- Volume 61 , Number 2
- Page: 199–204
Tuberculosis in leprosy patients detected between 1902 and 1991 in French Polynesia
ABSTRACT
From 1902 onward, notification and follow up of leprosy patients has been systematic in French Polynesia. Since 1960, a tuberculosis control program and a register has also been implemented. From 1902 to 1959, 673 cases of leprosy were detected [346 multibacillary (MB), 138 paucibacillary (PB), and 179 unclassified due to the loss of medical files by the time of classification which was done during the 1980s]. Of these 673 cases, 89 (13.2%) died f rom tuberculosis, giving a mean annual death rate of tuberculosis in leprosy patients of 232 per 100,000. Mortality f rom tuberculosis in leprosy patients detected between 1901 and 1930 was 20.7%, and decreased to 8.04% in patients detected f rom 1931 to 1959. In total, it was estimated that 26.4% of the leprosy cases had developed tuberculosis. From 1960 to 1991, 350 new cases of leprosy were detected (141 MB, 209 PB). Of them, 12 (3.4%) developed tuberculosis (7 before detection of leprosy, 5 after detection of leprosy). The dramatic decrease of the proportion of leprosy patients who developed tuberculosis between the periods 1902-1959(26.4%) and 1960-1991 (3.4%) might be related to the important decline of the tuberculosis situation since 1960. From 1902 to 1959, mortality f rom tuberculosis occurred significantly more frequently in MB patients (13%) than in PB patients [4%, relative risk (RR) = 3.21, p = 0.003]. From 1960 to 1991, the incidence of tuberculosis seemed more frequent in MB patients (RR = 2.96, p = 0.07) whatever the sequence of detection of the two diseases. Our study suggests that lepromatous patients could share factors of susceptibility to mycobacterial diseases with patients developing tuberculosis.RÉSUMÉ
Depuis 1902, la notification et le suivi des malades de la lèpre a été systématique en Polynésie française. Depuis 1960, un programme de lutte contre la tuberculose a également été mis en route, ainsi qu'un registre. De 1902 à 1959, 673 cas de lèpre ont été détectés [346 multibacillaires (MB), 138 paucibacillaires (PB) et 179 sans classification suite à la perte du dossier médical au moment de la classification, qui fut réalisée dans le années quatre-vingts]. De ces 673 cas, 89 (13,2%) sont morts de tuberculose, donnant un taux annuel moyen de mortalité par tuberculose de 232 pour 100.000 chez les malades de la lèpre. La mortalité par tuberculose chez les lépreux détectés entre 1901 et 1903 était de 20,7% et a diminué à 8,04% chez les patients détectés de 1931 à 1959. Au total, on a estimé que 26,4% des malades de la lèpre avaient développé une tuberculose. De 1960 à 1991, 350 nouveaux cas de lèpre ont été détectés (141 MB, 209 PB). Parmi ceuxci, 12 (3,4%) ont développé une tuberculose (7 avant et 5 après la détection de la lèpre). La diminution spectaculaire de la proportion des malades de la lèpre qui ont développé une tuberculose entre les périodes 1902¬ 1959 (26,4%) et 1960-1991 (3,4%) pourrait être liée au déclin important de la tuberculose depuis 1960. Entre 1902 et 1959, la mortalité par tuberculose survenait sigificativement plus souvent chez des patients MB (13%) que chez des patients PB [4%, risque relatif (RR) = 3,21, p = 0,003]. De 1960 à 1991, l'incidence de la tuberculose paraissait plus fréquente chez des patients MB (RR = 2,96, p = 0,07) quel que soit l'ordre dans lequel les deux maladies avaient été détectées. Notre étude suggère que les malades lépromateux pourraient partager avec les patients tuberculeux des facteurs de susceptibilité aux maladies à mycobactéries.RESUMEN
A partir de 1902, la notificación y el seguimiento de los pacientes con lepra ha sido una práctica sistemática en la Polinesia Francesa. Desde 1960 también se ha implementado un programa de control y registro para la tuberculosis. De 1902 a 1959, se descubrieron 673 casos de lepra [346 multibacilares (MB), 138 paucibacilares (PB), y 179 no clasificados debido a la pérdida de los archivos médicos correspondientes]. Ochenta y nueve de estos 673 casos (13.2%) murieron de tuberculosis, dando una mortalidad media anual por tuberculosis de 232 por 100,000 pacientes con lepra. La mortalidad por tuberculosis que en los pacientes con lepra descubierta entre 1901 y 1930 fue del 20.7%, disminuyó al 8.04% en los pacientes descubiertos entre 1931 y 1959. Se calculó que el 26.4% de los casos de lepra habían desarrollado tuberculosis. De 1960 a 1991, se descubrieron 350 nuevos casos de lepra (141 MB, 209 PB). De ellos, 12 (3.4%) desarrollaron tuberculosis (7 antes y 5 después de la detección de la lepra). La dramática disminución en la proporción de pacientes con lepra que desarrollaron tuberculosis entre los periodos de 1902-1959 (26.4%) y de 1960-1991 (3.4%) podría estar relacionada