- Volume 61 , Number 2
- Page: 214–7
Leprosy reversal reaction in HIV-Positive patients
ABSTRACT
We report two cases of leprosy in HIV-infected patients who, by their clinical, histological and immunological features, enhance the evidence that HIV-positive leprosy does not differ f rom nonHIV-positive leprosy. Moreover, extensive studies of reversal reactions in HIV-positive patients might be of great interest in determining the exact pathogenesis of this leprosy reactional state.RÉSUMÉ
Nous rapportons deux cas de lèpre chez des patients infectés par le VIH qui, par leurs caractéristiques cliniques, histologiques et immunologiqucs, renforcent l'évidence que la lèpre chez les patients séropositifs pour le VIH ne diffère pas de celle des séronégatifs. De plus, des études approfondies des réactions reverses chez des patients séropositifs pour le VIH pourraient être d'un grand intérêt pour déterminer la pathogénèse exacte de cet état réactionncl de la lèpre.RESUMEN
Informamos sobre dos casos de lepra en pacientes infectados con el virus de la inmunodcficicncia adquirida (HIV) quienes, por sus características clínicas, histológicas e inmunológicas, refuerzan la evidencia de que los pacientes con lepra HIV-positivos, no difieren de los pacientes con lepra HIV-negativos. El estudio cuidadoso y extensivo de las reacciones reversas en los pacientes HIV-positivos, podría resultar de gran valor para ayudar a entender la patogénesis exacta de este tipo de lepra reaccional.The relationship between the human immunodeficiency virus (HIV) and Mycobacterium leprae infections is still unclear. In contrast with tuberculosis or other mycobacterial diseases, no increased incidence of leprosy has been noted in both HIV- and leprosy-endemic countries. Indeed, the paucity of reported leprosy cases in HIV-infected patients is striking. We report two cases of leprosy in HIV-positive patients who, in addition, each developed a reversal reaction which is known as a delayed-type hypersensitivity reaction.
CASE REPORTS
Case 1. A 40-year-old Senegalese man was seen in May 1989 at the Infectious Diseases Center of the Fann Hospital of Dakar, Senegal, for weight loss, fever, dyspnea and cough. A chest X-ray showed miliary features. The diagnosis of tuberculosis was suspected and an antituberculosis treatment, including rifampin, isoniazid and ethambutol, was rapidly initiated.
After 3 weeks of treatment, the patient was seen at the Institute of Leprosy for a sudden skin eruption and pains in his right forearm and left leg. Clinical examination revealed numerous, symmetrical, erythemato-edematous and well-demarcated papular lesions over his entire body (Fig. 1). Some lesions were hypoesthetic. His right ulnar and left lateral popliteal (common peroneal) nerves were swollen, tender and painful with a striking motor deficit. A biopsy of a cutaneous lesion showed an edema of the papillary dermis and a dense perivascular and perineural granulomatous dermal infiltrate containing many lymphocytes and few epithelioid cells. Ziehl-Neelscn staining revealed numerous acid-fast bacilli with a bacterial index (BI) of 1 to 10 bacilli per oil immersion field (3+).
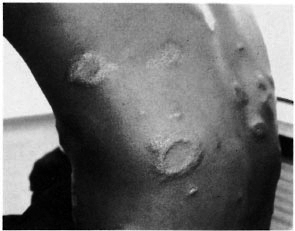
Fig. 1. Case 1. Clinical aspect at time of reversal reaction: annular, well-demarcated, edematous and slightly squamous lesions of the back.
The HIV-1 serological positivity was confirmed by both ELISA and Western-blot techniques, and the peripheral blood CD4 + lymphocyte count showed 10 CD4+ cells/ mm3.
The acute occurrence of both cutaneous and neurological features and the epithelioid infiltrate associated with a positive BI supported the diagnosis of borderline lepromatous leprosy in reversal reaction. Dapsone (100 mg/day) was added to the antituberculosis treatment as a second anti- M. leprae antibiotic. For the reversal reaction (RR), steroid therapy (prednisone 1 mg/kg/ day) was started and rapid clinical improvement was seen. The patient died 1 month later of Salmonella typhimurium septicemia.
