to species-specific antigens of M. leprae has opened new possibilities for study of infection by the organism. Although results differ from study to study, in general, the rate of seropositivity has been found to be higher among household contacts of leprosy patients than among non-contacts (8, 10, 18). In addition, the rate of seropositivity is higher among healthy individuals from leprosy-endemic areas than among those from non-endemic areas (7). Although these studies suggest that the presence of antibodies reflects infection with M. leprae, a number of findings are inconsistent with the notion that scropositivity can be taken simply as evidence of infection. Distribution of antibody titers in general populations are unirather than bimodal (12, 19). It is thus likely that antibody titers are associated with the degree of exposure to M. leprae. Serological assays do not detect all clinically-apparent infections, because the majority of PB patients do not demonstrate a humoral response. Follow-up studies have shown that the risk of developing leprosy is much higher among seropositive than among seronegative individuals, but new cases of leprosy arise from among the seronegatives (19), suggesting that not all subclinical infections are reflected by a serological response.
Thus, as a tool for detecting subclinical infection leading to disease, serological testing lacks both sensitivity and specificity. Nevertheless, serological testing may yield valuable information on the extent of infection in a population and on transmission of the infection (7). Assuming that the prevalence of scropositivity in a population roughly reflects rates of exposure or infection, the effects of control measures may be evaluated by repeated serological testing. Recently, it was shown that determination of the prevalence of antibodies to PGL-I in the course of school surveys may be a useful indicator of the true leprosy prevalence at the district level in a leprosy-endemic area of Indonesia (P.K.R., accompanying paper).
Conclusion
Serology of leprosy is clearly useful, although in a limited way. Serological tests can provide support for the diagnosis of the disease, especially in its early stages, as well as for follow-up after completion of treatment, for early detection of relapse, especially to distinguish relapse from reversal reaction. However, it must be emphasized that the serological results must be interpreted in combination with other diagnostic information.
An operational function for serology within the leprosy control services certainly requires a more simple test system than ELISA. The recently developed dipstick for detecting anti-PGL-I antibodies is a step toward this goal.
Seroepidemiological studies have increased our knowledge of the epidemiology of M. leprae infection, lending strength to the notion that M. leprae infection is more prevalent than is leprosy. Serological testing of populations may be of value in monitoring changes of the intensity of M. leprae infection, representing thereby a rapid method to establish the effects of control measures.
REFERENCES
1. ANDREOLI, A., BRETT, S. J., DRAPER, P., PAYNE, S. N. and ROOK, G. A. Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum. Int. J. Lepr. 52(1985)211-217.
2. BRITTON, W. J., HELLQVIST, L. BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
3. CHATTERJEE, D., Clio, S.-N., BRENNAN, P. J. and ASPINALL, G. O. Chemical synthesis and seroreactivity of 0-(3,6-di-O-methyl-D-glucopyranosyl(1 ->4)- 0-2,3-di-O-methyl-L-rhamnopyranosy I(l->9)-oxynonanoyl-bovine serum albumin- the leprosy-specific, natural disaccharide-octylneoglycoprotein. Carbohydr. Res. 156(1986)39-56.
4. CHIN-A-LIEN, R. A. M., FABER, W. R., VAN RENS, M. M, LEIKER, D. L., NAAFS, B. and KLATSER, P. R. Follow-up of multibacillary leprosy patients after discontinuation of treatment using PGL-I ELISA. Are increasing ELISA values indicative of relapse? Lepr. Rev. 63(1992)21 -27.
5. CHO, S.-N., FUJIWARA, T., HUNTER, S, w., REA, T. H., GELBER, R. H. and BRENNAN, P. J. Use of an artificial antigen containing the 3,6-di-O-methylbeta-D- glucopyranosyl epitope for the serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
6. CHO, S.-N., KIM, J. D., CELLONA, R. V., BALAGON, M. V. F., VILLAHERMOSA, L. G., FAJARDO, T. T., ABALOS, R. M, WALSH, G. P., THOLE, J. and BRENNAN, P. J. Association of antibodies to Mycobacterium leprae and nerve antigens with lepra reactions in leprosy patients. Proceedings, 30th U.S.-Japan Joint Conference on Tuberculosis and Leprosy, July 19-21, 1996, Fort Collins, Colorado, pp. 137-141.
7. CHO, S.-N., KIM, S. H., CELLONA, R. V., CHAN, G. P., FAJARDO, T. T, WALSH, G. P. and KIM, J. D. Prevalence of IgM antibodies to phenolic glycolipid I among household contacts and controls in Korea and the Philippines. Lepr. Rev. 63(1992)12-20.
8. DOUGLAS, J. T, CELLONA, R. V., ABALOS, R. M.,MADARANO, M G. and FAJARDO, T. T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Cebu, the Philippines. Int. J. Lepr. 55(1987)18-21.
9. DOUGLAS, J., CELLONA, R., A BALOS, R., FAIARDO, T., KLATSER, P. and WALSH, G. Prospective study on the early detection of leprosy in household contacts in Cebu. Abstracts, 96th General Meeting of the American Society for Microbiology, May 19-23, 1996, New Orleans, Louisiana, p. 129.
10. DOUGLAS, J. T., HIRSCH, D. S., FAJARDO, T. T., CELLONA, R. V., A BALOS, R. M., DELA CRUZ, E. C, MADARANG, M. G., DE WIT, M. Y. L. and KLATSER, P. R. Evaluation of Mycobacterium leprae antigens in the serological monitoring of a clofazimine based chemotherapeutic study of dapsone resistant lepromatous leprosy in Cebu, Philippines. Lepr. Rev. 60(1989)8-19.
11. DOUGLAS, J. T., STEVEN, L. M., HIRSCH, D. S., FU-JIWARA, T., NELSON, K. E., MADARANC;, M. G. and CELLONA, R. V. Evaluation of four semi-synthetic Mycobacterium leprae antigens with sera from healthy populations in endemic and non-endemic areas. Lepr. Rev. 63(1992)199-210.
12. FINE, P. E. M., PONNIGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. C. M. Seroepidemiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988) 243-254.
13. FUJIWARA, T. and IZUMI, S. Synthesis of the neoglycoconjugates of phenolic glycolipid-related trisaccharides for the serodiagnosis of leprosy. Agric. Biol. Chem. 51(1987)2537-2539.
14. IZUMI, S., FUJIWARA, T., IKEDA, M., NLSHIMURA, Y, SUGIYAMA, K. and KAWATSU, K. Novel gelatin particle agglutination tests for serodiagnosis of leprosy in the field. J. Clin. Microbiol. 28(1990)525-529.
15. KLATSER, P. R., DE WIT, M. Y. L. and KOLK, A. H. J. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
16. KLATSER, P. R., DE WIT, M. Y. L., FAJARDO, T. T., CELLONA, R. V., ABALOS, R. M., DELA CRUZ, E. C, MADARANG, M. G., HIRSCH, D. S. and DOUGLAS, J.T. Evaluation of Mycobacterium leprae antigens in the monitoring of a dapsone-based chemotherapy of previously untreated lepromatous patients in Cebu, Philippines. Lepr. Rev. 60(1989)178-186.
17. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by a monoclonal antibody-based immunoassay. Int. J. Lepr. 53(1985)33-38.
18. SOEBONO, H. and KLATSER, P. R. A seroepidemiological study of leprosy in high- and low-endemic Indonesian villages. Int. J. Lepr. 59(1991)416-425.
19. ULRICH, M., SMITH, P. G., SAMPSON, C, ZUNIGA, M., CENTENO, M., GARCIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to native glycolipid-I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-415.
20. VANDER WILLIGEN, A. H., CHIN-A-LIEN, R. A. M., VANJOOST, T. H., STOLZ, E. and NAAES, B. Lepra, je moet et aan denken! Ned. Tijdschr. Geneeskd. 133(1989)1345-1347.
21. WORLD HEALTH ORGANIZATION. Progress towards the elimination of leprosy as a public health problem. Weekly Epidemiological Record 68(1993)181-186.
Discussion of papers by Drs. Brennan, Klatzer and Cho
Dr. Dockrell: Although the skin-test antigens described by Dr. Brennan have not yet been tested in man, we appear, on the basis of the results from assays in vitro, to have increased recognition of these antigens by T-cells, by removal of carbohydrate and lipid components. About 87 per cent of TT patients reacted to these new antigens, compared to about 70 per cent who reacted to MLSA. It's interesting that MB patients reacted equally to MLSA and to the new antigens. We've been working with wholeblood assays, because we believe these simpler assays might be taken into the field, and be performed in parallel with the skin tests.
Prof. Britton: Do you see an increase of skin-test reactivity in the guinea pig to the antigen after removal of carbohydrate?
Dr. Brennan: Yes, we see a great increase of sensitivity in the guinea pig.
Dr. Pannikar: Do any of these tests discriminate between normals and PB patients? We have much less difficulty recognizing MB patients on clinical grounds.
Dr. Naafs: The lesions of MB patients are full of PGL-I, whereas those of PB patients don't contain the glycolipid; this may explain why we don't find anti-PGL-I antibodies in PB leprosy. On the other hand, the granulomas of patients with PB leprosy and those with sarcoid contain human HSP60, which is homologous to a degree with the HSP65 of M. leprae. It may be that the titers of antibodies to these antigens will enable discrimination between normals and PB patients-at least those that produce granulomas.
Dr. Smith: It appears much easier to validate diagnostic tests than it would be to validate tests of infection. How should we validate tests of infection.
Prof. Britton: One way to do this might be to compare endemic with non-endemic populations.
Dr. Klatser. It appears to me that, just as serological tests are much more likely to be positive in MB than in PB patients, the opposite is likely to be the case with respect to skin tests. Is infection MB or PB? Must a test of infection recognize 100 per cent of all patients?
Dr. Gupte: Validation of tests for infection will require surveillance without treatment for a long period of a population, in which both skin- and serological tests are performed repeatedly.
GENOME SEQUENCING AND ITS POTENTIAL APPLICATIONS
D. Young, Department of Microbiology, Wright Fleming Institute, St. Mary's Hospital Medical School, London, U.K.
Genome sequencing represents an essential step in achieving an understanding of microbial pathogenesis. In the 21st Century, establishment of the genome sequence of a pathogen will have a role analogous to that of taxonomy in the 20th Century. The genome sequence will represent the basic textbook for future research. In the context of the elimination of leprosy, several areas may benefit directly from the program of sequencing the genome of Mycobacterium leprae.
Strain typing
Bovine tuberculosis has been eradicated from Australia, but not from New Zealand. The reason for this difference appears to be that, in the final stages of an eradication campaign, the existence of an environmental reservoir of a human pathogen becomes increasingly important. In New Zealand, M. bovis continues to be spread through the population of wild opossums. The feasibility of the eradication of leprosy will depend upon knowing whether M. leprae is transmitted only from man to man, or whether there exists an environmental or feral animal reservoir of the organism.
Recently developed molecular genetic tools for typing M. tuberculosis and M. bovis have been particularly useful in studying the transmission of these organisms, and may be useful in the case of M. leprae. It will first be necessary to develop methods for typing strains of M. leprae, based on PCR rather than on chromosomal DNA. For example, it is likely that, from the sequence of the genome, we will be able to identify repetitive sequences that can be predicted to display strain polymorphism- sequences analogous to the DR repeat of M. tuberculosis, or to the microsatellites of mammalian DNA. These sequences may provide the basis of systems for typing strains, that could be used to monitor transmission of M. leprae, and to evaluate putative environmental isolates of the organism.
