- Volume 61 , Number 2
- Page: 218–26
Correlation between TNF production, increase of plasma C-Reactive protein level and suppression of T lymphocyte response to concanavalin a during erythema nodosum leprosum
ABSTRACT
The complex symptoms observed in lepromatous leprosy patients with reactive episodes of the erythema nodosum leprosum (ENL) type are associated with different serum components actively participating in the acute inflammatory reaction. Among them are the tumor necrosis factor (TNF) and the acute-phase protein C-reactive protein (CRP). TNF and CRP were found at significantly more elevated concentrations in the serum of patients with ENL, with a positive correlation of about 95% when compared with patients with nonreactive lepromatous leprosy (L) or tuberculoid leprosy (T) or with control individuals. Furthermore, in another series of experiments CRP had a specific and significant suppressive action on concanavalin A (ConA)-induced lymphoproliferation in cultures f rom patients and controls, the reduction being more marked (75%) in patients with ENL. By extrapolation f rom its known actions, production of TNF may have a number of potential consequences for the immunobiology of ENL. Thus, TNF may cause direct injury to compromised cells, facilitating mononuclear cell activation and production of cytokines such as interleukin-1 and interleukin-6, and upregulating hepatocyte expression of CRP. Both CRP and TNF in high serum concentrations have the ability to enhance the acute inflammatory process in ENL, favoring increased macrophage activation and phagocytosis, and contributing to the elimination of damaged cells and bacilli, as well as in the reduction of T-suppressor cells, with a consequent improvement in the immunologic response of ENL patients.RÉSUMÉ
Les symptômes complexes observés che/ les patients présentant une lèpre lépromateuse avec des épisodes réactionncls du type érythème noueux lépreux (ENL) sont associés avec différents composés du serum participant activement dans la réetion inflammatoire aigue. Parmi ceux-ci, il y a le facteur de nécrose des tumeurs (FNT) et la protéine C-réactive de phase aigue (CRP). Les FNT et CRP ont été trouvés à des concentrations significati vement plus élevées dans le serum de patients présentant un ENL, avec une correlation positive d'environ 95% par rapport à des patients présentant une lèpre lépromateuse non réactionnclle (L), une lèpre tuberculoidc (T) ou des témonis. De plus, dans une autre série d'expérimentations, la CRP avait une action spécifique et significativement suppressive sur la prolifération lymphocytaire induite par la concanavalinc A (ConA) dans des cultures provenant de patients et de témoins, la réduction étant plus marquée (75%) chez les patients avec ENL. Par extrapolation de ses actions connues, la production de FNT peut avoir plusieurs conséquences potentielles pour l'immunobiologic de l'ENL. C'est ainsi que le FNT peut causer un dommage direct à des cellules compromises, facilitant l'activation mononucléaire et la production de cytokines telles que rinterleukinc-1 et l'interleukine-6, et déregulant vers une augmentation la production hépatocytaire de CRP. La CRP et le FNT à hautes concentrations dans le serum ont le pouvoir de stimuler le processus inflammatoire aigu dans l'ENL, favorisant une activation macrophagique accrue et la phagocytose, et contribuant à l'élimination des cellules endommagées et des bacilles, avec comme conséquence une amélioration de la réponse immunologiquc des patients présentant un ENL.RESUMEN
Los complejos síntomas observados en los pacientes lepromatosos con reacción del tipo eritema nodoso leproso (ENL) están relacionados con la participación de diferentes componentes del suero dentro de los que se encuentran el factor necrosante de tumores (TNF) y la proteína C-reactiva (CRP) de fase aguda. El TNF y la CRP se encontraron a concentraciones significativamente más elevadas en el suero de los pacientes con ENL que en el suero de los pacientes lepromatosos no reactivos (L) o que en el suero de los individuos control. Además, en otro serie de experimentos, la CRP tuvo una significante y específica acción supresora sobre la linfoproliferación inducida por la concanavalina A (Con-A) en cultivos de pacientes y de controles, siendo la reducción más marcada (75%) la observada en los pacientes con ENL. Por extrapolación de sus efectos conocidos, la producción del TNF podría tener un gran número de consecuencias potenciales en la inmunobiología del ENL. Así, el TNF podría causar un daño directo a las células comprometidas, facilitando la activación de las células mononucleares y la producción de citocinas tales como intcrleucina-1 e interleucina-6, además de que podría estimular la expresión aumentada de CRP por los hepatocitos. Tanto el TNF como la CRP, en altas concentraciones tienen la habilidad de aumentar el proceso inflamatorio agudo en el ENL, favoreciendo la activación y la fagocitosis de los macrófagos, y contribuyendo a la eliminación de células y bacilos dañados, asi como a la reducción de células T supresoras, con la consecuente mejoría de la respuesta inmunológica en los pacientes con ENL.Erythema nodosum leprosum (ENL) is an immunopathologic complication seen in about half of the patients with lepromatous leprosy. Its pathological picture includes impaired general condition, high fever, neurological involvement (neuritis), edema, erythema and pain in the joints (arthritis), and erythematous skin nodules which are painful upon palpation, characterizing a severeintensity acute inflammatory reaction (30,43).
