- Volume 61 , Number 2
- Page: 185–91
BCG vaccination protects against leprosy in Venezuela: a case-control study
ABSTRACT
A total of 64,570 household and other close contacts of about 2000 leprosy cases were screened for eligibility for entry into a trial of a new leprosy vaccine. The screening procedure included a clinical examination for leprosy and for the presence of BCG and lepromin scars. Ninety-five new cases of leprosy were identified, and the prevalence of BCG and lepromin scars among them was compared with similar data f rom matched controls selected f rom among those with no evidence of leprosy. The difference in the prevalence of BCG scars in the two groups was used to estimate the protection against leprosy conferred by BCG vaccination. One or more BCG scars was associated with a protective efficacy of 56% (95% confidence limits 27% to 74%). There was a trend of increasing protection with four or more BCG scars, but this was not statistically significant. There was no evidence that the efficacy of BCG varied with age or according to whether or not the contact lived in the same household as a case. The protective effect was significantly higher among males, and was significantly greater for multibacillary than for paucibacillary leprosy.RÉSUMÉ
On a examine un total de 64.570 contacts domiciliaires et autres proches contacts d'environ 2000 malades de la lèpre pour leur éligibilite à 1'incorporation dans un essai d'un nouveau vaccin anti-lépreux. La procedure comprenait un examen clinique pour la recherche de lèpre et la presence de cicatrices de BCG et de lépromine. Nonante-cinq nouveaux cas de lèpre ont été identifiés, et la prevalence des cicatrices de BCG et de lépromine parmie eux a été comparée avec les observations chez, des témoins appariés sélectionnés parmi les contacts ne présentant aucun signe de lèpre. La différence de la prevalence des cicatrices de BCG dans les deux groupes a été utilisée pour estimer la protection vis-à-vis de la lèpre conférée par la vaccination au BCG. Une ou plusieurs cicatrices de BCG était associée à une efficacité protectrice de 56% (limites de confiance à 95% : de 27% à 74%). Il y avait une tendance à l'augcmentation de la protection avec quatre cicatrices de BCG ou plus, mais celle-ci n'était pas statistiquement significative. Il n'y avait pas de signe que l'efficacité du BCG variait avec l'âge ou avec le fait que oui ou non le contact vivait dans la même maison qu'un malade. L'effet protectur était significativement plus élevé parmi les hommes, et était significativement plus grand pour la lèpre multibacillaire que pour la lèpre paucibacillaire.RESUMEN
Se examinaron 64,570 contados (convivientes y no con vi vientes) de aproximadamente 2000 pacientes con lepra, con el fin de cstablecer su cligibilidad para participar en un proyecto sobre una nueva vacuna antileprosa. El procedimientode tamisaje incluyó una evaluación clínica, buscando signos y sintomas de lepra, así como el registro de cicatrices producidas por el BCG o por la lepromina. Se identificaron 95 casos nuevos de lepra y la prevalência de cicatrices por BCG o lepromina entre ellos, se comparo con la prevalência de cicatrices observada en controles apareados seleccionados de los indivíduos que no tuvieron evidencias de la enfermedad. La diferencia en la prevalência de cicatrices por BCG entre los dos grupos se utilizo para calcular la protección contra la lepra conferida por la vacunación con BCG. Una o más cicatrices por BCG estuvieron asociadas con una eficácia protectora dei 56% (95% de confianza entre los limites deh 27% al 74%). La protección tendió a aumentar con cuatro o más cicatrices por BCG, pero este aumento no fuc de significancia estadística. No hubieron evidencias de que la eficácia dei BCG variara con la cdad o de que pudicra estar en función dei grado de convivência, esto es, de que el contacto viviera o no en la misma casa que el paciente. El efecto protector fue significativamente mayor entre los hombres, y también significativamente mayor para la lepra multibacilar que para la paucibacilar.Four large controlled trials have been conducted to assess the protective efTect of BCG vaccination against leprosy (2,7,12,13). The efficacy observed for the vaccine has varied from approximately 20% in Myanmar (Burma) (7) to 80% in Uganda (12). The reasons for this variation are unknown and, therefore, it is difficult to assess the role that BCG vaccination might have in leprosy control, especially in those geographical areas such as Latin America where no trials have been carried out.