Skin-test reagents
Improved skin tests capable of identifying definitively individuals infected with M. leprae would be of considerable use in providing an early assessment of an intervention, such as MDT, designed to interrupt transmission of M. leprae. Such tests may also be useful in determining whether leprosy is generally a "primary" disease-one that progresses directly from infection, or whether it is a reactivation disease, in which case the M. leprae infected individual carries a subclinical infection for many years before becoming ill. For such a test, it will be necessary to identify the M. leprae specific antigens that trigger an immune response in a broad section of the population. By comparison of the genome sequence of M. leprae with that of M. tuberculosis, and by using powerful algorithms for identification of immunologically important motifs, it will be possible to identify the peptides or whole proteins that fit this requirement.
Vaccines for prevention of leprosy
An early immune response to antigens secreted by living Mycobacteria may be associated with protective immune responses. By analysis of the genome sequence of M. leprae, it will be possible to identify open reading frames encoding secreted proteins, based on the presence of a signal sequence. Such an analysis will provide candidate antigens for subunit vaccines, and also for potentially useful skin-test reagents.
Culture of M. lepraeand pathogenesis of nerve damage
By comparison of genome sequences of M. leprae with those of other Mycobacteria, and by moving genes among different Mycobacteria, it may be possible to identify genetic factors that underly the failure of M. leprae to grow in cell-free media, and to identify the genes responsible for its unique propensity to cause nerve damage.
THE ROLE OF INFLAMMATORY CYTOKINES IN THE TISSUE INJURY OF LEPROSY
E.N. Sarno and E.P. Sampaio, Oswaldo Cruz Institute, Rio de Janeiro, Brazil
Leprosy polyneuropathy is the most common treatable neuropathy in the world. Nevertheless, one third of all patients with leprosy may develop nerve lesions, often progressive and irreversible, even after chemotherapy (24). Nerve damage occurs across the entire spectrum of the disease, but is more often seen among patients with multibacillary (MB) leprosy, particularly among those with borderline leprosy. In some cases, irreversible nerve damage progresses insidiously for prolonged periods, in marked contrast to the acute or subacute injury to peripheral nerves that occurs in the course of reactions occurring during chemotherapy.
Reactions are seen in all forms of leprosy(2, 25). These acute episodes of inflammatory responses are associated with changes of immunological reactivity. Recent reports have strongly suggested that immunological reactivity occurs both in reversal reaction (RR) and, at least to some extent, in erythema nodosum leprosum (ENL) (7, 9). Although the clinical aspects of reactions are well-known to specialists, lack of a standard classification for use in the field, as well as the confusing nomenclature adopted by different schools of specialists, have made it difficult to carry out reliable analyses of incidence rates in endemic areas (8). Moreover, no standard system for grading nerve damage has yet been adopted, and even when an evaluation is performed by a health-care service, the accuracy of the data available from long-term studies may be open to question (20).
The objectives of our study were to investigate the role of inflammatory cytokines in the process of the nerve injury encountered in MB leprosy, and to determine if there is, in fact, an increased incidence of nerve damage among patients who suffer reactions. To reach these objectives, three strategies were adopted: 1) to investigate the possibility of a correlation between reactions and impairments; 2) to attempt to detect overproduction of tumor necrosis factor alpha (TNFα) during reactions; and 3) to demonstrate a correlation between high levels of TNFα and nerve damage.
As a first step, a retrospective cohort of patients was organized from our Outpatient Unit Databank, which has been managed since the early 1980s by a team of health workers (dermatologists, physiotherapists, microbiologists, etc.,) trained in multidrug therapy (MDT), from our National Reference Center. One hundred sixty-nine MB patients with bacterial index (BI) ranging from 1-5+, who had undergone 2-year fixed-duration MDT (the regimen recommended by the World Health Organization) between 1986 and 1991, were selected. All had completed chemotherapy within 2 years, and not one has experienced relapse. Reactions that occurred during MDT were classified as ENL or RR and treated by standardized physiotherapeutic interventions and recommended drug-regimens- corticosteroids and thalidomide-whenever necessary. The grade of disability (GD) was evaluated before and after treatment. The resulting data were analysed by analysis of variance (ANOVA), by means of the Epiinfo v.6.02 program (manuscript in preparation).
One hundred (59 per cent) of the patients experienced reactions during MDT-51 had ENL and 49 had RR. The distribution of ENL and RR among the clinical types of leprosy (BB, BL and LL) showed a significant predominance of RR among BB patients (67 per cent) and of ENL among LL patients (55 per cent). In this cohort, no correlation was observed between reactions during MDT on the one hand, and sex, age, BI or GD on the other. However, when we analyzed only those patients who began MDT with GD = 0 (70 patients), a meaningful correlation between reaction and development of disability was detected, most patients exhibiting RR (manuscript submitted). Analysis of the data retrieved from our files led us to determine that the risk of developing disability was 3 times greater among those patients who suffered reactions, and that RR was critical in this respect. In addition, it appeared that the most effective preventive measure against nerve impairment was early diagnosis.
Over the past few years, many of our studies as well as those of others have confirmed that TNFα is overproduced during reactions, and is a key mediator of systemic symptoms and of the tissue damage of leprosy, during both RR and ENL (11, 12). Extremely high concentrations of circulating TNFα (> 5000 pg per ml) were measured in some patients with ENL, in the absence of a life-threatening or fatal outcome (18). Both thalidomide and corticosteroids decrease the concentration of TNFα in the sera of some patients. Serum concentrations of TNFα were also found to be higher before treatment than at completion of MDT among some patients with no clinical evidence of reaction at diagnosis (15). These same patients presented systemic inflammatory manifestations-fever, malaise, influenza-like symptoms, lymphadenopathy, edema, etc., -with no signs of reaction in the skin. In patients with MB leprosy, intradermal injections of recombinant interferon gamma (IFNγ) induced ENL, in keeping with reports stating that ENL had been triggered by vaccination, PPD skin-tests or other immune stimulation (14, 16).
Experiments in vitro have also provided new information. Mycobacterium leprae and some of its components induced release of TNFα by human peripheral blood mononuclear cells (PBMC) and monocytes; cells of patients with ENL responded to TNFα agonist stimuli in a manner different from that of other leprosy patients; and the inhibitory effect of thalidomide was found to be specific in enhancing degradation of TNFα mRNA (17). No effect was noted on synthesis of other inflammatory cytokines. In addition to these data, studies employing reverse transcriptase-PCR (RT-PCR) in progress in our laboratory have demonstrated that the PBMC of patients in reaction showed a pattern of cytokine gene expression different from that of patients not in reaction. Patients in reaction expressed primarily TNFα, IL-8, IL-6, GM-CSF, IFNγ and perforin mRNA (manuscript in preparation). We concluded that there is, indeed, a correlation between overproduction of TNFα and reactions. In light of our hypothesis that inflammatory cytokines are directly involved in the process of nerve injury, it is logical to expect that patients with severe nerve damage present increased concentrations of TNFα in the serum. Two basic questions then come to mind: 1) do serum concentrations of inflammatory cytokines correlate with the degree of nerve damage? and 2) are the cytokines and cytokine-specific mRNAs produced locally in the skin and the lesions in the nerves? We proceeded to measure the concentrations of TNFα in the serum of patients with acute nerve injury, basing the clinical diagnosis of nerve damage on spontaneous pain in the nerve.
Since 1991, we have been following a prospective cohort of 114 patients with MB leprosy, all of whom were submitted to a strict clinical and immunological protocol as part of a project sponsored by the World Health Organization (WHO). The protocol allowed for close observation of each patient on an individual basis, and gave us an opportunity to collect sequential blood and tissue samples, beginning before the start of chemotherapy. None of these patients had been included in the retrospective cohort mentioned earlier. The patients were instructed to return to the clinic at the onset of a reaction. During monthly visits to the center, for the costs of which the patients were reimbursed, they were examined by a dermatologist, to detect early symptoms of reaction, and treatment with steroids or thalidomide was begun early, whenever necessary. Therefore, we believe that all reactions were detected.
Among the 114 patients, 83 (73 per cent) exhibited reactions, ranging in severity from very mild to severe. Reactions were more frequent in this cohort than in that described earlier, in whom only 59 per cent suffered reactions, although the two groups were very similar with respect to distribution of the types of leprosy, BI and other parameters, perhaps because the former group was observed more carefully. Forty-one patients developed ENL and 36 developed RR. Neuritis accompanied the reaction in 19 patients with ENL and 6 with RR. Neuritis occurred as an isolated event, without ENL or RR, in 6 additional patients. Nine of the 19 patients who exhibited ENL and neuritis presented with neuritis as their first manifestation, and developed classical ENL only later in the course of chemotherapy. Serum TNFα levels were assayed in 96 MB patients, including 23 patients not in reaction who were assayed at diagnosis, 19 reactional patients who exhibited neuritis during chemotherapy, 35 patients with ENL or RR who did not present neurological complaints, and the 6 MB patients who developed neuritis in the absence of any other evidence of reaction.
The concentrations of TNFα in the circulation were elevated in all of the patients who exhibited reactions, including those patients with isolated neuritis. In both groups, concentrations of TNFα were assayed during a neuritic episode. These patients were treated promptly with steroids, after which their neurological complaints subsided and their serum TNFα concentrations decreased. In general, serum TNFα concentrations were lower among the patients who exhibited RR than among those with ENL (manuscript in preparation). Serum concentrations of TNFα were elevated in all 13 MB patients without reaction who presented systemic symptoms at diagnosis in the absence of clinical evidence of reaction. Finally, levels of TNFα were higher before start of multidrug therapy (MDT) than after its completion (15).
Confirming our earlier data, a correlation between reaction and development of disability was observed. Seven patients-5 with ENL and 2 with RR-demonstrated worsening of the GD at the end of MDT. Four of the 7-one with RR and 3 with ENL-developed foot drop or claw hand in the course of MDT. Elevated levels of TNFα were detected in all of the patients studied. On several patients with reactions, a kinetic study of the concentration of TNFα was carried out. Treatment with pentoxifylline and thalidomide, drugs known to inhibit production of TNFα, reduced serum concentrations of TNFα, and led to clinical improvement of the reactions.
In summary: 1) MB patients suffering from neuritis presented elevated serum concentrations of TNFα, even in the absence of other clinical evidence of reaction; 2) MB patients who suffered from reaction, ENL or RR, demonstrated elevated serum levels of TNFα, even in the absence of neurological complaints; and 3) elevated levels of TNFα were detected among patients whose GD worsened in the course of MDT.
In addition, tissue studies also demonstrated that cytokines play a key role in both types of reaction. TNFα-positive cells, detected immunocytochemically, are a constant finding in biopsy-specimens of the tissue lesions of reactions, although a few such cells can also be detected in non-reactional lesions. Moreover, by semi-quantitative RT-PCR, it was possible to detect differences of expression of the TNFα gene in lesions of the same patient analysed before and at the onset of reaction. Thus, levels of TNFα appear to be increased in the same patient both in vitro, following stimulation of the cells with TNFα agonists, and in vivo (both serum levels of the cytokine and gene expression in situ) (manuscript submitted).
