- Volume 61 , Number 2
- Page: 205–13
Development of giant reaction in response to PPD skin test in lepromatous leprosy patients
ABSTRACT
The present study analyzes some clinical and immunological aspects of the giant reaction (GR) in lepromatous leprosy. Sixteen out of a total of 147 (10.9%) lepromatous patients developed the clinical features of GR upon the intradermal administration of PPD; most (14 of 16) GRs occurred in bacteriologically positive cases. GR precipitated an episode of erythema nodosum leprosum (ENL) in three patients. In addition, patients with GR showed enhanced in vitro response to PPD, by the lymphoproliferation test and interferon-gamma assay, as compared to either PPD-negative individuals or PPD-positive patients without GR. Therefore, cell-mediated-immune response to mycobacterial antigens is present in lepromatous patients with GR. It is suggested that the exacerbated in vivo response to PPD in lepromatous leprosy is the result of an increased immunoreactivity to the antigen, which well may be associated with the local and/or systemic release of cytokines [tumor necrosis factor-a (TNFα) and interferongamma (IFNγ)] by the inflammatory cells. These episodes may, in fact, play an important role in determining the development of disabilities and reactional states, thereby interfering with the prognosis of leprosy disease.RÉSUMÉ
Cette étude analyse quelques aspects cliniques et immunologiqycs de la réaction géante (RG) dans la lèpre lépromateuse. Seize patients parmi un total de 147 patients lépromateux (10, 9%) ont développé les signes cliniques d'une RG suite à l'administration intradermique de PPD; la majorité des réetions (14 sur 16) sont survenues chez des malades bactériologiquement positifs. La RG a précipité un épisode d'érythème noueux lépreux (ENL) chez trois patients. De plus, les patients avec une RG ont montré une stimulation de leur réponse in vitro au PPD par le test de prolifération lymphocytaire et le titrage de l'interferon-gamma, en comparaison avec des patients, aussi bien négatifs que positifs au PPD, mais sans RG. Donc, la réponse à médiation cellulaire aux antigènes mycobactériens est présente chez les patients lépromateux avec RG. On émet l'hypothèse que la réponse exacerbée au PPD in vivo dans la lèpre lépromateuse est le résultat d'une augmentation de la réactivité immunologiquc à l'antigène, qui pourrait bien être associée à une libération locale ou systémique de cytokines [le facteur a de nécrose des tumeurs (FNTα) et l'interferon-gamma (IFNγ)] par les cellules inflammatoires. Ces épisodes pourraient en fait jouer un rôle important dans le dévcloppement f incapacites et d'états réactionnels, influençant ainsi le pronostic de la maladie lépreuse.RESUMEN
El presente estudio analiza algunos aspectos clinicos e inmunológicos de la reacción gigante (RG) en la lepra lepromatosa. Diez y seis de un total de 147 pacientes lepromatosos (10.9%), desarrollaron las características clínicas de la RG después de la administración intradérmica de PPD; la mayoría de las RG (14 de 16) ocurrieron en casos bacteriológicamente positivos. La RG desencadenó un episodio de eritema nodoso leproso (ENL) en 3 pacientes. Comparados con los individuos PPD-negativos o con los individuos PPDpositivos sin RG, los pacientes con RG también mostraron respuestas in vitro incrementadas al PPD, tanto en los ensayos de linfoproliferación como en los del interferón gamma. Lo anterior indica que la respuesta inmune celular hacia los antigenos micobacterianos está presente en los pacientes lepromatosos con RG. Se sugiere que la exacerbada respuesta in vivo al PPD en la lepra lepromatosa es el resultado de una incrementada reactividad al antígeno, la cual bien podría estar asociada con la liberación local y/o sistémica de citocinas [factor nécrosante de tumores alfa (TNFα) e interferón gamma (IFNγ)] por las células inflamatorias. Estos episodios bien podrían tener un papel determinante del desarrollo de incapacidades y de estados rcaccionales y por lo tanto podrían interferir en el pronóstico de la enfermedad leprosa.Approximately 7 years ago, Waters and Stanford (28) reported an exacerbated response to tuberculin and purified protein derivative of tuberculin (PPD) denned by these authors as a giant reaction (GR). This type of reaction is characterized by erythema and edema at the site of the skin test with a mean induration larger than 22 mm, usually associated with symptoms such as fever, headache and lymphadenopathy. GR was limited to lepromatous leprosy patients with broad geographic and racial backgrounds. Although referred to in several other reports (8,27), this type of response has not yet been investigated fully.
