- Volume 61 , Number 2
- Page: 192–8
Cross-sectional assessment of ELISA reactivity in leprosy patients, contacts, and normal population using the semisynthetic antigen natural disaccharide octyl bovine serum albumin (ND-O-BSA) in Cebu, the Philippines
ABSTRACT
An indirect enzyme-linked immunosorbent assay (ELISA) using natural disaccharide octyl bovine serum albumin (ND-O-BSA) as antigen was used in testing leprosy patients, contacts and a normal population in Cebu, The Philippines, f rom 1985 to 1989. A total of 1413 persons were studied. The results suggested that ELISA reactivity and the bacterial index (BI) correlate in a general way. In multibacillary (MB) leprosy, positivity ranges f rom 54.2% to 92.3% among patients with a BI of < 2+ to > 4 + on the Ridley scale, with an overall average of 84.5%. Paucibacillary (PB) leprosy patients have a low degree of reactivity, with only 15.0% ELISA positive. The test is more efficient in detecting MB than PB leprosy. The contacts of MB leprosy showed 6.5% positivity; contacts of PB leprosy, 7.0% positivity. The normal population showed 1.7% positive ELISA or 17 per thousand population, which is very much less than that of the household contacts. However, because the normal population is a much larger population than the household contact population in a community, more new leprosy cases would emanate f rom it. Leprosy workers are concerned about the transmission of the disease to household contacts. However, for the reason stated above, we should be more concerned with the silent spread of the disease to the normal population in the community. Further studies are required along this line: One to determine whether there is a correlation between prevalence rates the rates of ELISA positivity in the normal population, the other is to find out if the rate of ELISA positivity in the normal population of a community can be used to monitor the efficiency of a leprosy control program.RÉSUMÉ
Un test immuno-enzymatique indirect (ELISA) prenant un disaccharide naturel octyl de l'albumine scrique bovine (ND-O-BSA) comme antigène a été utilisé chez des malades de la lèpre, des contacts et une population normale à Cebu, aux Philippines, de 1985 à 1989. Un total de 1413 personnes a été étudié. Les résultats suggéraient de manière générale une correlation entre la réactivité de l'ELISA et l'index bactérien (IB). Dans la lèpre multibacillaire (MB), la positivité s'étendait de 54,2% à 92,3% parmi les patients avec un IB allant de moins de 2+ à plus de 4+ sur l'échelle de Ridley, avec une moyenne générale de 84,5%. Les patients atteints de lèpre paucibacillairc (PB) avaient un faible degré de réactivité, avec seulement 15% d'ELISA positifs. Le test est plus efficient pour la détection de la lèpre MB que la lèpre PB. Les contacts de patients MB ont montré une positivité de l'ELISA de 6,5%; les contacts de patients PB une positivité de 7%. La population normale avait une positivité à l'ELISA de 1,7%, ou 17 positifs pour milie personnes, ce qui est beaucoup plus faible que les taux observés pour les contacts domiciliarics. Cependant, du fait que la population normale est beaucoup plus grande que la population de contacts domiciliares dans une communauté, un plus grand nombre de nouveaux cas de lèpre en sortira. Le personnel impliqué dans la lutte contre la lèpre se soucie particulièrement de la transmission de la maladie parmi les contacts domiciliaires. Cependant, pour la raison énoncée ci-dessus, nous devrions nous sentir plus concernés par la dissémination silencieuse de la maladie dans la population normale. D'autres études sont nécessaires en ce sens: une pour déterminer s'il existe une correlation entre les taux de prévalence et les taux de positivité à l'ELISA dans la population normale; l'autre pour voir si le taux de positivité à l'ELISA dans la population normale à l'intérieur d'une communauté peut être utilisé pour monitorcr l'efficience d'un programme de lutte contre la lèpreRESUMEN
De 1985 a 1989 se utilizó en Cebu, Filipinas, un inmunoensayo enzimático (ELISA) indirecto para analizar la reactividad de los sueros de pacientes con lepra, de sus contactos y de la población sana, contra el antígeno disacárido natural-octil-albúmina sérica bovina (ND-O-BSA). El estudio incluyó un total de 1413 personas. Los resultados del ELISA y del índice bacteriano (BI) correlacionaron bien de manera general. En los casos de lepra multibacilar (MB), la positividad osciló del 54.2% al 93.2% entre los pacientes con un BI de menos de 2+ a más de 4+ en la escala de Ridley, con un promedio general del 84.5%. Los pacientes con lepra paucibacilar tuvieron un bajo grado de reactividad, con sólo un 15% de positivos por ELISA. La prueba resultó más eficiente en la detección de casos MB que PB. Los contactos de los casos MB mostraron una positividad del 6.5% por ELISA; los contactos de los casos PB, un 7.0% de positividad. La población normal mostró una positividad del 1.7% (o 