The case-control approach may be used to make a retrospective assessment of vaccine efficacy (11), and although this method of evaluation is inferior to that of a controlled trial, if carefully applied it may enable a reasonable assessment to be made of the impact of vaccination. For example, using this and other methods in a study in Malawi, Fine and colleagues (6) obtained an estimate of the efficacy of BCG against leprosy of approximately 50%, not greatly different from the estimate of 80% obtained further north in Africa in a controlled trial in Uganda (12).
In Venezuela, BCG vaccination has been incorporated into leprosy control activities for many years, although without formal evidence of the effectiveness of this measure. The rationale for the use of BCG for this purpose was based on the postulate by Fernandez (5) of a protective effect of such vaccination. This postulate was supported by the observation that when household contacts of leprosy patients were vaccinated repeatedly with BCG, the Mitsuda reaction became positive (3). In the Venezuelan leprosy control program an attempt was made to give BCG every 1 or 2 years to the household contacts of leprosy patients. A similar policy was adopted for children under the age of 15 years who lived in areas where the prevalence of leprosy was relatively high.
During the recruitment phase of a randomized controlled trial to assess the efficacy of a new leprosy vaccine in Venezuela (4), we were able to conduct a case-control investigation of the efficacy of BCG against leprosy among the household and other close contacts of leprosy cases. We believe that this investigation is the first in which an assessment has been made of the efficacy of BCG against leprosy in a Latin American country. Preliminary results of this investigation have been presented elsewhere (4), and the findings are presented here in more detail.
METHODS
In 1984 a large-scale, randomized controlled trial was started in Venezuela to assess whether a vaccine consisting of a mixture of BCG and armadillo-derived killed Mycobacterium leprae offered better protection against leprosy than BCG alone (4). The incidence of leprosy in Venezuela is low, and it was not expected that there would be sufficient cases arising to include more than two "arms" in the trial. Thus, although it was not known if BCG alone protected against leprosy in the trial population, it was decided not to include a group in the trial who received only a placebo vaccination. The trial population was chosen from among those known to be at relatively high risk of leprosy, that is, from the household and other close contacts of known leprosy patients (e.g., neighbors, workmates, family members) in three states of Venezuela with the highest rates of leprosy. The eligible trial population was defined by public health inspectors who compiled a list of such contacts, according to detailed instructions, for the approximately 2000 known cases of leprosy in the study area. The contacts were invited to participate in the trial and those who agreed came for a physical examination and were given skin tests with tuberculin and leprosy soluble antigen, and a blood sample was taken. As part of the physical examination each contact was examined for BCG scars and lepromin scars and the numbers of each were noted. Each contact was also examined for evidence of leprosy, and any cases found were excluded from the trial but are the subject of the present investigation. A comparison was made of the distribution of the number of BCG scars in the new cases of leprosy found and in the remainder of the population examined. Because both BCG vaccination and leprosy rates vary with age, geographical location, and the closeness of the contact with the index leprosy case, an attempt was made to control for the potential confounding effects of these factors by selecting matched controls for each case. For each case, the controls were selected as all those contacts who were examined and who were of the same sex as the case, were in the same fiveyear age group, lived in the same municipality and were of the same contact "status" (household or nonhousehold contact).
Analyses were conducted of the data in the matched (case-control) sets to obtain relative risk estimates using the method of conditional logistic regression. Adjustments were made for potential confounding factors using the same strategy.
RESULTS
Among the 64,570 contacts who were screened for entry into the trial, 95 new cases of leprosy were found. For 692 contacts, including 3 cases, the numbers of BCG and lepromin scars were not recorded. The numbers of BCG scars noted for each of the remaining 63,878 contacts are shown in Table 1, together with the corresponding data for cases, classified according to the type of leprosy. There is an inverse association between the number of BCG scars and the prevalence of leprosy. Also, the cases whose disease was classified toward the lepromatous pole had fewer BCG scars, on average, than those classified toward the tuberculoid pole. The data in Table 1 must be interpreted with caution, however, since they are not adjusted for age and other potentially confounding factors.