A variety of evidence suggests synergy between IFNγ and TNFα in the lesions of both ENL and RR. There is evidence, for example, of the expression of activation molecules as a consequence of the induction of genes that are normally induced by these cytokines, including ICAM-1 and HLA-DR in keratinocytes and ICAM-1 in endothelial cells. It is now widely accepted that overexpression of integrins on the endothelial surface is one of the first steps in the immuno-inflammatory cascade (5). These events, in association with expression of the activation molecules in circulating leukocytes that are also induced by IFNγ and TNFα, determine, at least partially, the cellular characteristics of the inflammatory infiltrate present in the lesion. Why neutrophils are preferentially activated during ENL remains to be explained; however, variation of the expression of TNF-receptors on leukocytes modulates the autocrine as well as the paracrine effects of TNFα in these cells (3). This may explain the changes of composition of the infiltrate, and the degree of differentiation of mononuclear cells observed at any given moment. It has been observed that these immunological changes promptly regress upon administration of thalidomide or pentoxifylline (11, 19).
How does TNFα induce nerve damage in leprosy? In addition to its action of enhancing the pro-inflammatory activity of leukocytes and endothelial cells, TNF has been implicated as a cytotoxic or myelinotoxic molecule in some experimental systems and diseases of the nervous system of man, of which multiple sclerosis is perhaps the best example (21-23). TNFα exerts a pleiotrophic effect, resulting in death of the oligodendrocyte, a myelin-forming cell, while simultaneously inducing expression of the genes related to the activation state and proliferation of microglia, the analogs in the central nervous system of cells of the monocyte-macrophage lineage. Both effects appear to be mediated through activation of the TNF-receptor (26).
Although the BI is not directly correlated with nerve damage, intraneural M. leprae have been found in all forms of leprosy. Most of the endoneural organisms are found in the cytoplasm of the Schwann cell, although they are also present in macrophages and endothelial cells within the nerve (4). The effect of this long-lasting parasitism within the Schwann cell, a myelinforming cell, needs clarification. Because Schwann cells are functionally similar to oligodendrocytes, with respect to myelinsynthesis, they might express TNFα-receptors in a similar way. Assuming this to be so, one might speculate that TNFα exerts some specific effects on these cells; this might account for some of the intriguing characteristics of triggering and progression of nerve injury in MB leprosy.
We suggest that leprosy is a chronic disease of man in which tissue damage is intimately related to an immunological mechanism that is triggered by M. leprae during reactions. Because of the low aggressivity of this pathogen, immunoinflammatory mediators generated during the infection create their own internal regulation, which ceases only after the patient has been cured of the infection for a long time, or, in some cases, only after complete clearance of the antigen-load. Individual variation among MB patients with respect to production of TNFα must be further investigated (10, 13).
In conclusion, we have demonstrated that reactions are responsible for a three-fold increase of the risk of disability in the course of MDT In addition, we detected elevated TNFα production and TNFα mRNA expression in patients in reaction, especially at the time that neuritis was diagnosed (6). Immunohistological evidence suggested that TNFα was produced within the leprosy lesion, and, during reaction, cooperated in promoting inflammatory reactivity in tissue cells. Finally, it may be argued that TNFα, in addition to acting as a pro-inflammatory molecule, might also act on Schwann cells infected with living M. leprae.
REFERENCES
1. BARNES, P. F., CHATTERJEE, D., BRENNAN, P. J. and REA, T. H. Tumor necrosis factor production in patients with leprosy. Infect. Imnnm. 60(1992)1441-1446.
2. BECX-BLEUMINK, M. and BERHE, D. Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
3. CASSATEI.A, M. A. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16(1995)21-26.
4. DESIKAN, P. and DESIKAN, K. Persistence of lepromatous granuloma in clinically cured cases of leprosy. Int. J. Lepr. 63(1995)417-421.
5. HERTLE, M. D., JONES, P. H., GROVES, R. W., HUDSON, D. L. and WAIT, F. M. Integrin expression by human epidermal keratinocytes can be modulated by interferon- γ, transforming growth factor-p, tumour necrosis factor-β, and culture on a dermal equivalent. J. Invest. Dermatol. 104(1995)260-265.
6. KHANOLKAR-YOUNG, S., RAYMENT, N., BRICKELL, P. M., KATZ, D. R., VINAYAKUMAR, S., COLSTON, M. J. and LOCKWOOD, D. N. J. Tumour necrosis factor-alpha (TNF-α) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin. Exp. Immunol. 99(1995)196-202.
7. LAAL, S., BHUTANI, L. K. and NATN, I. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema! nodosum leprosum. Infect. Immun. 50(1985)887-892.
8. LIENHARDT, C. and FINE, P. E. M. Type 1 reaction, neuritis and disability in leprosy. What is the current epidemiological situation? Lepr. Rev. 65(1994)9-33.
9. LOCKWOOD, D. J., VINAYAKUMAR, S., STANLEY, J. N. A., MCADAM, K. P. W. J. and COLSTON, M. J. Clinical features and outcome of reversal (Type 1) reactions in Hyderabad, India. Int. J. Lepr. 61(1993)8-15.
10. MOLVIG, L., BAEK, L., CHRISTENSEN, P., MANOGUE, K. R., VLASSARA, H., PLATZ, P., NIELSEN, L. S., SVEJGAARD, A. and NERUP J. Endotoxin-stimulaled human monocyte secretion of interleukin I, tumour necrosis factor alpha, and prostaglandin E2 shows stable interindividual differences. Scand. J. Immunol. 27(1988)705-716.
11. MOREIRA, A. L., SAMPAIO, E. P., ZMUIDZINAS, A., FRINDT, P.. SMITH, K. A. and KAPLAN, G. Thalidomide exerts its inhibitor activity on tumor necrosis factor α by enhancing mRNA degredalion. J. Exp. Med. 177(1993)1675-1680.
12. PARIDA. S. K., GRAU, G. E., ZAHEER, S. A. and MUKHERJEE, R. Serum tumor necrosis factor and interleukin 1 in leprosy and during lepra reactions. Clin. Immunol. Immunopathol. 62(1992)23-27.
13. POCIOT, E. BRIANT, L., JOGENEEL, C V., MLOVIG J. WOKSAAI;, H., AniiAi., M., THOMSEN, M., NERI'P, J. and CAMBON-THOMSEN, A. Association of tumor necrosis factor (TNF) and class 11 major histocompatibility complex alleles with the secretion of TNF-α and TNF-β by human mononuclear cells: a possible link to insulin-dependent diabetes mellilus. Eur. J. Immunol. 23(1993)224-231.
14. SAMPAIO, E. P., DUPPRE, N. C., NERY, J. A. C, MOREIRA, A. L. and SARNO, E. N. Development of giant reaction in response to PPD skin lest in lepromatous leprosy patients. Int. J. Lepr. 61(1993)205-213.
15. SAMPAIO. E. P., KAPLAN. G. MIRANDA, A., NERY. J. A. C, MIGUEL, C P., VIANA, S. M. and SAKNO, E. N. The influence of thalidomide on the clinical and immunologic manifestation of erythema nodosum leprosum. J. Infect. Dis. 168(1993)408-414.
16. SAMPAIO. E. P.. MOKLIKA. A. L.. SAKNO. E. N., MALTA, A. M. and KAPLAN, G. Prolonged treatment with recombinant interferon gamma induces erythema nodosum leprosum in leproniatous leprosy patients. J. Exp. Med. 175(1992) 1729-1737.
17. SAMPAIO, E. P., SAKNO. E. N.. GALLH.Y. R., COHN, Z. A. and KAPLAN, G. Thalidomide selectively inhibits tumor necrosis factor α production by stimulated human monocytes. J. Exp. Med. 173(1991)699-703.
18. SARNO, E. N., GKAU, G. E., VIEIRA., L. M. M. and NERY, J. A. C. Serum levels of tumour necrosis factor-alpha and interleukin-β during leprosy reactional states. Clin. Exp. Immunol. 84(1991)103-108.
19. SARNO, E. N.. NERY, J. A. C. GARCIA, C C. and SAMPAIO, E. P. Is pentoxifylline a viable alternative in the treatment of ENL7 Int. J. Lepr. 63(1995)570-571.
20. SCOLLARD, D. M., SMITH. T. BHOOPAT, L.. THEE-TKANONT, C, RANGDAENG, S. and MOKENS, D. M. Epidemiologic characteristics of leprosy reaction. Int. J. Lepr. 62(1994)559-567.
21. SELMAJ, K. W. and RAINC, C S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vino. Ann. Neurol. 23(1988)339-346.
22. SELMAJ, K. W.. RAINE, C. S., CANELLA. B. and BROSNAN, C. F. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J. Clin. Invest. 87(1991)949-954.
23. SHARILI, M. K., PHIL, M. and HENTGES, R. Association between tumor necrosis factor-a and disease progression in patients with multiple sclerosis. N. Eng. J. Med. 325(1991)467-472.
24. SRINIVASAN. H. Deformities and disabilities-unfinished agenda in leprosy work. Lepr. Rev. 66(1995)193-200.
25. VAN BRAKEL. W. H.. KHAWAS. I. B. and LUCAS, S. B. Reactions in leprosy: an epidemiological study of 386 patients in west Nepal. Lepr. Rev. 65(1994)190-203.
26. WILT, S. G. MILWARD, E., ZHOU, J. M.. NAGASATO, K ., PATTON, H., HUSTEN, R.., GRIFFIN, D . E.. O'CONNOR, M. and DUHOIS-DALCQ, M. In vitro evidence for a dual role of tumor necrosis lactor-u in human immunodeficiency virus type I encephalopathy. Ann. Neurol. 37(1995)381-394.
Discussion of Dr. Sarno's paper
Dr. Naafs: Did I understand correctly that the level of TNF-α in the blood is higher during reaction without neuritis than during reaction accompanied by neuritis? Are you implying that, in the presence of neuritis, the quantity of TNF-receptors is greater, and that the receptors remove TNF from the blood, producing nerve damage?
Dr. Sarno: The TNF-α that causes nerve damage is not in the blood, but rather within the nerve. I believe that it is dangerous to draw conclusions from associations between serum concentrations of TNF and nerve damage. We don't know how much TNF is present in the nerve.
Prof. Ji: What are the latest developments with respect to anti-TNF drugs?
Dr. Sarno: During the last five years, there have appeared many reports that thalidomide is able to block production of TNF by blocking synthesis of TNF-α-mRNA.. A second drug is pentoxifylline, which also blocks TNF-production. I have used pentoxifylline in fertile females who suffer from ENL. with good effect.
Dr. Sampaio: I believe that any drug that inhibits production of TNF may be useful in ENL. including non-teratogenic analogs of thalidomide.
Dr. Naafs: There is much nerve damage in reversal reaction, which is not affected by thalidomide, suggesting that TNF-α may not play the major role in producing nerve damage.
Dr. Sarno: Histopathologically, there are major differences between the nerve lesion of ENL and that of reversal reaction. In addition, reversal reaction represents a cellmediated reaction of long duration, and is accompanied, within the nerve, by the release of many mediators in addition to TNF-α.