17 por 1000 habitantes) por ELISA, mucho menor que la encontrada entre los contactos convivientes. Sin embargo, debido a que la población normal en una comunidad es mucho mayor que la población de contactos convivientes, seguramente surgirán de ella más nuevos casos de lepra. Aunque los trabajadores de la lepra están procupados por la transmisión de la enfermedad a los contactos convivientes, de acuerdo los datos anteriores es claro que todos deberíamos estar también muy procupados de la silenciosa dispersión de la enfermedad a la población normal en la comunidad. Se requieren más estudios, y más extensos, para determinar si hay correlación entre los grados de prevalência y la positividad por ELISA en la población normal, y para establecer si el grado de positividad por ELISA en la población normal de una comunidad se puede usar para "monitorear" la eficiência de un programa de control contra la lepra.The optimal strategy for leprosy control consists of early detection and treatment to halt the transmission of the disease. Early detection will also prevent the development of deformities and disabilities (14,15). Current case-finding methods in leprosy are limited because the diagnosis can be made only after signs and symptoms of the disease have appeared. By that time, transmission to others already may have occurred, and in the patient deformities and disabilities may have started developing. For these reasons, a tool is urgently needed to diagnose leprosy before overt signs and symptoms of the disease appear. Many approaches have been devised for this purpose. Notable among them are the in vitro lymphocyte culture stimulation for detection of subclinical infection by Godal (8), the specific antibody radioimmunoassay by Harboe, et al. (11), and the fluorescent leprosy antibody absorption test (FLA-ABS) by Abe, et al. (1).
More recently, the enzyme-linked immunosorbent assay (ELISA) was developed as the result of the isolation of a Mycobacterium leprae-specihc antigen, phenolic glycolipid-I (PGL-I) (2,4,18). The modified, semisynthetic, natural disaccharide antigen octyl bovine albumin (ND-O-BSA) antigen representing the PGL-I molecule of M. leprae was later developed and is now in use. The ND-O-BSA antigen was reported to be at least equal to or even better than the other derivatives of the PGL-I antigen, whether natural or synthetic (7,17). This assay also has been used to monitor the development of leprosy in experimentally infected sooty mangabey monkeys and nine-banded armadillos. The studies in monkeys showed variable levels and patterns of anti-PGL-I antibodies (9), while in armadillos the establishment of infection could be detected earlier and more reliably than by the histologic method previously used (13).
One difficulty with the PGL-I ELISA is that there are significant variations both in ELISA technology and in the criteria for positivity which varies from laboratory to laboratory. However, it has been shown that while differences prevent direct comparison between different laboratories, there is good overall correlation (12).
The objective of the present study was to assess the ELISA reactivity of a cross-sectional group of populations (leprosy patients, their contacts and normal population) in Cebu, The Philippines, from 1985 to 1989 to the semisynthetic antigen (ND-O-BSA).
MATERIALS AND METHODS
ELISA. The ELISA used was an indirect assay in which 50 µ l of 0.15 µ g/ml concentration of ND-O-BSA is dried onto the "U" bottom of microtiter plates (Immulon I; Dynatech Laboratories, Chantilly, Virginia, U.S.A.). The details of the assay have been described elsewhere (5). An ELISA reading of 0.16 optical density (OD) and above was interpreted as positive. The ND-O-BSA antigen was provided by Dr. P. J. Brennan (Colorado State University, Fort Collins, Colorado, U.S.A.) under National Institutes of Health contract AI 52582.
Study populations. The populations studied included multibacillary (MB) and paucibacillary (PB) leprosy patients and their contacts. A normal population was also included. MB leprosy patients included all borderline and lepromatous patients with a bacterial index (BI) of at least 2+ on the Ridley scale at any one site. PB leprosy patients included indeterminate, tuberculoid (TT) and borderline tuberculoid (BT) with Bis of < 2+ (Ridley) at any one site. These parameters were defined in the World Health Organization (WHO) Study Group report of 1982 (15). Contacts were persons living in the same household as the MB and PB cases in the last 3 years. The normal population was composed of persons living in the same community as the patients but free of clinical disease and with no case of leprosy in their households.
RESULTS AND DISCUSSION
Table 1 shows the different population groups in the study. Blood samples were taken from a total of 1413 persons and tested for ELISA reactivity (720 males, 693 females). The mean age and the age range of each group are shown.