con la importante disminución de tuberculosis observada desde 1960. De 1902 a 1959, la mortalidad por tuberculosis ocurrió más frecuentemente en los pacientes MB (13%) que en los PB [4%, riesgo relativo (RR) = 3.21, p = 0.003]. De 1960 a 1991, la incidencia de tuberculosis pareció ser más frecuente en los pacientes MB (RR = 2.96, p = 0.07), independientemente de la secuencia de detección de las dos enfermedades. Nuestro estudio sugiere que los pacientes lepromatosos comparten factores de susceptibilidad a las enfermedades por micobacterias con los pacientes que desarrollan tuberculosis.In 1895, G. H. Armauer Hansen found tuberculosis to be the most common cause of death among leprosy patients in Norway (11). Since that early study, the demonstration of the association of tuberculosis and leprosy in individuals living in an area endemic for both diseases scarcely has been documented although it is likely to be common (17). As suggested by many authors, tuberculosis and leprosy might interact or interfere with one another (5,7). The objective of this paper is to report the mortality from tuberculosis and the incidence of tuberculosis in leprosy patients detected between 1902 and 1991 in French Polynesia.
MATERIALS AND METHODS
Background. French Polynesia consists of five main archipelagoes containing some 130 islands located in the South Pacific between latitudes 8º south and 28º south. It has a total emerged land area of4000 square kilometers in an area of 4 million square kilometers of ocean. The population was 12,000 in 1902, and increased to 81,000 in 1960 and to 202,000 in 1991. The population is multi-ethnic but most of it is native, and the ethnic origin of the inhabitants has not significantly changed over the study period.
Leprosy patients. The first leprosy control activities began with the construction of leprosaria: the first two were in Tahiti and in a remote valley of the Southern Marquesas in 1914; a third one was opened in 1934 in Reao (Tuamotu archipelago). Notification of leprosy cases has been mandatory since 1902. From 1902 to 1946, the diagnosis of leprosy was based on clinical examination. Classification of patients according to the type of leprosy [multibacillary (MB) or paucibacillary (PB)] was performed during the 1980s for cases detected between 1902 and 1946 using the medical files. Since 1946, the diagnosis also has been based on biological tests: the lepromin intradermal reaction, the search for acid-fast bacilli (AFB) in nasal mucosa and skin (earlobes and skin lesions), and a biopsy for pathological examination. From 1951 to 1982 dapsone monotherapy, prescribed lifelong for MB patients and for an average of 10 years for PB patients, was the basis of treatment. Since 1982, multidrug therapy, including daily administration of rifampin 10 mg/kg, has been implemented in French Polynesia. For each new case of leprosy, a patient form is filled out indicating civil status, date, and place of leprosy detection as well as the results of clinical, microbiological and pathological examinations, and the nature and duration of treatment. Each patient is followed up lifelong, and the cause of death is recorded by a physician. All data on leprosy patients living in the three leprosaria are centralized in Tahiti, where a central leprosy register is kept. Case detection rates for leprosy decreased from around 50 per 100,000 in 1902 (important interannual fluctuations were observed during the beginning of this century) to 25 per 100,000 in 1946 and to 8 per 100,000 in 1991, with an average annual rate of decrease of 2.5% between 1945 and 1991 (3) and 2% between 1902 and 1991.
Tuberculosis patients. A tuberculosis control program and a register were implemented in 1960. Since the 1950s, multidrug therapy has been available for tuberculosis patients. From 1902 to 1945, the diagnosis of tuberculosis was mainly based on clinical examination; from 1945 onward radiological examination has been available. Bacteriological examination of sputum samples (smears and cultures) has been performed systematically since 1975. All cases notified by physicians are considered as tuberculosis cases. Between 1960 and 1991, case-notification rates for tuberculosis decreased dramatically from 568 to 25 per 100,000, an average annual rate of decrease of 9.6% (10).
Source of data. The data analyzed in this study come from the leprosy register. This register was linked with the tuberculosis register for assessment of the incidence of tuberculosis after 1960 in patients with a history of leprosy. We report here "a) the mortality from tuberculosis between 1902 and 1959 in leprosy patients, b) the incidence of tuberculosis from 1960 to 1991 in leprosy patients, and c) the incidence of leprosy from 1960 to 1991 in patients with a history of documented tuberculosis.