Dr. Hussain: ENL develops among 80 per cent of MB patients during the first 6 months of treatment. Among these is a group of patients who continue to have multiple episodes of ENL. Do these two groups of patients demonstrate different levels of TNF? Also, can you tell me if ELISA-based and radioimmunoassay-based assays for TNF yield comparable levels?
Dr. Sampaio: In general, levels of TNF are lower after subsequent episodes of ENL than during first episodes. We use an ELISA-based kit, which gives results comparable to those of a biological assay.
IMMUNOPROPHYLAXIS OF LEPROSY-LESSONS F ROM THE TB PROGRAM
D. Young, Department of Microbiology, Wright Fleming Institute, St. Mary's Hospital Medical School, London, U.K.
Growing concern about the global epidemic of tuberculosis, together with opportunities provided by novel molecular approaches, have focused attention on the prospects for developing new vaccines against tuberculosis. At a meeting held in Madrid in 1995, a coordinated strategy for vaccine development was agreed upon by representatives of the World Health Organization, the pharmaceutical industry and major funding bodies, including the U.S. National Institutes of Health and the European Community. The tuberculosis vaccine program provides a useful framework for a discussion of the prospects for vaccines against leprosy. The overall strategy can conveniently be divided into three stages: generation of candidate vaccines, testing in animal models, and evaluation in man.
Generation of new candidate vaccines
Two general approaches are being taken to develop new, anti-mycobacterial vaccines, based on live, attenuated bacteria or on subunit preparations. The strategy of preparing improved live vaccines by manipulation of BCG is clearly relevant to leprosy. BCG already provides some protection against leprosy, and it is reasonable to suggest that addition of key genes of Mycobacterium leprae might result in greater efficacy. The limiting factor is in selection of the appropriate "key" antigens. Intensive research on M. leprae antigens during the 1980s did not result in identification of obvious "protective" antigens. The alternative live vaccine approach relevant to tuberculosis involves genetic manipulation of virulent M. tuberculosis; however, this approach is not possible in the case of the non-cultivable M. leprae. The development of subunit vaccines for leprosy may be feasible, although the problem of antigen selection will need to be solved. For tuberculosis, there is interest in approaches based upon screening the entire genome for protective antigens-for example, by nucleic acid vaccination. However, the lack of a convenient animal model suitable for screening large numbers of candidates precludes use of this approach for leprosy.
Screening in animal models
A range of experimental animal systems is available for screening tuberculosis vaccines. Whereas none of these is considered to provide an exact measure of vaccine efficacy in man, it appears likely that, at this stage, efficacy in animal systems will be an important criterion in the selection of candidates for eventual human trial. The lack of an appropriate animal model represents a fundamental limitation in any program to develop vaccines for use in leprosy. It is possible that non-human primates could be used in the late stages of preclinical evaluation of promising candidate vaccines.
Evaluation in man
It is probable that progress in vaccine development, both for tuberculosis and for leprosy, will depend upon identification of some correlate of protection, that may be used as a preliminary indication of vaccine efficacy in man. Lack of understanding of the mechanisms of protective immunity represents a formidable obstacle to progress in this area. In this context, the situation in leprosy is no worse than that in tuberculosis; in fact, because of the possibilities for immunological analysis provided by the leprosy spectrum, it might be argued that the chances of understanding the mechanisms of protection are greater in leprosy than in tuberculosis. Strategies for identification of suitable correlates of protection are based upon analysis of immune repertoire and immune function-generally as defined by cytokine profiles.
Vaccine profiles
An important discussion in relation to current strategies for vaccine development in tuberculosis is related to the characteristics required of the final product. At least three different vaccine profiles may be considered. The ideal vaccine for use in tuberculosis would replace BCG, being given at birth, and providing life-long protection. Such a vaccine for leprosy would also be ideal, but with the disease coming under control, it would be difficult indeed to set up clinical trials, by which to determine the efficacy of the vaccine, and it would be difficult to justify widespread vaccination economically.
A second profile in the case of tuberculosis involves a "booster" vaccine to be administered to high-risk, young-adult populations, who may already have been administered BCG or infected with M. tuberculosis. This profile appears more appropriate to leprosy, representing a vaccine that might be used in intensive vaccination campaigns among certain selected populations as part of a general eradication program.
The third profile envisages an immunotherapeutic vaccine. In the case of tuberculosis, the goal is to reduce duration of treatment. In leprosy, the goal might be to limit the extent of the disease in individual patients.
Conclusion
Leprosy is an immune-mediated disease, and it is certainly important to consider the prospects for immune-mediated prevention. However, at the present time, there are fundamental gaps in our understanding of mycobacterial pathogenesis that preclude rational vaccine design, and it is difficult to envisage a research program that is directed specifically at development of a leprosy vaccine. Nevertheless, it is important that we be aware of developments in the active area of research toward vaccines for tuberculosis. That BCG has consistently demonstrated efficacy against leprosy suggests that a better leprosy vaccine may actually prove a more feasible goal than a better tuberculosis vaccine.
IMMUNOPROPHYLAXIS AGAINST LEPROSY; THE CASE FOR IMPROVED VACCINES AGAINST LEPROSY W.J. BRITTON, UNIVERSITY OF SYDNEY, SYDNEY, AUSTRALIA
The case for improved vaccines against leprosy rests upon two premises. First, despite successful implementation of multidrug therapy and the "Elimination of Leprosy" campaign to reduce the prevalence of leprosy, complete eradication of the disease will be aided by effective primary prevention of infection. Second, BCG has had a demonstrable impact on the prevalence of leprosy, despite variable results with BCG in formal trials, and a more effective vaccine would be of greater benefit. Development of an improved vaccine depends upon: 1) design of a vaccine more effective than BCG; 2) the ability to monitor the effects of the vaccine in vitro with reliable correlates of a protective action prior to any vaccine trial; and 3) application of the vaccine in field trials, and then as part of the routine program of immunization.
Design of anti-leprosy vaccines
There are four possible approaches to the design of such a vaccine. First, the serendipitous approach is to use another cultivable, non-pathogenic Mycobacterium, such as Mycobacterium "w" or the ICRC bacillus for immunization.
Second, an "avirulent" form of M. leprae could be prepared by inactivation of the genes encoding virulence factors. As the leprosy organism has not been grown in culture, nor have virulence factors been defined, this approach is only theoretical at present.
Third, a subunit vaccine could be prepared, based on one or more M. leprae- specific protein antigens. This is unlikely to succeed, as no single protein antigen is recognized by all M. leprae- infected subjects. Furthermore, T-cell responses in man are directed to multiple and diverse epitopes in one protein, and the dominant T-cell epitopes vary among populations. This is illustrated by the responses of leprosy and tuberculosis patients to the M. leprae 70 kDa (1) and M. tuberculosis 23 and 30 kDa secreted proteins (3, 10).
Fourth, the effect of BCG could be enhanced by: 1) insertion into its genome of genes encoding M. Ieprae- specific antigens; 2) manipulation of the response to BCG by modifications of dose, timing and site of immunization, or by insertion of genes encoding immune-enhancing cytokines; or by 3) boosting BCG-induced memory T-cells to prevent waning of the protective effect of BCG.
The M. leprae antigens to be expressed in BCG should meet the following criteria: 1) they should be relatively specific to M. leprae; 2) they should be frequently recognized by cellular immune responses in contacts and patients with tuberculoid leprosy; and 3) they should induce responses to IFNγ in man, and elicit delayed-type hypersensitivity (DTH) in sensitized animals. Few of the 25 M. leprae proteins that have been characterized (6) meet these criteria, as many have homologs in the "M, tuberculo .v/.v-BCG" complex, or the cellular responses to them have not been studied in detail. A complicating factor is that, in the few comparative studies of cellular responses that have been carried out (7), relatively few contacts or patients with paucibacillary (PB) leprosy recognized recombinant mycobacterial proteins expressed in Escherichia coli. By comparison, larger proportions of subjects recognize native proteins purified from BCG, M. tuberculosis or other cultivable Mycobacteria. For example, recombinant M. tuberculosis 23 kDa protein expressed in M. smegmatis is more immunogenic in assays in vitro that employ human cells and more effective in eliciting DTH in guinea pigs than is the E. coli-derived r23 kDa protein (5). M. leprae antigens to be expressed in BCG include the 18 kDa protein, the putative secreted 25 and 27 kDa proteins (although both have homologs in BCG), the 35 kDa proline-rich protein, and the 45 kDa serine-rich protein (6). M. leprae 35 kDa protein (MMPI). This protein was first recognized by monoclonal antibodies (mAbs) directed to an M. leprae specific determinant on the antigen. Subsequently, an mAb-inhibition assay demonstrated serological responses to the protein in most multibacillary (MB) and one third of PB patients, but not in tuberculosis patients. Independently, the protein was purified from M. leprae, and characterized as membrane-associated. Recently, we cloned the gene for this protein (11) and expressed it in M. smegmatis under control of the pBlaF* high-expression promoter (8), to obtain "native" recombinant antigen for immunological studies. This protein is a candidate for expression in BCG, because: 1) it is not present in M. tuberculosis or BCG, although a homolog with 88 per cent ami no-acid identity is present in M. avium ; 2) immune responses to this antigen across the leprosy spectrum parallel those to M. leprae, with IFN-secreting T-cell responses dominant in contacts and PB patients, and antibody responses in MB patients. In all, 90 per cent of leprosy patients recognized this antigen, whereas only 20 percent of tuberculosis patients from an endemic environment did so (9); and 3) r35 kDa protein elicits DTH in M. leprae -sensitized guinea pigs. Enhanced expression of the protein in BCG was achieved, when the gene that encodes it was expressed under the control of the pBlaF* promoter rather than under the control of its own promoter. Also, rBCG-35 induces immune responses to the encoded antigen in mice. Therefore, rBCG-35 is a potential vaccine for use in preventing leprosy, but significant problems in its development include inadequacy of the M. lep roe-infected mouse to determine if candidate vaccines are superior to BCG, and the regulatory requirements surrounding use of recombinant BCG as vaccines in man.
Boosting Effects of Leprosy Vaccines. One common observation in BCG trials in tuberculosis and leprosy is waning of the protective effect after 10 years. Non-mycobactcrial vectors such as DNA, viral or multivalent subunit vaccines may have a role, either as agents to boost BCG-induced memory T-cells, or to prime Mycobacteria reactive T-cells prior to BCG vaccination. The effect is exemplified by the effect of rBCG-18 in boosting the effect of a vaccinia virus vector that expresses the same M. leprae 18 kDa protein (2).
Monitoring effects of immunization
More defined markers of the cellular immune responses to candidate vaccines are required, and measurements made with these markers should preferably be demonstrated to correlate with protective efficacy in an experimental M. leprae infection. Assays should measure the expansion of M. leprae -specific memory T-cells that produce IFN-γ and other Th I cytokines in response to stimulation. The vaccines should be able to induce DTH to M. leprae -specific skin-test reagents in subjects who have not been previously exposed to the organism. The development of simple, whole-blood assays to measure M. leprae -specific IFN-γ responses may facilitate comparative studies in the field. For example. Weir, el al., found that whole-blood IFN-γ responses were more sensitive lor detecting antigenspecific responses in leprosy patients than were proliferative assays (10). A 24-hour IFN-γ assay used for the diagnosis of bovine tuberculosis (12) has been modified to detect human T-cell responses to PPD and M. tuberculosis-specific secreted proteins (5). A multicenter study to compare whole-blood IFN-γ responses and tuberculin reactivity is currently in progress.