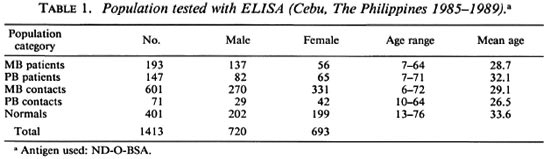
Table 2 depicts the percentages of ELISA-positive reactivity of the different population groups studied. The MB cases showed 84.5% positive reactivity; the PB cases, 15.0%; the MB contacts, 6.5%; the PB contacts, 7.0% and the normal population, 1.7%. MB patients had a high percentage of positives while a relatively small number of PB cases were positive. The majority of the PB cases (85.0%) were ELISA negative. These results demonstrate that the ELISA is much more efficient in detecting MB leprosy than PB leprosy. While the ELISA is highly specific for M. leprae, it appears to lack sufficient sensitivity to detect lower levels of antibody present in a few MB cases and most PB patients.
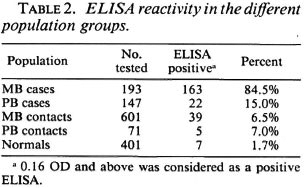
Table 3 depicts the degree of ELISA reactivity in newly diagnosed MB leprosy. In this table, reactivity has been stratified into groups of high positivity (reactivity > 0.6 OD), moderate positivity (reactivity between 0.31 to 0.60 OD), low positivity (reactivity between 0.16 to 0.30 OD), and negative reactivity (reactivity < 0.16 OD). The data show that of 193 MB patients tested, nearly half (47.2%) had high positivity, 22.3% moderate, and 15.0% low positivity. A total of 84.5% were positive and 15.5% were negative.
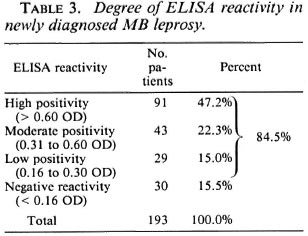
Table 4 correlates the Bis of the MB patients with their ELISA reactivity. The results show a high degree of correlation. The patients with high Bis (4+ and above) showed 75.4% high reactivity, 10.8% moderate, and 6.1% low reactivity. However, at this high BI it should be noted that 7.7% were ELISA negative. Patients with low Bis (< 2+) showed only 12.5% high positivity, 25.0% moderate, and 16.7% low positivity. A large number of the patients (45.8%) with low Bis had a negative ELISA.
It is difficult to explain why some patients with very high Bis have low reactivity or even a negative ELISA. One explanation is that perhaps ELISA reactivity may depend upon the presence or absence of antigenemia. Antigenemia stimulates the production of antibodies which plays a significant role in ELISA reactivity. When antigens are fixed in the tissues and are not found in sufficient amounts in the blood, the ELISA will be negative even in the face of a high BI. Conversely, the presence of adequate antigenemia could result in a positive ELISA even when the BI is low.
Table 5 shows the ELISA reactivity in newly diagnosed PB leprosy. Only 2% of the patients were highly positive, 4.8% were moderate, and 8.2% were of low positivity, for a total of only 15.0% with a positive ELISA. The great majority of the patients (85.0%) were negative. The findings demonstrate the inadequacy of the ELISA in detecting PB leprosy. This is consistent with the results in other studies in which only a small percentage of PB patients were ELISA positive (3,6,10,16).
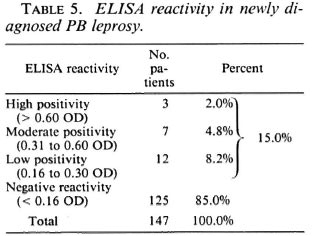
Table 6 shows the ELISA reactivity in contacts of PB and MB leprosy patients. No MB contacts showed high ELISA reactivity, 0.7% showed moderate, and 5.8% low positivity, for a total of 6.5% overall positivity. No PB contacts showed high ELISA reactivity, 1.4% showed moderate, and 5.6% low positivity. A total of 7.0% were ELISA positive. The great majority of the contacts (93.5% of MB and 93.0% of PB) were ELISA negative. The PB contacts were slightly more reactive than the MB contacts, but the difference is not statistically significant since the groups are not comparable. It is possible that PB contacts with positive ELISA were infected by MB cases outside their households rather than by the PB index cases within their households.