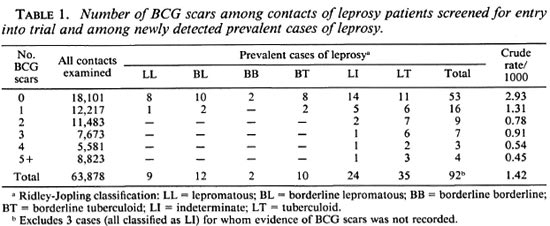
Further analyses were based, therefore, on the comparison of the cases with their matched controls. The number of controls who could be matched to each case varied greatly. For two cases no suitably matching controls could be found, and these cases were excluded from subsequent analyses. The greatest number of controls matched to a single case was 335. In five instances two cases shared the same matching characteristics, and they were preserved in pairs for the analysis together with their matched controls. In one other instance a pair of cases shared the same matching characteristics but they had different types of leprosy (one multibacillary and one paucibacillary). The 39 matched controls were divided randomly between these two cases (19 to one, 20 to the other). Thus, the logistic regression analyses were based on the analysis of 85 matched sets of cases and controls.
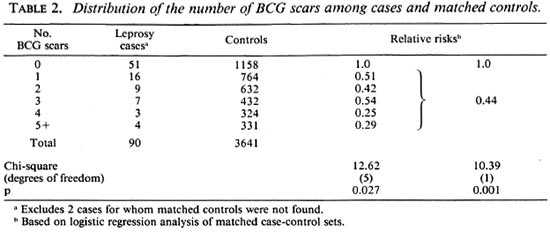
The overall distributions of the numbers of BCG scars recorded for the cases and the matched controls are shown in Table 2, together with estimates of the risk of leprosy relative to those with no BCG scars recorded. Overall, those with one or more BCG scars had a relative risk of leprosy of 0.44, corresponding to a vaccine efficacy of 56% (95% confidence limits 27% to 74%). The relative risk was about 0.5 for those with 1 to 3 BCG scars, and about 0.3 for those with 4 or more such scars, but this difference was not statistically significant. Eleven cases and 91 of the matched controls had one or more lepromin scars recorded, and the presence of such a scar was associated with a relative risk of leprosy of 2.9 (95% confidence interval 1.3 to 6.8).
The cases were classified according to various characteristics to determine if there was evidence that the protective effect associated with the presence of BCG scars varied in different subgroups. The results of these analyses are summarized in Table 3. There was no evidence that the protective effect varied with age or according to whether or not the case of leprosy lived in the same household as another case. The number of cases and controls with one or more lepromin scars was small and, although the decreased relative risk of leprosy among those with one or more BCG scars was apparent only in those without a lepromin scar, the difference in relative risk among those with and without a lepromin scar was not statistically significant. The efficacy among males was significantly higher than among females.
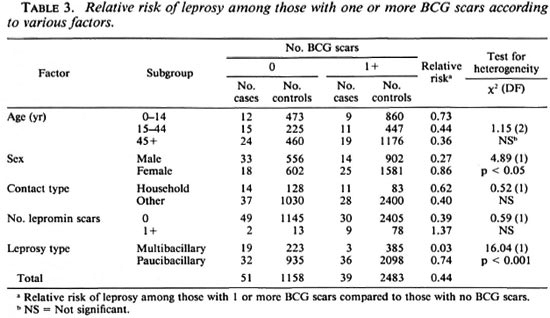
Among the cases, 22 were classified as multibacillary (Table 1: LL + BL + BB, less 1 with no controls) and 68 as paucibacillary (the remainder in Table 1, less 1 with no controls). Among the former group the relative risk of leprosy among those with one or more BCG scars was 0.03; whereas among the latter group, the corresponding relative risk was 0.74 (Table 3). This difference was statistically highly significant [ x 2 (1 DF) = 16.04; p < 0.001].