Implementing use of the vaccine
The protective efficacy of any new candidate leprosy vaccines should be tested in an improved animal model before testing is carried out in humans. Eventual application in a field trial would depend upon the outcome of the leprosy vaccine trials currently in progress, and future developments in the epidemiology of leprosy.
REFERENCES
1. ADAMS, E.. BRITTON, W. J., MORGAN, A., SERGEANTSON, S. and BASTEN, A. Individuals from different populations identify multiple and diverse T-cell determinants on mycobacterial HSP70. Stand. J. Immunol. 58(1994)588-594.
2. BAUMGART, K. W., MCKENZIE, K. R.. RADIORD. A. J., RAMSHAW, I. and BRITTON, W. J. Immunogenicity and protection studies with recombinant mycobacteria and vaccinia vectors co-expressing the 18 kDa protein of Mycobacterium leprae. Submitted for publication.
3. ROCHE, P. W.. FENG. C. G. and BRITTON. W. J. Human T-cell epitopes on the Mycobacterium tuberculosis secreted protein MPT64. Scand. J. Immunol., in press.
4. ROCHE, P. W., PEAKE, P.. Fins, T , BILLMAN-JA-COHE, H., DORAN, T. and BRIITON, W. J. T-cell determinants and antibody binding sites on the major secretory protein MPB59 of Mycobacterium bovis. Infect. Immun. 62(1994)5319-5326.
5. ROCHE, R W., WINTER, N., TRICCAS, J. A., FENG, C. G. and BRITTON, W. J. Expression of the Mycobacterium tuberculosis MPT64 in recombinant Myco. smegmatis: purification, immunogenicity and application to skin tests for tuberculosis, Clin. Exp. Immunol. 103(1996)226-232.
6. THOLE, J. E. R., WIELES, B., CLARK-CURTISS, J. E., OTTENHOFF, T. H. M. and RINKE DE WIT, T. F. Immunological and functional characterization of Mycobacterium leprae protein antigens: an overview. Mol. Microbiol. 18(1995)791-800.
7. THOLE, J. E. R., JANSON, A. A. M., KIFLE, A., HOWE, R. C, MCLEAN, K., NURILYGN, A., FILLEY, E., SHANNON, E. J., BULLA, J., HERMANS, J., DE-VRIES, R. R. P., F ROMMEL, D . and RINKE DE WIT, T. F. Analysis of T-cell and B-cell responses to recombinant M. leprae antigens in leprosy patients and healthy contacts: significant T-cell responses to antigens in M. leprae non-responders. Int. J. Lepr. 63(1995)369-80.
8. TIMM, J., PERILLI, M. G., DUEZ, C, TRIAS, J., OR-EFICI, G.. FATTORINI, L., AMICOSANTE, G., ORA-TORE, A., JORIS, B. and FRLRE, J. M. Transcription and expression analysis, using lacZ and phoA gene fusions of Mycobacterium fortuitum fj-lactamase genes cloned from a natural isolate and high-level β-lactamase producer. Mol. Microbiol. 12(1994)491-504.
9. TRICCAS, J. A., ROCHE, P. W., WINTER, N., FENG, C. G., BUTLIN, R. and BRITTON, W. J. A 35 kDa protein is a major target of the human response to Mycobacterium leprae. Submitted for publication.
10. WEIR. R. E., MORGAN, A. R., BRITTON, W. J., BUTLIN, C. R. and DOCKRELL, H. M. Development of a whole blood assay to measure T cell responses to leprosy: a new tool for immunoepidemiological field studies of leprosy immunity. J. Immunol. Methods 176(1994)93-101.
11. WINTER, N., TRICCAS, J., RIVOIRE, B., PESSOI.ANI, M. C, EIGLMIER, K., LIM, E. M., HUNTER, S., BRENNAN, P. J. and BRITTON, W. J.. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol. Microbiol. 16(1995)865-876.
12. WOOD, P. R., CORNER, L. A., ROTHEL, J. S., BAL-DOCK, C, JONES, S. L., COUSINS, D. B., Mc-CORMICK, B. S., FRANCIS, B. R., CREEPER, J. and TWEDDLE, N. B.. Field comparison of the interferon-gamma assay and the intradermal tuberculin test for the diagnosis of bovine tuberculosis. Aust. Vet. J. 68(1991)286-290.
Discussion of Prof. Britton's and Dr. Young's papers
Dr. Klatser. Prof. Britton stated that 20 per cent of tuberculosis patients recognize the 35kDa major protein antigen, which is consistent with dual infection.
Prof. Britton: I was speaking of a country in which both leprosy and tuberculosis are endemic. In this situation, one cannot distinguish between lack of specificity and dual infection. We should test the 35 kDa antigen on tuberculosis patients who live in a country in which leprosy is not endemic.
Dr. Cole: I was intrigued by the dichotomy between responses to M. leprae antigens expressed in E. coli and those to the same antigens expressed in a mycobacterial vector. It raises interesting questions with respect to post-translational modifications and the possibility of contamination by other mycobacterial molecules. What sort of quality control was done?
Prof. Britton: Your point is well taken. With respect to the two antigens we studied, we go to some length to check their purity with monoclonal antibodies, and screen them for other components. Although post-translational modification of some proteins is known to occur, we have no evidence that it occurs with the proteins with which we have worked.
Dr. Naafs: Mycobacteria have been incriminated in a number of autoimmune diseases, and probably also tuberculoid leprosy. When you express mycobacterial proteins, is there not a risk that you will induce an autoimmune process? In addition, what about the possibility that a vaccine employed as immunotherapy may, instead, produce tolerance?
Prof. Britton: Of course, considering your second point first, dosage, timing and route of administration of vaccines are important, particularly if one is attempting to boost vaccine-induced immunity. I think, however, that the way in which our animals were primed and administered BCG did not induce tolerance. I don't think that we need worry about autoimmunity; we can purify proteins so that they are not contaminated by heat-shock proteins.
Dr. Gitpte: Can someone enlighten us with respect to "super" BCG, which has just become newsworthy?
Dr. Young: There is now becoming available a family of engineered BCG vaccines that produce recombinant cytokines, such as IFN-γ, for example, and can potentiate the effect of BCG a bit.
CHEMOTHERAPY OF LEPROSY: PROGRESS SINCE THE ORLANDO CONGRESS, AND PROSPECTS FOR THE FUTURE
Baohong Ji1; Louis Levy2; Jacques H. Grosser
1. Faculté de Médecine Pitie-Salpetriere, Paris, France.
2. Hadassah University Hospital, Jerusalem, Israel.
Since 1982, the use of multidrug therapy (MDT) as a means of achieving control of leprosy has been aggressively promoted by the World Health Organization (WHO). By June, 1995, 76 per cent of the registered leprosy patients in the world had been brought under treatment by MDT, nearly 6.7 million leprosy patients had been cured by the treatment, and the global prevalence rate of leprosy had declined sharply (9). The MDT regimens for multibacillary (MB) and paucibacillary (PB) leprosy have been well tolerated, and adverse reactions have been rare and mild.
The relapse rate among patients with MB leprosy after completion of specific antimicrobial treatment is a crucial parameter in assessing the long term efficacy of chemotherapy. A survey organized by WHO reported 467 relapses among 92,194 MB cases, with an overall relapse rate of 0.23 per 100 patient-years (8), and another survey reported 67 relapses among 20,141 MB cases observed during the period 1984-1992, yielding a cumulative risk of relapse of 0.77 % by the end of 9 years after stopping MDT; the annual relapse rate ranged from 0.01 % to 0.14 % (8). Thus, data from routine leprosy control programs indicate that, after completion of MDT, relapses among MB cases have been very few, well below 1 per 100 patient-years. However, these low relapse rates must be interpreted with caution, because the mean duration of follow-up of the patients is only about 4 years, whereas relapse after treatment by a regimen that includes rifampicin (RMP) may occur late->5 years after stopping treatment.
The Marchoux Chemotherapy Study Group recently reported (3) that, among 35 MB patients who had completed two years of MDT between 1984 and 1986, 7 patients suffered relapse, after a mean duration of 72.7 ± 1.73 months of follow-up. Relapse was defined as an increase of bacterial index (BI) by > 2 + over the previous value from any single old lesion, together with the occurrence of definite new skin lesion(s) demonstrating a higher BI than any preexisting lesion, and was confirmed by the demonstration of viable Mycobacterium leprae by mouse foot-pad inoculation. The overall relapse rate was 20 per cent, or 3.3 per 100 patient-years; the mean incubation period of the 7 relapses, 62.7 ± 18.7 months, confirmed that relapses occurred late after stopping MDT. All of the isolates were susceptible to both RMP and clofazimine (CLO), and the patients responded very well to a second course of MDT.
The most important finding of this study was that relapse was significantly more frequent among the patients with BI > 4 before MDT or > 3 at the end of MDT. That RMPor CLO-resistance was not demonstrated among the strains of M. leprae isolated from the relapsed patients, and that relapse was closely correlated with the bacterial load of the patient, suggest that the relapses were not caused by the emergence of drug resistance, but by viable M. leprae ("persisters") that had survived treatment. Because MB patients with an initial mean BI > 4 are relatively few, the number of relapses in any control programme should be very small. And because the M. leprae remain susceptible to RMP and CLO, and the patients respond well to a second course of MDT, there is no need to modify WHO/ MDT, or to attempt to detect the few patients with mean BI > 4, provided that control programs are aware of the possibility of relapse among those patients with an initially high BI.
Because the WHO/MDT regimens are highly cost-effective, they will continue to be the standard regimens for the treatment of the majority of leprosy patients, even during the post-elimination era. However, new, more effective or operationally less demanding regimens are required for certain situations. It would be very helpful if the duration of treatment for MB leprosy could be significantly shortened, and if the regimens could be simplified, so that treatment could be easily delivered within the general health services. Ideally, all patients would be treated by drugs administered monthly under full supervision. Because skin smears, upon the results of which depends the classification of patients as PB or MB leprosy, are often either unreliable or unavailable, a common regimen for both PB and MB patients would be desirable. For those patients who are unable to attend monthly supervised treatment, a highly intensive, fully supervised, short-course, daily regimen might represent a solution. Those light-skinned patients, to whom CLO is unacceptable because of skin pigmentation, require a safe and effective alternative. Finally, special regimens are required for patients who cannot take RMP because of allergy or intercurrent disease such as chronic hepatitis, or whose M. leprae are resistant to RMP.
Unlike the regimens that have been used widely for control purposes, such as DDS monotherapy or WHO/MDT, new MDT regimens must be developed step-by-step. The effectiveness of the components and their combinations must first be measured in the laboratory. Then, the bactericidal effect of the individual components and their combinations and patients' tolerance to them must be confirmed in clinical trials among MB patients. And, finally, the long term efficacy of the regimens must be examined by field trials, in each of which at least 500 patients per regimen are treated and followed over a period of 5 to 7 years for evidence of relapse.