GR to PPD seems to constitute another unusual pattern of response of lepromatous leprosy patients. These patients frequently exhibit exacerbated inflammatory reactions, expressed clinically as reversal reaction, erythema nodosum leprosum (ENL), or Lucio's phenomenon, that usually are accompanied by neuritis, orchitis, arthritis, edema and other systemic symptoms. These episodes can be induced by a variety of stimuli, namely, vaccination, drugs, viruses, associated infections, and mycobacterial skin tests (14). The mechanisms involved in these inflammatory and/or necrotic responses and the role of mycobacterial antigens in triggering reactional episodes remain unknown.
Many studies have shown that lepromatous patients, who are unable to mount a cell-mediated immune (CMI) response to specific Mycobacterium leprae antigens, still remain capable of showing a normal in vitro immune response toward other T-cell-dependent antigens (18,23). On the other hand, early reports have analyzed the in vivo response to PPD in leprosy patients (7,8,15,16). A smaller reactivity in multibacillary (MB) as compared to paucibacillary (PB) patients and household contacts has been described. In our population, reactivity to PPD by these groups was found to be 40.8%, 55.5% and 63.2%, respectively (5).
The aim of the present study is to analyze the clinical and immunological aspects of the giant reaction in lepromatous leprosy patients together with their in vitro immune responses to mycobacterial antigens in order to clarify the nature of this unusual in vivo reaction to PPD.
MATERIALS AND METHODS
Patient population. A total of 246 subjects [age range of 16 to 67 years (mean ± S.D. = 38 ± 16)] selected from the Leprosy Outpatient Unit, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil, were included in this study. Leprosy patients diagnosed according to the Ridley-Jopling classification (20) were distributed as follows: 147 MB patients [62 polar lepromatous (LL), 8 5 borderline lepromatous (BL)]; 99 PB patients [57 borderline tuberculoid (BT); 42 with indeterminate leprosy (LI)]. None of the patients on admission showed either X-ray or clinical symptoms of active tuberculosis. Eighty-four individuals were newly diagnosed patients and had not received any treatment prior to skin testing. The 162 treated patients had been treated for 2 to 24 months (mean ± S.D. = 13 ± 4 months) with multidrug therapy (MDT) (MB patients: rifampin 600 mg and clofazimine 300 mg monthly supervised, clofazimine 100 mg every other day, and dapsone 100 mg daily for 24 months unsupervised; PB patients: rifampin 600 mg monthly and dapsone 100 mg daily for 6 months unsupervised). Medication was continued throughout the study. Among these 162 treated patients, 36 patients were tested after being discharged from MDT (patients on surveillance; mean posttherapy period ± S.D. = 16 ± 12 months, ranging from 6 to 20 months). For in vitro tumor necrosis factor-alpha (TFN α ) measurements, blood cells were tested from 5 normal donors (laboratory personnel), 8 unreactional lepromatous patients, 8 lepromatous patients with reactions (ENL), 3 patients on surveillance, and 4 patients with GR. ENL patients were treated with thalidomide (300 mg/day, progressively reduced by 100 mg) until total remission of the inflammatory symptoms.
Enumeration of bacilli. Slit-skin smears for obtaining a bacterial index (BI) (19) were taken from six sites at the time of diagnosis and were taken again to indicate their rate of reduction during the 24 months of MDT. The Bis of patients tested ranged from 0 to 5+ (mean ± S.D. = 3.5 ± 1.0).
Serum collection. Serum samples for the determination of TNF α levels were collected from lepromatous leprosy patients without GR and from three GR patients before and following the PPD skin test. The sera were collected aseptically, processed under sterile conditions, stored in 0.5-ml aliquots, and kept frozen (-20ºC) until used.
Culture stimulation. Armadillo-derived M. leprae antigen was provided Dr. R. J. W. Rees (IMMLEP Bank, National Institute of Medical Research, London, U.K.). The optimal antigen-stimulating concentration was found to be 20 µ g/ml. BCG (bacillus Calmette Guerin, 5 mg) was provided by the Ataulfo de Paiva Foundation (Rio de Janeiro, Brazil) and, after heat-killing, was used at 25 µ g/ml. PPD (Statens Serum institut, Copenhagen, Denmark) was used at 10 µ g/ml. Single batches of each antigen preparation were used throughout the entire study. The concentrations of contaminating endotoxin in stock solutions and antigens were estimated by the Limulus amebocyte lysate assay (LAL; Whittaker M.A. Bioproducts, Walkersville, Maryland, U.S.A.). The reagents contained less than 10 pg/ml endotoxin.