Case 2. In 1987, a 34-year-old Senegalese man was started on WHO-multidrug therapy (MDT) (rifampin + dapsone + clofazimine) for a multibacillary borderline lepromatous (BL) relapse (BI = 5+) (Fig. 2). The patient previously had been treated by dapsone from 1970 until 1980 for an indeterminate form of the disease. With MDT the lesions improved and 1 year later the patient was clinically cured and had a BI of 4 + . In January 1989 during ongoing treatment, the patient exhibited an acute worsening of his pre-existing cutaneous lesions without signs of neuritis. The histology of a cutaneous lesion (Fig. 3), in comparison to the histology performed in 1987, showed an increased number of lymphocytes and an epithelioid differentiation of histiocytes. His BI was 3 +. Signs of reaction quickly subsided under steroid therapy (prednisone at an initial daily dose of 0.5 mg/kg).
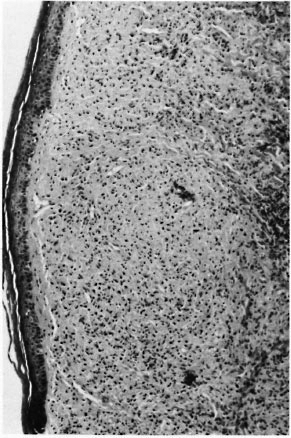
Fig. 2. Case 2. Histological features at time of 1987 relapse: borderline lepromatous leprosy.
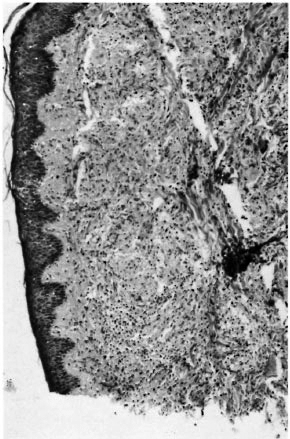
Fig. 3. Case 2. Histological features at time of 1989 reversal reaction: increased number of lymphocytes and few epithelioid cells.
The HIV serological studies (ELISA and Western blot) performed on the patient's scrum taken in 1987 and in 1989 showed an HIV-2 positivity. At the time of his RR, his CD4+ T-cell count was about 560 cells/ mm3. The patient did not suffer from other diseases and, more importantly, had had no opportunistic infection.
DISCUSSION
Up until the present time, only 14 cases of leprosy occurring in HIV-positive or AIDS patients have been reported (2,5,4,9) . All of the cases except one (9) were borderline, either borderline tuberculoid or borderline lepromatous patients. This suggests that as in "normal" patients, HIV-infected patients may exhibit varying levels of anti- M. leprae T-cell-mediated immunity (CMI). Moreover, although the number of cases is low, it seems that there is no correlation between the severity of the HIV infection and the form of leprosy. Of the 11 AIDSrelated complex (ARC) or Stage II [according to the Centers for Disease Control classification (1)] patients, an equal number of tuberculoid and lepromatous patients has been reported and of the 3 patients classified as Stage IV, 2 patients were tuberculoid cases. In the same way, it seems that the number of circulating CD4+ T lymphocytes does not influence the form of leprosy since 3 of the 4 patients with detailed T-cell counts were borderline tuberculoid patients and exhibited a CD4+/CD8+ ratio lower than 0.5.
The reversal reaction (RR) is a well-known immunological leprosy complication which occurs in 10% to 30% of borderline leprosy patients, sometimes spontaneously but more often during the first months of treatment (7). This type of reaction is attributed to a delayed-type hypersensitivity reaction resulting in the enhancement of anti- M. leprae CMI and a shift toward the tuberculoid pole of the disease. It is currently attributed to an increased CD4+ T-lymphocyte response.