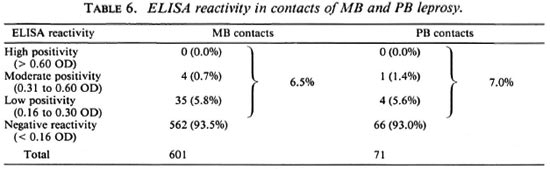
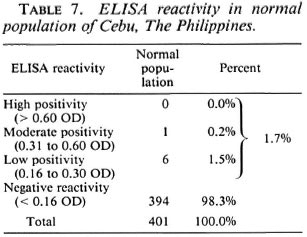
Table 7 shows the ELISA reactivity in the normal population. As previously described, this population comes from the same community as the patients and contacts in the study. They have no clinical disease and no case of leprosy in their households. Of the 401 tested randomly, 1.7% were ELISA positive. None of the normal population had high positive reactivity, 0.2% had moderate reactivity, and 1.5% were of low reactivity. The large majority of this population (98.3%) were ELISA negative. In 1985, from statistics compiled at the Cebu Skin Clinic (a government leprosy center), the prevalence of leprosy in the Cebu area was estimated to be approximately 1 per thousand population. The 1.7% ELISA positivity in the normal population converts to 17 per thousand population which is significantly less than the ELISA positivity in contacts of MB and PB patients (6.5% or 65 per thousand contacts for MB and 7.0% or 70 per thousand contacts for PB).
The spread of leprosy directly from patient to household contact has been of grave concern to leprosy workers. However, the spread of the disease to the normal population should be a more serious concern, because this represents a much greater number of the total community as compared to the household contact population. It is possible that more new cases could emanate from the normal population than from contacts, in spite of the normal population's lower ELISA positivity rate.
More studies are necessary along this line. One study would be to determine if communities with high, moderate or low prevalence rates also have high, moderate or low ELISA-positivity rates in the normal population. In other words, to determine if a correlation exists between the degree of prevalence rates and ELISA positivity in the normal population. Another would be to ascertain if ELISA positivity in the normal population of a community can be used to monitor the efficiency of a leprosy control program.
CONCLUSION
Generally speaking, the ELISA is able to detect most MB leprosy cases, with efficiency increasing as the bacterial load increases. There are, however, occasional MB cases with high bacterial load but with little or negative ELISA reactivity. There are also MB cases with low bacterial load but with moderate or even high ELISA reactivity. In PB leprosy, ELISA reactivity is usually of low degree, and most PB cases are ELISA negative. The findings demonstrate the inadequacy of the ELISA in detecting PB leprosy. Because the assay is generally efficient in detecting PGL-I antibodies, we conclude that low antibody levels in the blood are the cause of low reactivity or even negative ELISA in leprosy patients. We speculate that PGL-I antigens may be fixed in the tissues of these patients and not sufficiently released into the blood circulation. The contacts of MB and PB leprosy cases also showed a low degree of ELISA reactivity, and only 6.5% of the MB contacts and 7.0% of the PB contacts were positive.
Of the normal population, 1.7% or 17 per thousand were ELISA positive; this is much less than that of the leprosy household contacts. However, due to the much greater number of people representing the normal population in the community compared to the household contact population, more new leprosy cases would emanate from the normal population. Leprosy workers are justly concerned about the spread of the disease to the household contacts. However, we should be more concerned with the silent spread of the disease to the normal population because more potential new leprosy cases can emanate from it, especially when efficient methods of control of the disease are wanting. More studies are needed along this line: One would be to establish whether there is a correlation between varying degrees of prevalence rates and the rates of ELISA positivity in the normal population. The other would be to find out whether the rate of ELISA positivity in the normal population of a community can gauge the efficiency of leprosy control activities.
Acknowledgment. We acknowledge with thanks Mr. Junie F. Abellanaand Mrs. Maria Teresa Lee for taking the blood samples, Miss Manuela Luisa Parrilla for performing the ELISA, Mr. Bernardo Mendoza for his help with the computer work on the tables that are presented, and to Mr. Fernando B. Arriola and Miss Sheila Ann Reynes for their clerical help in the preparation of this manuscript.
REFERENCES
1. ABE, M. , MINAGAWA, F., YOSHINO, Y. , OZAWA, T., SAIKAWA, K. and SAITO, T. Fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Int. J. Lepr. 48(1980)109-119.
2. BRENNAN, P. J. and BARROW, W . W . Evidence for species specific lipid antigens in Mycobacterium leprae. Int. J. Lepr. 48(1980)382-387.
3. CHANTEAU, S., CARTEL, J.-L., ROUX, R., PLICART, R. and BACH, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid I in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-776.