Recently, three new, very active antileprosy drugs-ofloxacin (OFLO), clarithromycin (CLARI) and minocycline (MINO), all acting by different antimicrobial mechanisms, have become available. Their bactericidal activity against M. leprae has been demonstrated in mice, and confirmed in clinical trials. Employment of these new drugs may make it possible to increase the effectiveness of antileprosy chemotherapy, to shorten its duration, and to develop regimens based upon the monthly administration of the drugs, which may therefore be fully supervised. Finally, regimens that include these drugs may replace CLO in the current regimen, and may be used to treat leprosy patients who cannot benefit from RMP.
Work in nude mice
The many possible combinations of the new drugs with the standard antileprosy drugs cannot all be tested in clinical trial; there are simply not enough suitable patients or qualified institutions in which trials might be carried out. This is the case even for particularly useful regimens, such as those that might be fully supervised and administered monthly, or that might be effective in very short-course therapy. In addition, ethical considerations prohibit the inclusion in clinical trials of important control regimens, such as RMP monotherapy or the combination DDS-CLO, making it difficult to ascertain the role of each component in the combinations to be tested. At the same time, delaying the testing of these combinations would delay by many years the application of truly useful new regimens to the control of loprosy. For these reasons, the Scientific Working Group on Chemotherapy of Leprosy (THELEP) of the UNDP/ World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) decided to carry out experimental studies in mice of various combinations of these drugs.
Earlier, the bactericidal activities of regimens consisting of multiple doses of RMP and the new drugs had been demonstrated to be so powerful that comparison of these regimens was beyond the sensitivity of the M. leprae -infected normal mouse. However, unlike normal mice, in which the maximal inoculum is 104 organisms per foot-pad, multiplication of M. leprae from inocula containing as many organisms as can be inoculated may be recognized in congenitally athymic (nude) mice, because of their immune deficiency. After the infection has been established in nude mice, the mean number of M leprae per g of tissue, and the proportion of viable organisms in the bacterial population are significantly greater than those in normal mice, thereby reducing by 2 to 3 orders of magnitude the minimal proportion of viable M. leprae that can be measured.
In a typical experiment (5), each hind foot-pad of 180 female Swiss nude mice was inoculated with 0.03 ml of a bacterial suspension containing 7.5 x 105 M. leprae. By 12.5 months after inoculation, only 10 mice had died, and 4 mice had been sarificed for enumeration of acid-fast bacilli (AFB). Among the remaining mice, swollen foot-pads containing 107 to 108 AFB per foot-pad were observed in 155, which were selected for the experiment. The mice were randomly allocated to an untreated control group and 12 treated groups. As shown in Table 1, the treatments fall into three categories: intermittent (once every 4 weeks-groups 1, 2, 4, 6, and 7); daily (groups 5 and 8-12); and daily plus intermittent (group 3). The mice of group 3 were administered a regimen modeled on the WHO/MDT regimen for MB leprosy. The longest duration of treatment was 24 weeks for intermittent therapy and 8 weeks for daily therapy.
Bactericidal activity was assessed by comparing the proportion of viable organisms in treated mice with that measured before treatment; the proportion of viable organisms was titrated by subinoculating the bacteria into foot-pads of normal or nude mice, as is done in clinical trials. At regular intervals, AFB were harvested from 4 footpads (2 mice) of each control or treated group, the suspensions prepared from each of the 4 foot-pads were pooled, and the AFB were counted; serial 10-fold dilutions of the pooled suspensions were then made in Hanks' balanced salt solution for subinoculation. Each dilution of each suspension was subinoculated into both hind foot-pads of 6 normal mice or 10 nude mice. The AFB harvested from untreated control mice and from mice that had been administered only a single dose of a regimen were subinoculated only into normal mice; the AFB harvested from mice that had been administered more than a single dose of treatment were subinoculated into both normal and nude mice. The largest inoculum for normal mice was 104 AFB per foot-pad; nude mice were inoculated with 105 AFB per foot-pad or the maximal available inoculum-always > 106 AFB per 0.03 ml. Harvests of M. leprae were carried out from the subinoculated foot-pads 12 months later. In those foot-pads in which the number of AFB inoculated was < 104 the organisms were considered to have multiplied- i.e., the inoculum included viable organisms-if > 105 AFB were harvested; in foot-pads in which the inoculum had been > 105 AFB, the criterion of multiplication was a > 2-fold increase over the number inoculated. A bactericidal effect of the treatment was defined as a significant decrease of the proportion of viable M. leprae in the treated group from the pretreatment value.
The proportion of viable organisms in the suspensions and the significance of their differences were calculated in terms of the median infectious dose (ID50) of Spearman and Karber from the results of multiplication of M. leprae in the mouse foot-pads that had been inoculated with serially diluted suspensions prepared from the same sample. If only normal mice are inoculated with no more than 104 AFB per foot-pad, a proportion of viable M. leprae as small as 0.003 per cent can be measured; if nude mice are inoculated with the maximal inoculum of 104 AFB per foot-pad, the smallest proportion of viable organisms that can be measured is 0.00003 per cent, 2 orders of magnitude smaller. Typical results of such measurements are shown in Table 2.
The mean log 0 number of M. leprae per foot-pad in untreated control mice gradually increased from 7.60 ± 0.27 on Day 380, the day treatment was begun, to 8.49 ± 0.18 on Day 548, 24 weeks after starting treatment. The populations of M. leprae of all treated groups remained virtually at the pretreatment level, suggesting that neither intermittent therapy for as long as 24 weeks nor daily therapy for 8 weeks caused a significant decrease of the size of the bacterial populations in nude mice. As shown in Table 2, the mean proportion of viable organisms in untreated control mice, the pretreatment value, was 6.90 per cent.
The mice of groups 11 and 12 were administered daily regimens that did not include RMP-CLARI-MINO and CLARI-MINO-OFLO. As shown in Table 3, a single dose of each regimen displayed bactericidal activity against M. leprae in nude mice. The proportions of viable organisms remained at the same level after 6 daily doses as after a single dose (p > 0.05), and declined significantly thereafter, reaching almost the lower limit of detectability- i.e., 0.00003 per cent-after 4 weeks of daily treatment by either regimen, and no viable organisms were detected after daily treatment for 8 weeks. Except on Day 6, the proportion of viable M. leprae in the two groups did not differ significantly, indicating that the addition of OFLO did not enhance the bactericidal activity of CLARI-MINO.
The mice of groups 8, 9 and 10 were treated by daily RMP-containing regimens. As is also shown in Table 3, a single dose of all of the regimens displayed significant bactericidal activity against M. leprae, multiple daily doses showed greater activity than single doses, and 6 daily doses were more active than 3 daily doses (p < 0.01), as expected. That the proportions of viable organisms on Day 1 and Day 6 were smaller in the mice of group 10 than in the mice of groups 1 1 and 12 indicates that the bactericidal activity of RMP alone was greater than that of the combinations CLARI-MINO or CLARI-MINO-OFLO. On the other hand, there was no evidence that the addition of CLARI-MINO, with or without OFLO, enhanced or antagonized the bactericidal activity of RMP against M. leprae. A tiny proportion of viable organisms was always detected after 6 daily doses of all of the three RMP-containing regimens, a situation different from that in patients and in normal mice. As shown in Table 4, after 3 or 6 daily doses of RMP-CLARI-MINO-OFLO or RMP alone, the proportions of viable organisms decreased significantly (p < 0.01) during the first 12 weeks after treatment, and then increased (p < 0.01) between 12 and 24 weeks after treatment, suggesting that the bactericidal effect continued during the first 12 weeks after stopping treatment, but the persisting organisms multiplied thereafter.
The bactericidal activities OF four intermittent combined regimens were compared with those of RMP alone administered intermittently, DDS-CLO administered daily, OR the standard MDT regimen. As shown in Table 3, the proportions of viable M. leprae decreased significantly in every group by week 4, indicating that single doses OF ALL of the intermittent regimens displayed a degree OF bactericidal activity. After single doses of any RMP-containing regimen, the proportions OF viable M. leprae decreased virtually to the lower limit detectable by inoculation of normal mice. Treatment by DDS-CLO daily for 4 weeks killed 98.7 % OF the viable M. leprae; this degree OF bactericidal activity was smaller than that of a single dose of RMP (p < 0.01), but did not differ significantly from that of a single dose of CLARI-MINO-OFLO. The apparently greater activity of a single dose of CLARI-MINO in the mice of group 6 was probably an error, because no study has revealed evidence of antagonism between CLARI-MINO and OFLO. At week 12, the proportions of viable M. leprae in all seven groups of mice were significantly smaller than those at week 4, and the proportion of viable organisms in the mice treated by the four RMP-containing regimens had decreased nearly to the lower limit detectable by inoculation of nude mice. These findings indicate that three doses of intermittent treatment displayed greater bactericidal activity than did single doses of the same regimens (p < 0.01). The activities of intermittent administration of the four RMP-containing regimens were indistinguishable.
As expected, three intermittent doses of the two regimens that did not include RMP-CLARI-MINO (group 6) and CLARI-MINO-OFLO (group 7)-were less actively bactericidal than the RMPcontaining regimens (p < 0.01); nevertheless, the two former regimens killed at least 99.9 % of the viable M. leprae. The activity of DDS-CLO administered daily for 12 weeks was greater than expected; that no viable M. leprae were detected even after subinoculation of the maximal available inoculum in nude mice indicates that the treatment killed > 99.999 % of the viable organisms originally present. Because three intermittent doses of RMP-containing regimens or 12 weeks of daily DDS-CLO were so active, it was not possible to detect additional bactericidal effects resulting from the administration of additional doses of these regimens. However, viable M. leprae were still detected in mice after six intermittent doses of CLARI-MINO, with or without OFLO.
In summary, this experiment showed that RMP was more bactericidal than any combination of the new dru«s. A simile dose of CLARI-MINO, with or without OFLO, displayed as much bactericidal activity as four weeks of daily treatment by DDS-CLO; thus, monthly CLARI-MINO, with or without OFLO, may replace DDS and CLO as components of WHO/MDT. A 12week course of daily DDS-CLO was more bactericidal than expected, suggesting that the duration of the current WHO/MDT regimen for MB leprosy may be shortened to significantly less than 24 months, without risk of RMP-resistance.
Clinical trials of the new drugs
In a trial of OFLO alone and its combination with DDS-CLO, 24 patients with newly diagnosed lepromatous leprosy were allocated randomly to three groups and treated for 56 days by 400 mg OFLO daily, 800 mg OFLO daily, or 400 mg OFLO combined with 100 mg DDS and 50 mg CLO daily plus 300 mg CLO once every 28 days (6). The bactericidal activities of the treatments were measured by titrating the proportion of viables in normal and nude mice. More than 99 %, > 99.99 %, and > 99.99 % of the viable M. leprae had been killed by 14, 28, and 56 days of treatment, respectively. Bactericidal activity did not differ significantly among the three groups. Thus, OFLO, displayed a powerful bactericidal effect against M. leprae in leprosy patients; its optimal dosage appears to be 400 mg daily; and combination with DDS-CLO did not enhance its activity.