Of the 14 reported cases, three patients seem to have developed a RR (2,9). However one case (2) had been treated previously and cured and, thus, the diagnosis of relapse cannot be excluded. Our two patients clearly exhibited a typical RR. In one case this RR occurred despite a strikingly decreased level of circulating CD4+ T cells. This shows that despite a failure of the CD4+ T-lymphocyte subset, HIV-positive patients may develop a hypersensitivity reaction. However, it also might be emphasized that, as suggested by some authors (6), RR does not result from this mechanism. This last hypothesis is strengthened by the fact that HIV infection is usually known to be associated with negative delayed-type hypersensitivity reactions.
Case 1 presents another feature of interest: the absence of clinical signs of leprosy before the RR and, thus, a delayed diagnosis. Such a situation of subclinical leprosy might be of major epidemiological impact for two reasons. First, it might explain the apparent low incidence of leprosy in HIVendemic areas (6,8) which might result from the rapidly fatal outcome of HIV infection while the period of leprosy incubation is longer, on average 2 to 6 years. Secondly, of the antituberculosis drugs, rifampin is the only one with anti-Af. leprae activity and rifampin monotherapy is known to rapidly induce M. leprae resistance (3). So, in leprosy-endemic areas, the treatment of tuberculous HIV patients might induce the emergence of rifampin-resistant M. leprae strains if given to symptom-free leprosy patients.
REFERENCES
1. CENTERS FOR DISEASE CONTROL. Classification system for human T lymphotrophic virus type III/lymphadenopathy associated virus infection. Ann. Med. 105(1986)234-237.
2. JANSSEN, F., WALLACH, D., KHUONG, M., PENNEC, J., PRADINAUD, R., SAID, G. and COTTENOT, F. Association de maladie de Hansen et de l'infection par le virus de l'immunodéficience acquise; deux observations. Presse Med. 17(1988)1652-1653.
3. JI, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
4. KENNEDY, C, CHIN A LIEN, R. A. M., STOLZ, E., VAN JOOST, T. and NAAFS, B. Leprosy and human immunodeficiency virus infection. Int. J. Dermatol. 29(1990)139-140.
5. LAMFERS, E. J. P., BASTIAANS, A. H., MRAVUNAC, M. and RAMPEN, F. H. J. Leprosy in the acquired immunodeficiency syndrome. Ann. Intern. Med. 107(1987)111-112.
6. MILLER, R. A. Leprosy and AIDS: a review of the literature and speculations on the impact of CD4 + lymphocyte depiction on immunity to Mycobacterium leprae. Int. J. Lepr. 59(1991)639-644.
7. PFALTZGRAFF, R. E. and BRYCESON, A. Clinical leprosy. In: Leprosy. Hastings, R. C, cd. Edinburgh: Churchill Livingstone, 1985, pp. 165-168.
8. PONNIGHAUS, J. M., MWANJASI, L. J., FLNE, P. E. M., SHAW, M. A., TURNER, A. C, OXHORROW, S. M., LUCAS, S. B., JENKINS, P. A., STERNE, J. A. C. and BLISS, L. IS HIV infection a risk factor for leprosy? Int. J. Lepr. 59(1991)221-228.
9. VREEBURG, A. Clinical observations on leprosy pa tients with HIV 1 infection in Zambia. Lepr. Rev. 63(1992)139-140.
1. M.D.; Institut de Léprologie Appliquée, B. P. 11023, Dakar, Senegal.
2. M.D., Institut de Léprologie Appliquée, B. P. 11023, Dakar, Senegal.
3. M.D.; Service de Dermatologie du Pr. Dubertret, Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, Paris 75010, France.
4. M.D., Service de Dermatologie du Pr. Dubertret, Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, Paris 75010, France.
5. M.D.; Service des Maladies Infectieuses, Hôpital Fann, Dakar, Senegal.
6. M.D., Service des Maladies Infectieuses, Hôpital Fann, Dakar, Senegal.
7. M.D., Institut Pasteur, B. P. 220, Dakar, Senegal.
Reprint requests to Dr. Flageul.
Received for publication on 11 December 1992.
Accepted for publication on 8 January 1993.