Statistical analysis. Frequencies and means were compared using standard bivariate analysis. Adjustment for covariates was performed using analysis of variance. All p values < 0.05 were considered statistically significant.
RESULTS
From 1902 to 1991, 1023 leprosy patients (634 males, 389 females) were detected. Of them, 844 patients could be classified into the PB or MB categories (357 and 487 patients, respectively). The mean age of patients at detection of leprosy was 27 years (S.D. 16), significantly lower for MB (25.8) than for PB (29.8, p < 0.05).
From 1902 to 1959 (before implementation of the tuberculosis register), 673 new cases of leprosy were detected. Of them, 89 (13.2%) died from tuberculosis, giving a mean annual death rate from tuberculosis in leprosy patients of 232 per 100,000. To date, 29 of the 673 cases are still living (11 MB, 18 PB). Assuming that 50% of the cases of tuberculosis would die if not treated, it could be estimated that 178 (26.4%) of the 673 leprosy patients developed tuberculosis.
The Table shows the mortality from tuberculosis in leprosy patients according to the type of leprosy. Among the 673 leprosy cases, 179 (27%) could not be classified into PB or MB categories due to missing data at the time of classification (loss of the medical file); these 179 cases were detected before 1931. Among the others, mortality from tuberculosis was more frequent in MB (13%) than in PB patients (4%), [relative risk (RR) = 3.21, p = 0.003 (95% confidence interval (CI) 1.4-7.36)]. Nonclassified patients could be a source of bias affecting the observed relation between type of leprosy and mortality from tuberculosis. However, those patients were not classified because of the loss of their medical files at the time of classification (in the 1980s), and this is unlikely to be related to the mortality from tuberculosis. Furthermore, during the period 1931-1959, 398 cases of leprosy were detected and all could be classified into the MB (66%) or PB (34%) categories. Among them, mortality from tuberculosis was significantly more frequent in MB (10.3%) than in PB (3.7%) patients (RR = 2.77, p = 0.02, 95% CI 1.09-7.04).
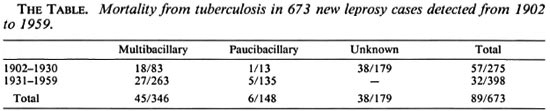
In total, mortality from tuberculosis in leprosy patients detected between 1901 and 1930 was 20.7% and decreased to 8.04% in leprosy patients detected from 1931 to 1959. Mortality from tuberculosis was not related to gender. The mean age at detection of leprosy in patients who died from tuberculosis was lower (19.9 years) than that of the other leprosy patients (24.3 years, p < 0.05), after adjustment for the type of leprosy. The median length of the interval between leprosy detection and death from tuberculosis was 7.5 (range 0-47) years. This length of interval was not related to the type of leprosy.
From 1960 onward, 350 new cases of leprosy were detected (141 MB and 209 PB, mean age at detection of leprosy 40 years, S.D. 18). Of them, 5 (1.4%) developed tuberculosis 1 to 23 (median 4.5) years after detection of leprosy. These 5 cases were MB patients (4 males, 1 female) who developed pulmonary tuberculosis (2 sputum-smear positive, 3 sputum-smear negative and culture positive). In addition, of the 350 cases of leprosy, 7 (2%) had a previous history of documented tuberculosis at detection of leprosy (6 males, 1 female). These 7 patients suffered pulmonary tuberculosis; all were sputum-smear negative and culture positive. The median length of the interval between detection of tuberculosis and detection of leprosy was 2 (range 1-9) years. Of the 7 patients who suffered successively tuberculosis and leprosy, 3 developed MB leprosy and 4 PB leprosy. In total, 12 patients (3.4%) developed both diseases (8 MB and 4 PB) (RR = 2.96, 95% CI 0.9-9.66, p = 0.07), whatever the sequence of detection of the two diseases.
DISCUSSION
Our observational study might be considered unsatisfactory because we could quantify the mortality from tuberculosis in leprosy patients detected from 1902 to 1959 but could not quantify the incidence of tuberculosis. The mean annual death rate for tuberculosis of 232 per 100,000 in our leprosy patients can be compared to the mean annual death rate for tuberculosis in Czechoslovakia around the year 1900 (about 380 per 100,000 general population) (20) or to the highest rate ever reported in the world: 653 per 100,000 general population in Alaska natives in 1950 (12). No reliable data on causes of death are available for French Polynesia for the period 1902-1985, and we could not compare the mean annual mortality rate of tuberculosis in leprosy patients to that in the general population. The institutionalization of leprosy patients until the 1950s probably could result in an increased opportunity for the spread of tuberculosis. But, also, one could argue that mortality rates of tuberculosis in leprosy patients could be of the same magnitude as mortality rates of tuberculosis in the general population with a similar socioeconomic environment. Nevertheless, tuberculosis was the first cause of death in Polynesian leprosy patients before implementation, by the end of the 1950s, of the so-called "100%-successful therapy" for tuberculosis. Such data are consistent with a study published one century ago, the results of which indicated tuberculosis as a main cause of death in leprosy patients (11).