Experiments in vitro and in vivo have all demonstrated that, on a weight-for-weight basis, sparfloxacin (SPFX) is more active than OFLO against M. leprae. The minimal effective dosages (MEDs) of daily OFLO or SPFX against 10 strains of M. leprae, measured in the mouse foot-pad, were 25 mg/kg for OFLO and only 6.25 mg/kg for SPFX (7). Thus, SPFX was four times more active than OFLO. However, because the manufacturer of SPFX recommends that patients be treated by no more than 200 mg daily, only one-half or one-fourth the dosage of OFLO, the greater activity of SPFX is likely to be offset by the lower dosage. In a clinical trial in which nine previously untreated lepromatous patients were treated by 200 mg SPFX daily for 12 weeks, most patients showed reduction of mouse foot-pad infectivity and bacillary radiorespirometric activity (1). Although 200 mg SPFX daily displayed rapid bactericidal activity against M. leprae in patients, its activity was no greater than that of 400 mg OFLO daily. Therefore, there is not yet convincing evidence that SPFX is more active than OFLO in the treatment of leprosy.
Because fusidic acid is active against M. leprae in vitro, its activity was tested in 9 lepromatous patients (2). Patients were treated by fusidic acid in a dosage of either 500 mg daily for 12 weeks or 750 mg daily for 4 weeks, followed by 500 mg daily for 8 weeks. After 8 weeks of treatment, the inlcctivity of the patients' M. leprae for the mouse was reduced significantly in 5 of the 9 cases. Thus, fusidic acid displayed only weak bactericidal activity, similar to that of DDS, and was less active than RMP and the other new drugs. Therefore, it is very unlikely that fusidic acid will become an important component of new MDT regimens.
Experiments in normal and nude mouse had demonstrated that a single dose of CLARI-MINO, with or without OFLO, exhibits bactericidal activity against M. leprae. As a further step in the development of an MDT regimen in which all drugs are administered monthly, a clinical trial was carried out to confirm the bactericidal effect of a single dose of CLARI-MINO, with or without OFLO, against M. leprae in previously untreated MB patients, and to evaluate the risk of adverse effects of the treatments; the WHO/MDT regimen for MB leprosy, and its RMP- or DDS-and-CLO components served as controls (4). Fifty newly diagnosed MB patients with large bacterial loads and active skin lesions were randomly allocated to one of five groups. The patients of group I were treated by one month of WHO/MDT; those of group II were administered a single dose of 600 mg RMP on Day 1; those of group III were treated by 30 days of DDS-CLO in the dosages used in WHO/MDT; the patients of group IV were administered a single dose of 2000 mg CLARI plus 200 mg MINO on Day 1; and those of group V were treated by a single dose of 2000 mg CLARI plus 200 mg MINO plus 800 mg OFLO. The patients treated by a single dose received a daily placebo from Day 2 to Day 30.
The pretreatment biopsy specimens from 49 of the 50 patients harbored sufficiently large concentrations of viable M. leprae that the bacilli multiplied in the foot-pads of normal mice. As shown in Table 5, by Day 31, the proportion of viable organisms in the biopsy specimens had decreased by at least 90 per cent from the pretreatment value in 39 patients, decreased slightly in 6 patients, and remained unchanged in 4. That such a significant decrease of the proportion of viable organisms was observed in the great majority of cases of all five groups indicates that all five of the regimens tested displayed some degree of bactericidal activity. After treatment, the organisms recovered from 28 (57 %) of the 49 patients had lost their infectivity for normal mice; included among these 28 patients were all 19 patients who had been treated with a single close of RMP, either alone (group II) or in combination with DDS-CLO (group I), and only 9 (30 %) of the 30 patients who had been treated by regimens that did not include RMP (p < 0.01). As is also shown in Table 5, a single dose of CLARI-MINO or CLARI-MINO-OFLO exhibited a degree of bactericidal activity against M. leprae similar to that of a month's daily treatment with DDS-CLO. No difference was discerned between the two single-dose regimens. A bactericidal effect of 30 days of treatment with daily DDS-CLO was observed in 8 of the 10 patients, and the organisms of 4 of them lost their infectivity for normal mice.
The results of this clinical trial clearly confirmed in MB patients the findings in nude mice. First, RMP is the most actively bactericidal drug against M. leprae; its activity is greater than that of any combination of the currently available new drugs. Second, a single dose of CLARI-MINO, with or without OFLO, displays a degree of bactericidal activity against M. leprae that does not differ significantly from that of a month of daily DDS-CLO, suggesting that it may be possible to replace the DDS-and-CLO components of WHO/MDT by a monthly dose of CLARI-MINO, with or without OFLO. Finally, a month of daily treatment with DDS-CLO displays significant bactericidal activity against M. leprae in the majority of MB patients.
Field trials of a newer generation of MOT regimens (10)
Because of the relatively small numbers of AFB that can be inoculated into each mouse foot-pad, one can measure at best only the initial 99.999 per cent killing of M. leprae in a nude mouse experiment or clinical trial, whereas a previously untreated lepromatous patient may begin treatment with as many as 1010 to 1011 viable organisms. As described in the previous sections, the bactericidal activity of multiple doses of RMP is so powerful, that it cannot be measured by any of the available techniques, including inoculation of nude mice. For this reason, the efficacy of RMP-containing combinations cannot be adequately tested either in nude mice or in classic clinical trials. The main objective of a field trial is to evaluate the efficacy of a new regimen over the long term, by measuring the relapse rate of the new regimen, in comparison with that of a standard regimen, such as WHO/ MDT In patients with MB leprosy, it is assumed that the relapse rate is directly correlated with the number of viable M. leprae present at the end of treatment, reflecting the bactericidal effect of the regimen. Taking into account the low relapse rate after treatment by WHO/MDT and the long incubation period of relapse after stopping MDT, a field trial requires about 500 new patients per regimen, the patients to be followed for at least 7 years after stopping treatment. Patients with PB leprosy may also be studied in the field trial, to see if they may be cured by significantly shortened treatment by the new regimen; in that case, about 1000 active PB patients must be treated by each regimen. Because a field trial is very expensive and time-consuming, only a very few, carefully chosen regimens may be tested in field trials. Beginning in 1991, the THEMYC Steering Committee has sponsored two large, multicentre field trials of new MDT regimens.
Fifteen centres from 8 different countries are participating. Patient-intake was completed in June, 1994, by which time 1651 MB and 1816 PB patients had been recruited. MB patients were allocated randomly to treatment by one of four regimens: i) WHO/MDT for MB leprosy for 24 months; ii) daily treatment with 600 mg RMP plus 400 mg OFLO for 4 weeks; iii) WHO/MDT for MB leprosy for 12 months; and iv) WHO/MDT for MB leprosy for 12 months plus an initial 4 week period of 400 mg OFLO daily. PB patients were allocated randomly to treatment by one of two regimens: i) WHO/MDT for PB leprosy for 6 months; or ii) 600 mg RMP and 400 mg OFLO daily for 4 weeks. The regimens have been well tolerated, and no relapse had been detected by the end of 1995.
In the second field trial sponsored by THEMYC, the new regimen consists of 600 mg RMP, 400 mg OFLO and 100 mg MINO; the dosages are reduced by half for children. As in the first field trial, the efficacy of the regimen is being tested among both PB and MB leprosy patients.
Because of the tremendous improvement of case-detection in the course of implementing MDT, the number of new cases (many of them children) detected each year has increased steadily, and more than half of these cases are detected at so early a stage that the only visible sign of the disease is a single skin lesion of PB leprosy. Because single-lesion PB cases have a strong tendency to self-healing, and the risk of developing nerve damage among such patients is minimal, it may be possible to cure these patients by a minimal course of chemotherapy, such as a sintzle dose of RMP-OFLO-MINO, instead of 6 months of WHO/MDT. In 1994, 1483 newly diagnosed single-lesion PB patients were recruited in 9 different centres in India and allocated randomly to treatment by either WHO/MDT for PB leprosy for 6 months, or a single dose of RMP-OFLO-MINO followed by placebo. To date, the trial is progressing smoothly and the treatments are well tolerated. Because the trial has been organized in double-blind fashion, the results of the trial will not become available until the end of 1997, at which time the code will be broken.
The RMP-OFLO-MINO trial is also being conducted in other countries. In Myanmar, 1000 PB and 1000 MB patients are being recruited; PB patients are to be treated by either 3 or 6 monthly doses of RMP-OFLO-MINO. and MB patients by either 12 or 24 monthly doses of RMP-OFLO-MINO. In Guinea, 400 PB and 100 MB patients are being recruited; PB patients are to be treated by 6 monthly doses of RMP-OFLO-MINO. and MB patients by 24 monthly doses of RMP-OFLO-MINO. Patients from other countries will also be included in the trial. After stopping treatment, patients will be followed for at least 7 years; the results of the new regimens will be compared with those of treatment by WHO/MDT in the national leprosy control programme.
Acknowledgment. Most of the results presented in this paper were derived from research projects supported by the UNDP/ World Bank/WHO Special Programme for Research and Training in Tropical Diseases and the Association Française Raoul Follereau.
REFERENCES
1. CHAN, G. P., GARCIA-IGNACIO, B. Y., CHAVEZ, V.E., LIVELO, J. B., JIM INEZ, C. L., PARRIKKA, M. L. P. and FRANZBLAU, S. Clinical trial of sparlloxacin for lepromatous leprosy. Antimicrob. Ag. Chemother. 38(1994)61-65.
2. FRANZBLAU, S.. CHAN, G. P.. GARCIA-IGNACIO, B. Y. CHAVEZ, V. E.. LIVELO, J. B., JIMINEZ, C. L., RIKKA, M. L. P.. CALVO, R. E, WILLIAMS, D. L. and Gil.I.is. T. P. Clinical trial of fusidic acid for lepromatous leprosy. Antimicrob. Ag. Chemother. 38(1994 )1651-1654.
3. JAMET, P., Jl, B., and THE MARCHOUX CHEMOTHERAPY STUDY GROUP. Relapse after long-term follow up of multibacillary patients treated by the W.H .O. multidrug regimen. Int. J. Lepr. 63(1995)195-201.
4. Ji. B., JAMET, P., PERANI, E. G., SOW. S.. LlEN-HARDT, C., PETINON, C. and GROSSET, J. 11. Bactericidal activities of a single dose of clarithromycin plus minocycline, with or without ofloxacin, in patients. Submitted for publication.
5. Ji. B.. PERANI. E. G., PHTINON, C. and GROSSIT, J. H. Bactericidal activities of combinations of new drugs against Mycobacterium leprae in nude mice. Antimicrob. Ag. Chemother. 40 (1994) 393-399.
6. Ji, B., PERANI, E. G., PETINON, C, N'DELI, L. and GROSSIT, J. H. Clinical trial of ofloxacin alone and in combination with dapsone plus clofazimine for treatment of lepromatous leprosy. Antimicrob. Ag. Chemother. 38(1994)662-667.
7. TRAORE, I., Ji, B., LlENHARDT, C, BOBIN, P. and GROSSET, J. Determination of the minimal effective dosages of ofloxacin and sparlloxacin against Mycobacterium leprae in the mouse foot pad system. Int. J. Lepr. 64(1996)142-145.