Monocyte cultures. Heparinized venous blood was collected for in vitro tests, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque (Pharmacia Fine Chemicals, Piscataway, New Jersey, U.S.A.) density centrifugation (1). For monocyte enrichment, PBMC were rosetted with neuraminidase-treated (Vibrio choleras neuraminidase; Calbiochem-Behring Corp., La Jolla, California, U.S.A.) sheep erythrocytes (Scott Laboratories, Friskeville, Rhode Island, U.S.A.) (15); 106 nonrosetted cells were cultured in 24-well plates (Corning Glass Works, Corning, New York, U.S.A.) in 1 ml RPMI 1640 medium (Gibco Laboratories, Grand Island, New York, U.S.A.), supplemented with 10% pooled AB+ serum, 100 U/ml penicillin, 100 µ g/ml streptomycin, and 2 mM L-glutamine (complete medium). The cells were stimulated with TNF α -inducing agonists for 18- 20 hr, at which time the supernatants were harvested and kept frozen (-20º C) until used.
Lymphocyte transformation test (LTT). PBMC, obtained as described above, were incubated in triplicate at 2 x 105 cells/well at 37ºC in 96-well, U-bottom plates (Costar Corporation, Cambridge, Massachusetts, U.S.A.) in 200 µ l of complete medium. The cells were cultured in the presence or absence of antigen, and the assay was performed as described (11). The results obtained as counts per minute (cpm) are expressed as ∆cpm (cpm obtained in stimulated cultures minus cpm obtained in control cultures) ± S.E.M.
Interferon gamma (IFN γ ) assay. The supernatants from each triplicate of the PBMC cultures (130 µ l per well) were recovered on day 5, pooled, and kept frozen (-20ºC) until used. The levels of IFN γ in the supernatants were determined using a commercial immunoradiometric assay (IRMA) kit (IMRX Corporation, Malvern, Pennsylvania, U.S.A.) specific for the active human IFN γ (2). The supernatants were diluted 1:2 and processed in duplicate according to the manufacturer's specifications. Units/ml (U/ ml) of IFN γ for each sample were obtained on a standard curve from standards provided with the kit, and were multiplied by the dilution factor. Levels of IFN γ are expressed as U/ml in the stimulated cultures minus U/ml obtained in the control cultures. The cut-off point for a positive response was 40 U/ml which is 4 standard deviations above mean values obtained in unstimulated cultures. The limit of detection for the assay is 0.1 U/ml.
TNF α ELISA. TNF α concentrations in serum samples and in cell culture supernatants were determined using a commercial TNF α -specific ELISA kit that was processed according to the manufacturer's recommendation (N. V. Innogenetics S.A., Antwerp, Belgium). The detection limit of the assay is 4 pg/ml. No increased TNF α levels were detected in the serum or plasma of 10 normal individuals tested (average value = 15 pg/ml). The cut-off point for a positive response was 45 pg/ml.
Skin test. An in vivo response to PPD was tested by injecting intradermally 0.1 ml of the reagent into the volar surface of the right forearm. Five tuberculin units (TU) of PPD (provided by Ataulfo de Paiva Foundation) were used. Induration was measured 48-72 hr later, and diameters of > 10 mm were regarded as positive.
A lepromin skin test was performed by intradermal injection of 0.1 ml of the standardized crude antigen preparation (lepromin A, 1.6 x 108 bacilli/ml; GWL Hansen's Disease Center, Carville, Louisiana, U.S.A.) into the volar surface of the left forearm. Readings were performed after 21-28 days, and reactions of > 3 mm were considered positive (10). All patients were lepromin tested before initiating specific leprosy treatment. The same batches of lepromin and PPD were used throughout the study. All injections and readings were done by the same trained person.
Statistical analysis. Parametric and nonparametric tests were used for statistical evaluation of significance. Student's t test, Kruskal-Wallis test, Chi-squared test and Fisher's exact test were used when appropriate. Standard deviation (S.D.) or standard error of the mean (S.E.M.) were used to express variances.