In the acute-phase inflammatory response, changes in the concentrations of many plasma proteins are characteristically observed, reflecting reorganization of the gene expression of hepatocyte secretory proteins after the inflammatory stimulus (21). C-Reactive protein (CRP) is the acute-phase protein occurring in humans which has been studied most extensively (16,23) and also was the first to be discovered (40). Normally, these proteins are present in small amounts in plasma, and an exacerbated increase in their rate of synthesis occurs after stimulation (23). Indeed, 1000-fold higher serum levels can be observed in individuals with serious infections (31). A progressively increasing number of hepatocytes are recruited for CRP synthesis during the first days after the inflammatory reaction (22). Thus, persistently elevated CRP levels reflect active disease, and have been reported to occur in several infections, inflammatory reactions, tumors, and tissue lesions (21,23,24).
Studies conducted to determine the extracellular signs that increase or decrease the expression of genes coding for acute-phase proteins in hepatocytes have revealed that several cytokines released from activated macrophages, such as tumor necrosis factor (TNF), interleukin-1 (IL-1) or IL-6 in combination with IL-1, are involved in this process (23). These cytokines, by performing a regulatory function, may act directly on the hepatocytes, inducing CRP synthesis (1,9,10,13,17,32,39). These data suggest that there may be a positive correlation between serum TNF and CRP levels in individuals with infectious diseases. Elevated TNF levels are frequently detected in the sera of patients with infectious (36,37,42) and parasitic (35) diseases, and play an important role in the response to tissue damage (4,41).
In addition to playing a critical role in the response to tissue damage (21), CRP modifies the behavior of effector cells, especially polymorphonuclear leukocytes (7,28), lymphocytes (18,44,45), monocytes (49,50) and platelets (11) during the inflammatory process. In view of the above considerations, the objective of the present investigation was to study the correlation between serum CRP and TNF levels in different groups of leprosy patients (15), and to determine the immunomodulating activity of CRP on the lymphoproliferative response of the various groups of patients, particularly those with ENL.
MATERIALS AND METHODS
Patients and controls. Seventy individuals from the Ribeirão Preto-SP region were selected. Of these, 19 were normal subjects and 51 were patients with leprosy who were divided into three groups as described below. The diagnosis of leprosy was based on clinical, histological and bacterioscopic examinations and on the evolutionary aspects of the disease. All patients and controls were examined at the time of blood collection. The Mitsuda test was performed in all cases, and tests for the presence of alcohol-acidresistant bacilli were performed in smears of material obtained from the skin lesions and nasal mucus of the leprosy patients. The control subjects were examined to determine whether they presented a lesion suggestive of leprosy and submitted to the Mitsuda test using the same antigen as used for the patients.
The patients were treated at the University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo, São Paulo, Brazil, and classified as having lepromatous (L) or tuberculoid (T) leprosy according to the criteria adopted at the VI International Congress of Leprology held in Madrid, Spain in 1953.
Group I consisted of 28 L patients aged 21 to 60 years (mean 46 years), and most of them (86%) had been under treatment with dapsone (DDS) at a dose of 100 mg/ day for varying periods of time. In relation to the sex distribution, males predominated (19 patients, 63%) in relation to females. The results of earlobe bacilloscopy were positive in 13 patients (43%). Moreover, all patients were negative to the Mitsuda test, and 11 of them presented a reaction of the ENL type. None of the ENL patients was being treated with an immunosuppressive drug at the time of blood collection.
Group II consisted of 23 T patients aged 18 to 60 years (mean 52.5 years). The sex distribution was similar in this group (12 males, 11 females). All patients were lepromin-reactive, with responses ranging from 1 + (12) to 2+ (9) and 3+ (2); all were negative for skin lesion bacilloscopy. Only two patients presented positive earlobe bacilloscopy (1+) (47).
Group III (controls) consisted of 19 normal individuals aged 20 to 50 years (mean 28.2 years); 12 males and 7 females, most of them medical students in operational contact with leprosy patients. The controls were divided into lepromin-positive (11 individuals) and lepromin-negative (8 individuals) subjects on the basis of their responses to the Mitsuda test. The lepromin utilized contained 60 x 106 bacilli/ml, and was prepared from a human leproma (Hospital Lauro de Souza Lima, Bauru, São Paulo, Brazil).