A decrease in detection rates has been observed for leprosy from 1946 to 1991 (3) and for tuberculosis from 1960 to 1991 (10). These reductions in detection rates most likely reflect an improvement in the epidemiological situation for both diseases rather than a failure of the case-finding methods. An important economic development has been observed in French Polynesia, but it was not likely to be the only responsible factor for the improvement of the leprosy and tuberculosis situations. We observed in our population of leprosy patients a dramatic decrease in the proportion of patients who developed tuberculosis between the period 1902-1959 (estimated at 26.4%) and the period 1960-1991 (3.4%). Such a decrease might be related to the important decline of the tuberculosis situation since 1960. In the same way, a decrease in mortality from tuberculosis was observed in leprosy patients detected between the periods 1901-1930 (20.7%) and 1931-1959 (8.04%), suggesting either an improvement in the tuberculosis situation before the implementation of a multidrug therapy for tuberculosis or improvement in the living conditions of the leprosy patients, resulting in a decrease of the lethal rate of tuberculosis.
Of greater interest is the observation in leprosy patients that mortality from tuberculosis was more frequent in MB than in PB patients. This difference in mortality rates could not be attributed to a difference in the proportions of MB and PB patients living in a leprosarium (from 1902 to the 1950s, every one of the known leprosy cases was institutionalized). Also, the difference in mortality rates could not be attributed to a difference in all-cause mortality rates between MB and PB patients, since almost all of them had died when the study was conducted. Death information could vary with time, according to fashions or to newly available diagnostic methods, but we could not identify any systematic reason for a physician to ascertain similar causes of death differently, according to the type of leprosy.
We also found a higher (but not a significantly higher) proportion of patients developing both lepromatous leprosy and tuberculosis during the second period of study (1960-1991), the relative odds being close to those for mortality from tuberculosis in leprosy patients of the first period. The level of statistical significance was not reached, but leprosy and tuberculosis have become unfrequent conditions in French Polynesia over time. A lack of power of the statistical test, due to the small number of patients involved, could be invoked. Several theories have been raised concerning the interaction between tuberculosis and leprosy. The first ones suggested an antagonism between the two diseases (5,15); more recently, Gatner, et al. (9) and Kumar, et al. (14) reported that leprosy might encourage the development of tuberculosis. However, well known correlates of leprosy and tuberculosis, such as poor socioeconomic conditions, certainly need to be considered. Before the 1960s, the life style in French Polynesia was rural, and important differences in access to health services existed among the islands. However, there was no evidence that a higher proportion of MB cases came from remote islands as compared to the main island.
One might expect some crossprotection between leprosy and tuberculosis, but it has been difficult to demonstrate in human populations (4). However, Kaklamani, et al. (13) found a negative correlation between tuberculosis and the tuberculoid form of leprosy. A defective cellular immunity against mycobacteria in lepromatous patients could be a cause of this negative association, but this has never been demonstrated. Many studies on protective efficacy of BCG vaccine against the two forms of leprosy have also given contradictory results: BCG seems protective only against the nonlepromatous forms in some studies (1,8), the protection seemed higher against the lepromatous form of leprosy in another recent study (19), while no differences in protection between the two types of leprosy have been observed in other studies (2,16,18). One might speculate on the genetic factors that lepromatous patients could share, among other factors such as socioeconomic and ethnic factors, with patients developing tuberculosis (6), those factors inducing a higher susceptibility to both diseases.
REFERENCES
1. ABEL, L., CUA, V. V., OBERTI, J., LAP, V. D., DUE, L. K., GROSSET, J. and LAGRANGE, P. H. Leprosy and BCG in southern Vietnam. (Letter) Lancet 335(1990)1536.
2. BAGSHAWE, A., SCOTT, G. C, RUSSELL, D. A., WIGLEY, S. C, MERIANOS, A. and BERRY, G. BCG vaccination in leprosy: final results of the trial in Karimui, Papua New Guinea. Bull. WHO 67(1989)389-399.