RESULTS
GR development in lepromatous leprosy patients. Among the 147 MB patients tested, 16 patients (8 BL and 8 LL) (10.9%) developed skin-test responses to PPD with an induration size of > 22 mm surrounded by a large, swollen erythematous area exceeding, overall, 60 mm or more in diameter which occasionally involved the full circumference of the forearm. The central area, often vesiculated, sometimes showed necrosis. This reaction was accompanied by local pain, fever, headache, arthritis and axillary lymphadenopathy with or without lymphangitis (giant reaction, GR) (28). The peak of induration occurred at 48-96 hr. In some cases, the reaction (erythema and edema) started as early as 12 hr after injection. The indurated area gradually diminished in size but, in many cases, was still present after 21 days. All 16 individuals with GR were negative to the lepromin skin test (data not shown).
Five out of these 16 patients (31.2%) reported previous contact with tuberculosis. However, all patients had a normal chest X-ray and normal sputum examination, and none of them developed clinical tuberculosis during the 5 years of follow up. Confirming previous observations (27,28), GR was not observed in PB patients, although some of them (6 individuals) presented indurations at the PPD test site above 20 mm (Fig. 1B).
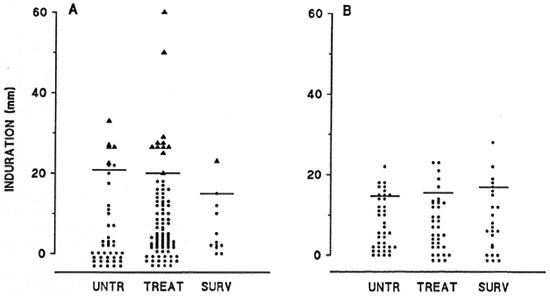
Fig. 1. In vivo reactivity to PPD in (A) multibacillary and (B) paucibacillary leprosy patients according to their treatment status. Untr = Untreated patients (A, N = 43; B, N = 39); Treat = patients treated with MDT for 2 months to 2 years (A, N = 92; B, N = 36); Surv = patients after 2 years of specific treatment who are now under surveillance (A, N = 12; B, N = 24); horizontal bars represent mean of positive induration (PPD > 10 mm) for each group tested; each point represents a single patient; ▲ = patients who developed giant reaction.
In spite of the fact that the percentage of reactivity to PPD was found to be lower among the lepromatous population (5), the mean induration size at the skin-test site was significantly higher in MB than in PB patients (p < 0.001, Student's t test) (Fig. 1). The incidence of GR among lepromatous patients was not associated with the duration of MDT. Approximately 11.6% (5 of 43) of the individuals developed GR in the untreated population as compared to 10.8% (10 of 92) of the treated patients and 8.3% (1 of 12) of the patients under surveillance (Fig. 1A). Although most (87.5%) GR cases occurred in bacteriologically positive patients, no significant difference in the occurrence of GR among patients with a negative or positive BI was noted (p > 0.02, Chi-squared test or Fisher's exact test). Two out of 35 individuals (5.7%), as compared to 14 of 112 (12.5%), developed GR in the Bl-negative or -positive populations, respectively (Fig. 2).
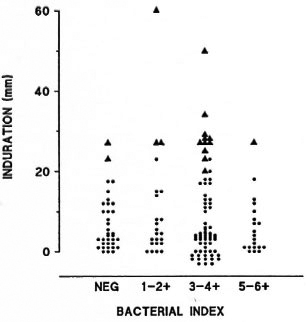
Fig. 2. In vivo reactivity to PPD in multibacillary leprosy patients according to the presence of M. leprae bacilli in slit-skin smears (bacterial index). Each point represents a single patient (N = 147); ▲ = patients who developed giant reaction.
In order to compare patient reactivity to repeated PPD injections, 13 lepromatous patients were retested for their in vivo responses to PPD within 2-17 months after the first PPD injection. The response to the second test was not significantly different from the previous one: 6 individuals were repeatedly skin-test negative, 5 presented GR in both tests, and only 2 patients (strongly positive in the first test) presented GR (systemic symptoms) in the second test. In addition, three patients with GR were retested after completion of MDT. Although all three patients presented induration and erythematous areas of > 24 mm (mean ± S.D. = 29.6 ± 4.0 and 33.6 ± 13 mm, respectively), none of them showed the systemic manifestations of GR as seen in the first PPD test.