DISCUSSION
The main finding in this study is evidence of a substantial protective effect of BCG vaccination against leprosy among household and other close contacts of leprosy cases. The examination of the contacts was undertaken to screen them for eligibility for entry into a trial of a new leprosy vaccine, and at the time the contacts were seen, it was not planned to conduct the analyses described in this paper. We have no reason to believe that suspected cases of leprosy were examined more carefully for BCG scars than other contacts. Indeed, if there had been such a tendency it is likely that it would have biased against finding a deficit of scars among cases compared to the controls. Evidence of previous BCG vaccination was based on the finding of vaccination scars. Scars are not formed among all those who have been vaccinated, and an examiner may mistakenly identify a scar as due to BCG that was due to some other cause. The effect of such misclassification is to bias the estimation of the relative risk of leprosy, associated with BCG vaccination, toward unity (11). Thus, it is likely that the protective efficacy of 56% estimated in this study (95% confidence interval 27% to 74%) is an underestimate of the true efficacy. It is important to emphasize, however, that the estimate of efficacy derived is not based on results from a randomized controlled trial, and there may have been differences in the risk of leprosy among those with and without BCG scars which were unrelated to BCG vaccination.
Our results could be explained, for example, if those at greatest risk of leprosy, for whatever reason, were less likely to be vaccinated with BCG. In normal circumstances such biases are not hard to imagine since leprosy is a disease which is associated with poverty and deprivation, and it is in such groups that the utilization of vaccination service is often poorest. We have tried to control for this in the analysis by selecting controls from the same subgroup as the cases (i.e., contacts of leprosy cases) and matching them for age, sex, closeness of contact with an index leprosy case and municipality of residence. No assessment was made of the socioeconomic status of cases or controls and, although we think it unlikely that differences of this kind can be entirely responsible for the protective effect found, we cannot exclude this possibility of bias completely.
Generalization of our findings should be undertaken with caution since the study population was atypical in two major respects. Firstly, it consisted of close contacts of leprosy patients and, thus, their exposure to leprosy is likely to have been greater than that of members of the general population. A significant number of contacts of leprosy patients may be exposed to M. leprae over extended periods, and the resulting immunological stimulation may act to extend the duration of any protection conferred by BCG vaccination. Secondly, many of the contacts in this study had received multiple doses of BCG, given periodically over a number of years. The protection associated with a single BCG scar was not significantly different from that associated with multiple scars, but there was a tendency for the risk of leprosy to be lowest among those with four or more BCG scars (Tables 1 and 2).
Multiple BCG vaccination had been part of the Venezuelan leprosy control activities, and it should be noted that some of those who received several doses of BCG also will have been tested repeatedly with lepromin. We cannot rule out the possibility that this antigen affected the subsequent risk of leprosy. This may be the explanation for the finding that a lepromin scar was associated with an increased risk of leprosy. In the Venezuelan leprosy control program the application of lepromin was limited to household contacts, whose risk of acquiring leprosy is much higher than in the general population. Thus, a lepromin scar may be a marker of an individual who has been a household contact of a leprosy case at some time in the past, even though at the time of the study they may not have resided in such a household.
Our finding that the efficacy of BCG against leprosy was higher among males than among females was also present in the BCG trial conducted in Myanmar (7), but a sex difference in protection has not been reported in other studies (2,6,12), and it may be a chance finding. A finding of special interest was the high protection that BCG vaccination appeared to have conferred against multibacillary leprosy (Table 3). Only 3 of 19 cases of multibacillary leprosy had a BCG scar, compared to 36 of 68 cases of paucibacillary disease. The numbers on which this finding was based were small, although the difference in the protection conferred by BCG with respect to the two types of leprosy was highly significant (Table 3).
In the controlled trials of BCG against leprosy, the incidence of multibacillary disease has been too small to make an accurate assessment of protection against such disease. In a recent case-control study in Brazil, Rodrigues, et al. (10) reported an overall protective efficacy of BCG against leprosy of 81%, but found the protective effect was near zero among paucibacillary cases and over 90% among multibacillary cases. Ponnighaus, et al. (9) reported a protective effect of 50% against all forms of leprosy in a prospective study in Malawi, and 84% against multibacillary disease, although the confidence limits on the latter estimate were wide. In a large case-control study in Tamil Nadu, South India, Muliyil, et al. (8) found no significant protection of BCG against all forms of leprosy combined, but reported that BCG appeared to increase the risk for indeterminate leprosy while protecting against borderline disease. Conversely, Abel, et al. (1), in a study in Vietnam, found evidence of protection only among nonlepromatous cases.