3. CARTEL, J. L., BOUTIN, J. P., SPIEGEL, A., GLAZIOU, P., PLICMART, R., CARDINES, R. and GROSSET, J. H. Leprosy in French Polynesia; epidemiological trends between 1946 and 1987. Lepr. Rev. 63(1992)211-222.
4. CHAUDHURI, S. and GHOSH, S. Leprosy and tuberculosis: immune allergic relationship. Lepr. India 47(1975)295-306.
5. CHAUSSINAND, R. Tuberculose ct lèpre, maladies antagoniques; eviction de la lèpre par la tuberculose. Int. J. Lepr. 16(1948)431-438.
6. FINE, P. E. M. Immunogenetics of susceptibility to leprosy, tuberculosis, and leishmaniasis; an epidemiological perspective. Int. J. Lepr. 49(1981)437-454.
7. FINE, P. E. M. Leprosy and tuberculosis-an epidemiological comparison. Tubercle 65(1984)137-153.
8. FINE, P. E. M. BCB vaccination against tuberculosis and leprosy. Br. Med. Bull. 44(1988)671-703.
9. GATNER, E. M. S., GLATTHARR, E., IMKAMP, F. M. J. H. and KOK, S. H. Association of tuberculosis and leprosy in South Africa. Lepr. Rev. 51(1980)5-10.
10. GLAZIOU, P., MARTIN, P. M. V. and CARTEL, J. L. Surveillance épidémiologique de la tuberculose en Polynésic française de 196 0 à 1990. Bull. Soe. Pathol. Exot. Filiales 85(1992)130-135.
11. HANSEN, G. A. and LOOFT, C. Leprosy-in Its Clinical and Pathological Aspects. Bristol: John Wright & Co., 1895.
12. JOHNSON, M. W. Results of 20 years of tuberculosis control in Alaska. Health Serv. Rep. 88(1973)247-254.
13. KAKLAMANI, E., KOUMANDAKI, Y., KATSOUYANNI, K. and TRICHOPOULOS, D. BCG, tuberculosis and leprosy. (Letter) Lancet 337(1991)304.
14. KUMAR, B., KAUR, S., KATARIA, S. and ROY, S. N. Concomitant occurrence of leprosy and tuberculosis-a clinical, bacteriological and radiological evaluation. Lepr. India 54(1982)671-676.
15. LONG, E. R. Leprosy; some analogies and contrasts with tuberculosis. Arch. Environ. Health (Chicago) 14(1967)242-243.
16. MULIYIL, J., NELSON, K. E. and DIAMOND, E. L. Effect of BCG on the risk of leprosy in an endemic area: a case control study. Int. J. Lepr. 59(1991)229-236.
17. PARIKH, D. A., MANIAR, J. K., DHARNE, L. L. and GANAPATI, R. Association of leprosy and tuberculosis. J. Assoc. Physicians India 35(1987)131-133.
18. PONNIGHAUS, J. M. , FINE, P . E. M. , STERNE, J. A. C, WILSON, R. J., MSOSA, E., GRUER, P. J. K., JENKINS, P. A., LUCAS, S. B., LIOMBA, N. G. and BLISS, L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet 339(1992)636-639.
19. RODRIGUES, M . L. O., SILVA, S. A., NETO, J . C. A., DE ANDRADE, A. L. S. S., MARTELLI, C. M . T. and ZICKER, F. Protective effect of intradermal BCG against leprosy; a case-control study in central Brazil. Int. J. Lepr. 60(1992)335-339.
20. STYBLO, K. Recent advances in epidemiological research in tuberculosis. Adv. Tuberc. Res. 20(1980)1-63.
1. M.D.; Institut Territorial de Récherches Médicales Louis Malardé, 13. P. 30, Papeete, Tahiti, French Polynesia.
2. M.D., Institut Territorial de Récherches Médicales Louis Malardé, 13. P. 30, Papeete, Tahiti, French Polynesia.
3. M.D.; CHU Pitié Salpétrière, 91 Blvd. de l'Hôpital, 75634 Paris 13, France.
4. M.D.; CHU Pitié Salpétrière, 91 Blvd. de l'Hôpital, 75634 Paris 13, France.
5. Ph.D.; CHU Pitié Salpétrière, 91 Blvd. de l'Hôpital, 75634 Paris 13, France.
6. Computer Scientist; CHU Pitié Salpétrière, 91 Blvd. de l'Hôpital, 75634 Paris 13, France.
7. M.D., Professor, CHU Pitié Salpétrière, 91 Blvd. de l'Hôpital, 75634 Paris 13, France.
Received for publication on 16 October 1992.
Accepted for publication in revised form on 8 February' 1993.