Twelve of the 16 patients with GR (75%) developed ENL episodes during the course of their disease. Interestingly, in three patients, GR precipitated an ENL episode. Among the 131 MB patients who did not develop GR, the incidence of ENL (disseminated subcutaneous nodules associated or not with systemic symptoms) was 42% (55 patients). A significant difference in the incidence of ENL between the two groups (with or without GR) was noted (p < 0.01, Chisquared test).
Correlation between in vitro response to pp d in lepromatous patients and development of GR. The in vitro immune response to M. leprae, BCG, and PPD was evaluated in 31 lepromatous leprosy patients (11 LL and 20 BL) using the LTT and the IFN γ assay (24 patients). Patients were distributed into three groups according to their in vivo responses to PPD (GR, PPD + and PPD-). A significant association between the in vivo and in vitro responses to PPD was noted (p < 0.001, Kruskal-Wallis test) (Fig. 3). Lepromatous patients who developed a GR showed higher T-cell proliferation (mean ∆cpm ± S.E.M. = 42,071 ± 12,820) as compared to patients with positive (PPD+) or negative (PPD-) skin-test responses (mean ∆cpm ± S.E.M. = 14,063 ± 4157 and 2345 ± 1078, respectively) (Fig. 3 A, open bars). The in vitro response to BCG followed the same pattern as that to PPD (Fig. 3A). Patients with GR were more responsive to BCG antigen in the lymphoproliferation assay than those without GR. Accordingly, levels of IFN γ in response to PPD were higher in patients with GR (mean U/ml ± S.E.M. = 138 ± 42) than in PPDpositive (mean U/ml ± S.E.M. = 36.5 ± 23.2) or -negative individuals (mean U/ml ± S.E.M. = 17.2 ± 12) (Fig. 3B). The in vitro response to M. leprae was not influenced by the in vivo response to PPD. As shown in Figure 3B (double hatched bars), patients in all groups remained nonresponsive to M. leprae antigen in spite of their high responsiveness or nonresponsiveness to PPD, both in vivo and in vitro.
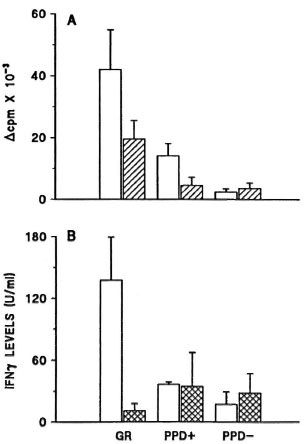
Fig. 3. A: In vitro LTT response to PPD ( ) or BCG (
) or BCG ( ) antigens among 31 lepromatous leprosy patients grouped according to their in vivo reactivity to PPD skin test. GR = Patients who developed the exacerbated in vivo response to PPD (N = 8); PPD+ = individuals who presented positive skin test (PPD > 10 mm) without GR (N = 8); PPD -= individuals who were skin-test negative (N = 15); results are expressed as mean ∆cpm ± S.E.M. for each group tested. B: In vitro IFN γ production in response to PPD (
) antigens among 31 lepromatous leprosy patients grouped according to their in vivo reactivity to PPD skin test. GR = Patients who developed the exacerbated in vivo response to PPD (N = 8); PPD+ = individuals who presented positive skin test (PPD > 10 mm) without GR (N = 8); PPD -= individuals who were skin-test negative (N = 15); results are expressed as mean ∆cpm ± S.E.M. for each group tested. B: In vitro IFN γ production in response to PPD ( ) or M. leprae (
) or M. leprae ( ) among 24 leprosy patients grouped according to in vivo response to PPD. GR = Patients who developed giant reaction (N = 9); PPD+ = patients with positive skin test without GR (N = 5); PPD = patients nonreactive to PPD in vivo (N = 10); results are expressed as mean U/ml of IFN γ ± S.E.M. for each group tested.
) among 24 leprosy patients grouped according to in vivo response to PPD. GR = Patients who developed giant reaction (N = 9); PPD+ = patients with positive skin test without GR (N = 5); PPD = patients nonreactive to PPD in vivo (N = 10); results are expressed as mean U/ml of IFN γ ± S.E.M. for each group tested.