8. WORLD HEALTH ORGANIZATION. Risk of relapse in leprosy. WHO document WHO/CTD/LEP/94.1.
9. WORLD HEALTH ORGANIZATION. Progress towards the elimination of leprosy as a public health problem. Weekly Epidemiol. Rec. (1995) 177-182 and 185-188.
10. WORLD HEALTH ORGANIZATION. THELEP and THEMYC Steering Committee Documents.
Discussion of the paper by Profs. Ji, Levy and Grosset
Dr. Cole: The data on relapse from the Institut Marchoux appear to provide the most compelling reason to continue research on a leprosy vaccine. If the patients relapsed, despite good compliance, and if we extrapolate these results to the world, we may expect a huge number of relapses.
Prof. Ji: The patients were hospitalized during MDT, and every dose, even the daily doses, was administered under supervision. I have not observed this myself, but I have been so informed by my colleagues at the Institut Marchoux.
Dr. Cole: Is it possible that the patients were reinfected after release from chemotherapy? After all, leprosy is highly endemic in Mali.
Prof. Ji: I know of no evidence that suggests that the relapses in fact represented the results of new infections by M. leprae. And I should emphasize that the relapses occurred only among those patients with initially high Bis. Reinfection should be as likely to occur among the patients with initially low Bis.
Dr. Pannikar: Certain aspects of the Institut Marchoux study require comment. More than 80 patients had originally been recruited into the study for treatment by MDT. Fifty of these were subsequently lost, leaving only 35 to be followed. I guess that, if we were to wait an additional live years, the relapse rate would be even higher, because more patients would have dropped out, leaving primarily those who had relapsed to be followed.
Dr. Noordeen: A preliminary study of patients with initially high Bis in other areas has not confirmed the greater risk of relapse among them.
Prof. Ji: Perhaps it is a matter of definition of a high BI. The Institut Marchoux is a referral center, to which a disproportionately large number of the most advanced cases are referred. Our data showed that no relapses occurred among the patients whose initial BI was no greater than 3+. We believe that this is a very special group of patients at great risk of relapse; we certainly do not recommend changing the MDT regimen so as to be able to deal better with a tiny minority of patients. On the other hand, we have the impression that, in many areas, the possibility of relapse of MB leprosy is simply ignored; however, patients do relapse.
Dr. Gupte: In the community, as opposed to the referral center, the situation appears much different. In our community-based program, only 5 per cent of the cases are smear-positive, and fewer than 10 per cent of the smear-positive patients have BI > 4.
Prof Ji: I believe that far fewer than 10 per cent of the smear-positives-perhaps only 1 or 2 per cent-have BI > 4.
Dr. Feenstra: I wish only to point out that a relapsed patient is not a total failure. The patient can easily be retreated. We are not deterred from doing cancer surgery by relapse rates of 80 per cent. Prof. Lechat: Perhaps this should be a research priority-it may be important to know the proportions of patients with initially high Bis in various parts of the world.
Dr. Waters: Another research priority might be to compare risk of relapse in previously untreated patients with that among patients who had been treated for some time with DDS monotherapy and then administered MDT for two years.
Dr. Pannikar: Prof. Ji referred to our paper which was based on routine program reporting. The patients were begun on MDT at the time MDT was implemented. Some of them had been treated in the past with DDS; all new patients were treated with MDT only. Prof. Ji did not refer to a second paper on fixed-duration chemotherapy, which included approximately 6000 patients, all of them new, and all of them with BI > 2+ at the time of recruitment; the relapse rate is less than 1 per cent.
Prof. Lechat: I am a bit concerned about the 7 million patients who Dr. Noordeen said had been cured. Do we know how many of these were patients who had already been cured by DDS monotherapy who were retreated with MDT without having relapsed, before the register had been cleaned? If, in fact, 5 million of the patients had already been cured by DDS monotherapy, and were given MDT to be sure, then I think it is important to take this into account in predicting trends.
Dr. Noordeen: The 7 million cured patients represent a mixture of new cases and patients who had previously been cured by DDS monotherapy. But even if we assume that the proportion of patients previously treated with DDS is high, the risk of relapse after DDS monotherapy is 2 per cent per year, a well documented fact, so that many of these patients would have relapsed, had they not been retreated with MDT.
Prof. Lechat: Perhaps, then, the number of relapses prevented is more important than the decline of prevalence or the number of patients cured by MDT.
Dr. Noordeen: Two factors contributed to the decline of prevalence: the number of patients cured by MDT, and improvement of the system of leprosy control, with removal from the registers of patients who did not exist, or whose disease was inactive. In fact, 7 million patients were treated with MDT and only then removed from the registers.
Dr. Louhenapessy. Perhaps patients should be followed longer than 5 years, if relapses may occur so late after MDT has been completed. Also, what should be done with those MB patients who are lost after having completed only 75-80 per cent of their treatment?
Dr. Feenstra: A research priority that I forgot to list is a urine dip-stick that will identify the patient whose disease has been cured.
Prof.Ji: This is a very serious suggestion. If we had a means of detecting the presence of a tiny number of viable M. leprae in the individual's body, it would be unnecessary for Dr. Pannikar to organize the held trials which have absorbed so much of his time and effort. We must carry out field trials because we have no other means by which to detect a tiny number of viable organisms. Although I pointed out in my paper that we can measure the killing of 99.999 per cent of the viable organisms originally present in the patient, this is not nearly good enough. The MB patient who begins chemotherapy harboring 1011 viable M. leprae will still harbor as many as 106 viable M. leprae after 99.999 per cent have been killed.
Dr. Naafs: What does this number of persisting viable M. leprae matter? What is important is that the patient is not sic-k, and does not infect his contacts. Consider, for example, the Malta trial. Biopsy specimens obtained from the Malta patients contained acid-fast bacilli, despite which the patients did not relapse. Why then do we need Dr. Feenstra's dip-stick, or why must you detect the last living M. leprae?
Prof. Ji : I am not speaking of detecting bacilli, but rather of detecting tiny proportions of viable M. leprae among the large numbers of dead bacilli; this is quite different. Consider that now we are recommending MDT of fixed duration; I am certain that, at the end of the 2 years of treatment, a proportion of the patients will still be smear-positive. Patients who are seen to harbor organisms may harbor viable organisms; except by awaiting relapse, we have no means of determining which of the patients harbor viable M. leprae.
Prof. Lechaf. Perhaps these small numbers of viable M. leprae are of no account. Perhaps we can live quite comfortably with 105-106 viable M. leprae. After all, all of us live with viable cancer cells.
Prof. Levy: Perhaps it is necessary to review a bit of ancient history. Those of us who are interested in the proportion of viable M. leprae, and who have been measuring them over the years did this because we know no other way to demonstrate that we had interrupted transmission of M. leprae. In our minds, the purpose of chemotherapy in the context of control is not to cure patients but rather to interrupt transmission. We began measuring drug-effects with the BI and the MI, because we did not know what to expect of the available drugs. We have progressed to field trials and to measuring the risk of relapse, but we recognize these to be intermediate measures. What truly interests us is the possibility of interrupting transmission. Perhaps, then, rather than a dip-stick, we need a means of detecting subclinical infection. Such a test would indeed save Dr. Pannikar much time, because field trials would not be needed at all, or could be greatly shortened. Certainly, well within the 5-7 years' duration of a field trial, we could recognize by such a test that transmission of M. leprae had indeed been interrupted. We expend a great deal of effort in field trials to measure very small rates of relapse, because we really have no alternative. But our purpose has been to interrupt transmission. How else can leprosy be controlled?
Dr. Noordeen: I thought that Prof. Levy would talk about the THELEP controlled clinical trials, which demonstrated that, no matter what the regimen, nor how long it had been administered, persisters were detected in approximately 7 per cent of patients, and were probably present in all. Despite the presence of persisters, very few patients relapse.
Prof. Ji: We don't understand very well the phenomenon of microbial persistence. We believe that the persisters represent a fairly constant proportion of the total, so that as we reduce the BI, we decrease the number of persisting M. leprae.
SETTING PRIORITIES
The participants in the Workshop were assigned each to one of three groups, and the groups were assigned the following responsibilities: Group I-priorities for research in chemotherapy, chaired by Prof. B. Ji; Group II-priorities for basic research, chaired by Prof. W. J. Britton; and Group III-priorities for epidemiological and operational research, chaired by Dr. W.C. Smith. The groups met individually, to discuss and prepare lists of research priorities in their respective areas of responsibility, after which, at a plenary session, the Workshop reconvened to discuss the several lists, and to produce an integrated list of research priorities. The reports of each group are summarized here.
Group I
High priority was given to an alternative to relapse as a predictor of treatment failure, in order that we might apply the results of ongoing field trials before the year 2000. Equally high priority was given to research into the treatment and prevention of reactions-reversal reactions, ENL and neuritis. In particular, the group felt that we need to know better how to use steroids to prevent reactions. In addition, the group believed that alternatives to steroids and thalidomide are urgently required. Second priority was given to research into methods by which early assessment may be made of the results of ongoing trials in PB leprosy, so that we might apply the results of these trials before the year 2000. Finally, the group believed that there is no need for trials of regimens including the new drugs for treatment of either MB or PB leprosy in addition to those already in progress.
Group II
First priority was given to completing the sequencing of the M. leprae genome, and exploitation of the information gained. The group believed that this work could be facilitated by establishment of a working group, which would be responsible for overseeing completion of the sequencing, and its applications to the most pressing needs of the community of leprosy workers. Second priority was given to the development of tests to recognize subclinical infection by M. leprae and to diagnose early leprosy. Lesser priority was given to development of tests for drug resistance and for bacterial viability, and to predictors of reactions. In addition, the group felt that research was needed into the mechanisms of early nerve damage, and into management of reactions. Finally, priority was given to development of a vaccine to be used in groups of individuals at high risk for leprosy.
Group III
Highest priority was given to studies of the epidemiology of infection by M. leprae, of the epidemiology of nerve damage, and of the epidemiology of relapse after completion of MDT, with particular reference to the pre-treatment BI, treatment prior to MDT, compliance with MDT, and the duration of MDT. Research priorities in the area of rehabilitation included development of methods to assess needs, development and assessment of different models of community-based rehabilitation, and development of methods to assess the effectiveness of programs for prevention and limitation of impairments and disabilities. Priorities for operational research in the post-elimination era included development of the optimal organization of leprosy services and the development of program indicators.
The integrated list of research priorities is presented in order of priority in the accompanying table.
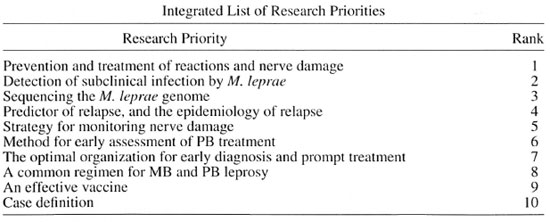
* Strictly speaking, incidence rates can serve only to prove false the hypothesis that MDT interrupts transmission-that is, to show that it does not, in case the incidence does not decline.
**These same components of the "poverty complex" might operate, rather, at the level of susceptibility of the M. leprae -infected to develop the disease. L. Levy, ed.
*** Based on a draft statement of the ILEP Medical Commission, December 1995.
**** The exchange rate is approximately US$1 = 25 Baht. L. Levy, ed.