4. CHO, S.-N., YANGIHERA, D. L., HUNTER, S. W. , GELBER, R. H and BRENNAN, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
5. DOUGLAS, J. T. , NAKA, S. O. and LEE, J. W. Development of an ELISA for detection of antibody in leprosy. Int. J. Lepr. 52(1984)19-25.
6. FINE, P. E. M., PONNIGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. C. Serocpidcmiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
7. FUJIWARA, T. , HUNTER, S. W. , CHO, S.-N., ASPI-NALL, G. O. and BRENNAN, P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of disaccharide protein conjugate for serodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
8. GODAL, T . Immunological detection of subclinical infection in leprosy. Lepr. Rev. 45(1974)22-30.
9. GORMUS, B. J., OHASHI, D. K. , OHKAWA, S., WALSH, G. P., MEYERS, W. M., BRENNAN, P. J. and TRYGG, C. Serologic responses to Mycobacterium leprae specific phenolic glycolipid-I antigen in sooty mangabcy monkeys with experimental leprosy. Int. J. Leprs. 56(1988)537-545.
10. GROENEN, G., PATTYN, S. R., GUYS, P., TSHILUMBA, K. , KUYKENS, L., COLSTON, M. J. and THE YA -LISOMBO STUDY GROUP. A longitudinal study of the incidence of leprosy in a hyperendemic area in Zaire, with special reference to PGL-antibody results. Int. J. Lepr. 58(1990)641-650.
11. HARBOE, M., CLOSS, O. , BJUNE, G., KRONWALL, G. and AXELSEN, N . Mycobacterium leprae H. specific antibodies detected by radioimmunoassay. Scand. J. Immunol. 7(1978)111-120.
12. MEEKER, H. C, LEVIS, W. R., SERSEN, E., SCHULLER-LEVIS, G., BRENNAN, P. J. and BUCHANAN, T. M. ELISA detection of antibodies against phenolic glycolipid-I in the management of leprosy; a comparison of laboratories. Int. J. Lepr. 54(1986)530-539.
13. TRUMAN, R. W. , MORALES, M. J., SHANNON, E. J. and HASTINGS, R. C. Evaluation of monitoring antibodies to PGL-I in armadillos experimentally infected with M. leprae. Int. J. Lepr. 54(1986)556-559.
14. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Scr. 768.
15. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Scr. 675.
16 WORLD HEALTH ORGANIZATION. Meeting of the Working Group on Rapid Diagnostic Test for Leprosy, World Health Organization, Regional Oflice for the Western Pacific, Manila. Philippines, 7-8 July 1987. Geneva: WHO. WP CHD/ICP/LEP/ 001-E.
17. Wu, Q., YE . G. and Li, X. Serological activity of natural disaccharide octyl bovine serum albumin (ND-O-BSA) in sera from patients with leprosy, tuberculosis and normal controls. Int. J. Lepr. 56(1988)50-55.
18. WU, Q., YE, G., LI, X., LIU, Q. and ZHOU, L. A preliminary study on serological activity of phenolic glycolipid and its application in diagnosis of leprosy. Proc. Chin. Acad. Sci./Peking Union Medical College 1(1986)58-61.
1. M.D., D.P.H. (Lond.), Assistant Director and Chief Epidemiologist; Leonard Wood Memorial Center for Leprosy Research, Cebu City 6000, The Philippines.
2. Ph.D., Director; Leonard Wood Memorial Center for Leprosy Research, Cebu City 6000, The Philippines.
3. M.D., M.P.H., Senior Clinical Consultant; Leonard Wood Memorial Center for Leprosy Research, Cebu City 6000, The Philippines.
4. M.D., Pathologist; Leonard Wood Memorial Center for Leprosy Research, Cebu City 6000, The Philippines.
5. D.V.M., Chief, Vivarium; Leonard Wood Memorial Center for Leprosy Research, Cebu City 6000, The Philippines.
6. M.D., Acting Chief, Clinical Research Branch; Leonard Wood Memorial Center for Leprosy Research, Cebu City 6000, The Philippines.
7. M.D., Clinical Assistant, Epidemiology Branch, Leonard Wood Memorial Center for Leprosy Research, Cebu City 6000, The Philippines.
8. M.D., M.P.H., First Secretary (Health) for Southern Africa, Royal Netherlands Embassy, Harare, Zimbabwe.
9. Ph.D., Associate Professor, Department of Microbiology, University of Hawaii at Manoa, Honolulu, Hawaii, U.S.A.
Received for publication on 28 July 1992;
Accepted for publication in revised form on 16 April 1993.