Detection of TNF α in serum samples obtained from g r patients. As described previously, lepromatous patients with ENL presented higher TNF α levels in their circulation than did patients without reaction (22,24). In the present study, patients who developed GR had their sera collected before and at the time of PPD skin-test reading. For all three patients tested, the amount of TNF α in the serum was increased following the PPD skin test (mean TNF α levels ± S.E.M. = 23.3 ± 14.5 vs 76.6 ± 14.5 pg/ ml before and after testing, respectively). This was not observed for lepromatous patients who did not develop GR (data not shown). However, determinations of TNF α levels during the GR episodes still need to be performed.
Synthesis of TNF α by peripheral blood monocytes. Monocyte-enriched cultures were examined for their capacity to produce TNF α following in vitro exposure to BCG or M. leprae. In the absence of any stimulant, no spontaneous release of TNF α was detectable, even from cells of patients undergoing an ENL reaction. However, after in vitro stimulation of monocytes with M. leprae or BCG (Fig. 4), a higher TNF α response was detected in untreated reactional patients compared to normal individuals, lepromatous patients without ENL, or thalidomide-treated ENL patients (data not shown). In addition, patients with GR showed enhanced production of TNF α in vitro compared to the amount of TNF α released by cells from ENL patients (Fig. 4). Similar results were obtained when whole PBMC were used in vitro. Interestingly, PBMC obtained from ENL patients, GR patients, or unreactional lepromatous patients released more elevated TNF α levels than did monocytes obtained from the-same individuals (data not shown).
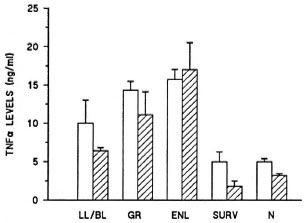
Fig. 4. Levels of TNF α released by monocytes from multibacillary leprosy patients and normal controls following in vitro stimulation with M. leprae ( ) or BCG (
) or BCG ( ). LL/BL = Lepromatous patients without reaction (N = 8); GR = lepromatous patients who developed giant reaction (N = 4); ENL = lepromatous patients who had developed ENL during the study (N = 8); Surv = patients who were tested after being discharged from MDT (N - 3); N = normal individuals (N = 5); cytokine levels are expressed in ng/ml of protein and represent mean ± S.E.M. for all individuals tested.
). LL/BL = Lepromatous patients without reaction (N = 8); GR = lepromatous patients who developed giant reaction (N = 4); ENL = lepromatous patients who had developed ENL during the study (N = 8); Surv = patients who were tested after being discharged from MDT (N - 3); N = normal individuals (N = 5); cytokine levels are expressed in ng/ml of protein and represent mean ± S.E.M. for all individuals tested.
DISCUSSION
In the present study, 16 lepromatous leprosy patients (10.9%) developed the clinical features of GR upon the intradermal administration of the purified protein derivative of tuberculin (PPD). In general, strong responses to PPD have been associated with previous contact with tuberculosis (25). However, it is difficult to believe that only 16 individuals among the population studied have had prior contact with tuberculosis. The mechanisms involved in the exacerbated response to PPD seen only in lepromatous leprosy patients raise several hypotheses that may help to understand the clinical course of lepromatous disease. Although no difference in the development of GR was noted between the MDT-treated and the untreated population, most patients with GR (87.5%) showed a positive BI. These data suggest that GR can occur whether the bacteria are alive or not, and that the antigenic load seems to play an important role in this phenomenon.
Thirteen patients were submitted to a second PPD skin test within a 1-year period. The response to the second test was not different from the previous one. It would seem that a booster phenomenon cannot explain the exacerbated response to PPD since only those patients who developed GR in the first PPD skin test did so after the second test.
Lepromatous patients have been considered by many authors as being anergic to specific M. leprae antigen, thereby excluding any role that cell-mediated immunity (CMI) may have in determining the pathogenesis of the disease. It has been suggested that the high bacillary load is implicated in the lower reactivity to PPD among lepromatous patients (15,16). Treatment of patients enhances the delayed-type hypersensitivity (DTH) responses to both common and specific mycobacterial antigens (3,6). We herein propose that cell-mediated immunity exists in lepromatous leprosy and, although it is not able to prevent M. leprae multiplication, it probably interferes with the course of the disease. In the present study, it was demonstrated that the CMI response to mycobacterial antigens, expressed by the strong response to PPD in vivo and in vitro, is present in lepromatous patients with GR.