We have postulated elsewhere that our failure to find that a mixture of BCG and killed armadillo-derived M. leprae gave substantially greater protection against leprosy than BCG alone (4) may be due to the fact that in Venezuela, at least, the latter vaccine itself confers high protection against the disease. It will be important to assess whether such protection can be demonstrated in other Latin American countries. The case-control approach provides a rapid and relatively inexpensive means of making such assessments.
REFERENCES
1. ABEL, L., CUA, V. V., OBERTI, J., LAP, V. D., DUE, L. K.., GROSSET, J. and LAGRANGE, P. H. Leprosy and BCG in southern Vietnam. Lancet 335(1990)1536.
2. BAGSHAWE, A., SCOTT, G. C, RUSSELL, D. A., WIGLEY, S. C, MERIANOS, A. and BERRY, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea. Bull. WHO 67(1989) 389-399.
3. CONVIT, J. and RASSI, E. Lepromin and tuberculin tests in Venezuelan leprosy foci; induction of lepromin reaction by BCG vaccination. Int. J. Lepr. 22(1954)303-310.
4. CONVIT, J., SAMPSON, C, ZUNIGA, M., SMITH, P. G., PLATA, I., SILVA, J., MOLINA, J., PINARDI, M. E., BLOOM, B. R. and SALGADO, A. Immunoprophylactic trial with combined Mycobacterium leprae /MCG vaccine against leprosy; preliminary results. Lancet 339(1992)446-450.
5. FERNANDEZ, J. M. The early reaction induced by lepromin. Int. J. Lepr. 8(1940)1-14.
6. FINE, P. E. M., PONNIGHAUS, J. M., MAINE, N., CLARKSON, J. A. and BLISS, L. The protective effects of BCG against leprosy in northern Malawi. Lancet 2(1986)499-502.
7. LWIN, K., SUNDARESAN, T., GYI, M. M., BECHELLI, L. M., TAMONDONG, C, GARBAJOSA, P. G., SANSARRICQ, H. and NORDEEN, S. K. BCG vaccination of children against leprosy: fourteen-year findings of the trial in Burma. Bull. WH O 63(1985)1069-1078.
8. MULIYIL, J., NELSON, K. E. and DIAMOND, E. L. Effect of BCG on the risk of leprosy in an endemic area; a case-control study. Int. J. Lepr. 59(1991)229-236.
9. PONNIGHAUS, J. M., FINE, P. E. M., STERNE, J. A. C, WILSON, R. J., MSOSA, E., GRUER, P. J. K., JENKINS, P. A., LUCAS, S. B., LIOMBA, N. G. and BLISS, L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 339(1992)636-639.
10. RODRIGUES, M. L. O., SILVA, S. A., NETO, J. C. A. and DE ANDRADE, A. L. S. S., MARTELLI, C. M. T. AND ZICKER, F. Protective effect of intradermal BCG against leprosy; a case-control study in central Brazil. Int. J. Lepr. 60(1992)335-339.
11. SMITH, P. G. Retrospective assessment of the effectiveness of BCG vaccination against tuberculosis using the case-control method. Tubercle 62(1982)23-35.
12. STANLEY, S. J., HOWLAND, C STONE, M. M. and SUTHERLAND, I. BCG vaccination of children against leprosy in Uganda; final results. J. Hyg. (Camb). 87(1981)233-248.
13. TRIPATHY, S. P. The case for BCG. Ann. Natl. Med. Sci. (India) 19(1983)11-21.
1. M.D.; Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
2. M.D.; Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
3. M.D.; Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
4. Ph.D.; Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
5. M.D.; Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
6. M.D.; Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
7. M.D.; Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
8. M.S., Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
9. D.Sc, Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K..
Received for publication on 1 December 1992;
Accepted for publication on 13 January 1993.