GR precipitated ENL in three patients. Although it is believed that ENL is due to an immune-complex-mediated injury, definitive proof is still lacking. There is evidence, however, of the emergence of T-cell reactivity to M. leprae antigen in ENL (12 17), which might explain a temporary production of cytokines and the consequent induction of monocyte activation (4,13). ENL is characterized by the appearance of disseminated subcutaneous nodules in the body, which may also be associated with systemic symptoms. It has been demonstrated that lepromatous patients with ENL showed high levels of TNF α and interleukin-1 β (IL-1 β ) in their sera during ENL episodes (24). The systemic features of GR and their similarity to reactions to endotoxin or to ENL suggest that those GR symptoms might be TNF α -mediated symptoms. IFN γ has been shown to be involved in the amplification of the TNF α response by monocytes after antigen or lipopolysaccharide stimulation in vitro or in vivo (9,21,23). Therefore, we propose that cytokines released by inflammatory cells could amplify inflammatory responses leading to hyper-reactivity, such as the exacerbated response to PPD and/or the reactional episodes. As demonstrated in this study, the increased levels of IFN γ and TNF α released by cells of patients with GR, in vitro or in vivo, support this hypothesis. Because of the multifunctional effects played by TNF α on several cells and tissues in the body (26), the occurrence of these episodes may play a major role in determining the development of reactions and, therefore, interfere with the prognosis of the disease. These proposed mechanisms are being investigated further.
Acknowledgment. We thank Dr. M. E. N. Gallo and M. F. S. Alvim for their support throughout the study and T. S. Guedes for technical assistance. Thanks are also due to A. R. de Moura for the graphic work. This study was supported in part by the WHO/UNDP/World Bank/TDR.
REFERENCES
1. BOYUM, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Invest. 21Suppl.(1968)97-105.
2. CHANG, T. W., MCKINNEY, S., LIU, V., KUNG, P. C, VILCEK, J. and LE, J. Use of monoclonal antibodies as sensitive and specific probes for biologically active human 7-interferon. Proc. Natl. Acad. Sci. U.S.A. 81(1984)5219-5222.
3. CRÉE, I. A., SMITH, W. C. S., REES, R. J. and BECK, J. S. The influence of antimycobacterial chemotherapy on delayed hypersensitivity skin test reactions in leprosy patients. Lepr. Rev. 59(1988)145-151.
4. DÉBETS, J. M. H., VAN DER LINDEN, C. J., SPRONKEN, I. E. M. and BUURMAN, W. A. T cellmediated production of tumour necrosis factor-a by monocytes. Scand. J. Immunol. 27(1988)601-608.
5. DUPPRE, N. C, ALVIM, M. F. S., GALLO, M. E. N., NERY, J. A. C. and SARNO, E. N. Fatores envolvidos na reatividade do PPD em pacientes com doença de Hansen. Cadernos Saúde Publica 6(1990)175-185.
6. ESQUENAZI, D. A., SAMPAIO, E. P., MOREIRA, A. L., GALLO, M. E. N., ALMEIDA, S. M. R. and SARNO, E. N. Effect of treatment on immune responsiveness in lepromatous leprosy patients. Lepr. Rev. 61(1990)251-257.
7. FERNANDEZ, J. M. M. Estúdio comparativo de la reaccion de mitsuda con las reacciones tuberculinicas. Rev. Argent. Dermatol. 23(1939)425-451.
8. HALE, J. H., MOLESWORTH, B. D., GROVE-WHITE, R. J., SAMBAMURTHI, C. M. and RUSSEL, D. A. A relationship and significance of the Mantoux and lepromin reactions in leprosy. Int. J. Lepr. 23(1955)193-147.
9. HART, P. H., WHITTY, G. A., PICCOLI, D. S. and HAMILTON, J. A. Control by IFN-7 and PGE2 of TNF α and IL-1 production by human monocytes. Immunology 66(1989)376-383.
10. INTERNATIONAL CONGRESS OF LEPROSY. Immunology, technical resolutions. Int. J. Lepr. 21(1953)527-535.
11. KAPLAN, G., WEINSTEIN, D. E., STEINMAN, R. M., LEVIS, W. R., ELVERS, V., PATARROYO, M. E. and COHN, Z . A. An analysis of in vitro T cell responsiveness in lepromatous leprosy. J. Exp. Med. 159(1985)917-929.
12. LAAL, S., BHUTANI, L. K. and NATH, I. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect. Immun. 50(1985)887-892.
13. NATHAN, C. F., KAPLAN, G., LEVIS, W. R., NUSRAT, A., WITMER, M. D., SHERWIN, S. A., JOB, C. K., HOROWITZ, C. R., STEINMAN, R. M. and COHN, Z. A. Local and systemic effects of intradermal recombinant interferon-7 in patients with lepromatous leprosy. 1986. N. Engl. J. Med. 315(1986)6-15.
14. PETTIT, J. H. S. and WATERS, M. F. R. The etiology of erythema nodosum leprosum. Int. J. Lepr. 35(1967)1-10.
15. PINTO, M. R. M., ARSECULERATNE, S. N. and WEL-LIANGA, L. V. The differential tuberculin test in leprosy. Lepr. Rev. 44(1973)13-21.
16. QUINTO, R. S. and MABAIAY, M. C. A note on the tuberculin reactions in leprosy. Int. J. Lepr. 30(1962)278-283.
17. RAO, R. D. and RAO, P. R. Enhanced cell-mediated immune response in erythema nodosum leprosum reactions of leprosy. Int. J. Lepr. 55(1987)36-41.
18. REITAN, L. J. The specificity of the immunodeficiency in lepromatous leprosy. Lepr. Rev. 57(1986)203-205.
19. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. 2nd edn. Cochrane, R. G . and Davey, T., eds. Bristol: John Wright and Sons, 1964, Appendix III, p. 620.
20. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-271.
21. ROOK, G. A. W., TAVERNE, J., LEVETON, C. and STEELE, J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology 62(1987)229-234.
22. SAMPAIO, E. P., MOREIRA, A. L., SARNO, E. N., MALTA, A. M. and KAPLAN, G. Prolonged treatment with recombinant interferon- γ induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175(1992)1729-1737.
23. SARNO, E. N., ESPINOSA, M., SAMPAIO, E. P., VIEIRA, L. M. M., FIGUEIREDO, A. A., MIRANDA, C. F., ESQUENAZI, D., SALGADO, J. L. and NOGUEIRA, N. Immunological responsiveness to M. leprae and BCG antigens in 98 leprosy patients and their household contacts. Braz. J. Med. Biol. Res. 21(1988)461-470.
24. SARNO, E. N., GRAU, G. E., VIEIRA, L. M. M. and NERY, J. A. C. Serum levels of tumour necrosis factor-alpha and interleukin-1/? during leprosy reactional states. Clin. Exp. Immunol. 84(1991)103-108.
25. STANFORD, J. L. and LEMA, E. The use of a sonicate preparation of Mycobacterium tuberculosis (new tuberulcin) in the assessment of BOG vaccination. Tubercle 64(1983)275-282.
26. WAKEFIELD, P. E., JAMES, W. D., SAMLASKA, C. P. and MELTZER, M. S. Tumor necrosis factor. J. Am. Acad. Dermatol. 24(1991)675-685.
27. WATERS, M. F. R. and RIDLEY, D. S. Necrotizing reactions in lepromatous leprosy, a clinical and histological study. Int. J. Lepr. 31(1963)418-436.
28. WATERS, M. F. R. and STANFORD, J. L. Giant reactions to tuberculin in lepromatous leprosy pa tients. Int. J. Lepr. 53(1985)546-553.
1. M.D.; Leprosy Unit, Oswaldo Cruz Foundation, Avenida Brasil 4365, Manguinhos, Rio de Janeiro, RJ, Brazil 21040.
2. Nurse; Leprosy Unit, Oswaldo Cruz Foundation, Avenida Brasil 4365, Manguinhos, Rio de Janeiro, RJ, Brazil 21040.
3. M.D.; Leprosy Unit, Oswaldo Cruz Foundation, Avenida Brasil 4365, Manguinhos, Rio de Janeiro, RJ, Brazil 21040.
4. M.D.; Leprosy Unit, Oswaldo Cruz Foundation, Avenida Brasil 4365, Manguinhos, Rio de Janeiro, RJ, Brazil 21040.
5. M.D., Head, Leprosy Unit, Oswaldo Cruz Foundation, Avenida Brasil 4365, Manguinhos, Rio de Janeiro, RJ, Brazil 21040.
Reprint requests to Dr. Sarno.
Received for publication on 21 October 1992.
Accepted for publication in revised form on 26 January 1993.