Extraction and purification of human serum CRP. CRP-positive serum samples were collected from patients with different conditions who had been admitted to the University Hospital, and stored at - 20ºC until use. CRP was extracted and purified as described previously (29). Briefly, serum samples were precipitated with ammonium sulfate (50%-70% saturated, at 5ºC), and the precipitate was applied to a column of Sepharose-4B (0.8 x 4.0 cm) and then to an affinity column of Sepharose-4B-bis-epoxy-phosphoryl-ethanolamine. The CRP solution was twice crystallized with a saturated sodium sulfate solution (50%, w/v), and a CRP concentration of 8.2 mg/ml was obtained. For use in the lymphocyte cultures, the CRP crystals were suspended in sterile culture medium and filtered through Sephadex G-25 on a 0.9 x 5.0-cm column immediately before use.
CRP quantification in patient and control sera. CRP concentration in patient and control sera was measured by the nephelometric method using an ICS Analyzer II (Immunochemistry System; Beckman). The results are reported as mg CRP/dl serum.
TNF determination in sera of patients and controls. TNF concentration in the sera of the groups studied was determined using a kit for immunoradiometric analysis (Medgenix Diagnostics, Belgium) according to the manufacturer's directions.
In vitro lymphoproliferation test. The lymphoproliferation assay was carried out as described previously (12). Briefly, blood samples (7 ml) were diluted with an equal volume of sterile RPMI 1640 culture medium containing glutamine, 2.0 g/l sodium bicarbonate, 15% heat-inactivated human AB serum, 150 U/ml penicillin, 8 µ g/ml streptomycin, and 20 mM HEPES. Samples were then applied to a Ficoll-Hypaque gradient and centrifuged at 400 x g x 30 min at room temperature and the interface, predominantly consisting of peripheral blood mononuclear cells (PBMC), was harvested. The PBMC were washed twice in RPMI 1640, resuspended in 1.0 ml culture medium, and counted in an automatic counter (CC510; Celm). Lymphocyte viability was determined in the presence of 2% Trypan blue. The cell suspension was then diluted with RPMI 1640 culture medium to a final concentration of 2.5 x 106 lymphocytes/ml. A 0.1 -ml sample of each cell suspension was added to each well (2.5 x 105 cells/well) of flat-bottom, 96-well plates (Corning). The cells from each individual were cultured separately in triplicate in the presence of 20 µ g/ml phytohemagglutinin (PHA; Difco Laboratories), 10 µ g/ml concanavalin A (ConA), and 5 µ g/ml CRP, or in the presence of RPMI medium as a control. In some experiments, 5 µ g/ml CRP was added to cultures previously stimulated with PHA or ConA. The plates were incubated at 37ºC with 5% CO2 for 3 days. Sixteen hr before the end of the incubation period, 0.5 µ Ci3 [H]-thymidine (specific activity, 6.7 Ci/mM; New England Nuclear) was added to each well. Cells were then harvested using an automatic collector (Cambridge Technology Inc.) and analyzed in a Beckman LS150 liquid scintillation spectrometer. Lymphoproliferation was quantified by determining the stimulation index (SI) which represents the mean quotient between counts of radiation emission per minute in the presence and absence of stimulating agents. An SI > 2.0 was considered to indicate blastogenesis.
Statistical analysis. Data were analyzed statistically by the Wilcoxon, Mann-Whitney, and Friedman tests and by the Spearman correlation coefficient.
RESULTS
Determination of TNF concentration. TNF concentrations (pg/ml) in the sera of patients and controls are shown in Figure 1. It can be seen that serum TNF concentrations were higher in group I (median, M: 9.4; Fig. 1 A) than in group III (M: 6.9; Fig. 1 C). Comparison of these medians (Mann-Whitney test) showed a 95% probability of detecting higher TNF levels in L patients than in controls (p < 0.0001). Among L patients, those with ENL (10 patients) presented the highest TNF concentrations (M: 112.9). In most T patients (Fig. 1 B), serum TNF levels oscillated around the mean value (M: 12.4). Only four of these patients had elevated levels (ranging from 48.0 to 52.0 pg/ml) and two of them presented a reactive bout. In the control group (Fig. 1 C), TNF levels were lower and uniformly distributed, favoring the hypothesis that TNF is spontaneously secreted at low concentrations by normal individuals.
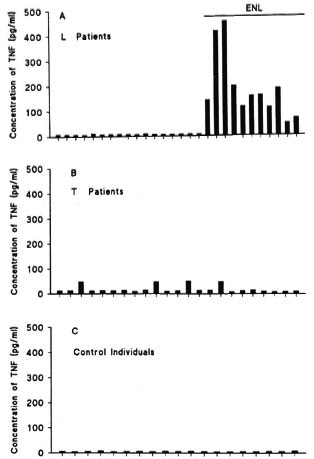
Fig. 1. TNF concentration in the serum of L (A) and T (B) leprosy patients and of control individuals (C). Each bar represents the concentration of TNF expressed as pg/ml of serum for each individual of corresponding group. Among 28 L patients, 11 of thempresented ENL reaction (as depicted in A).
Determination of CRP concentration. The distributions of CRP levels reported as means ± S.E.M. (mg/dl) were quite different for the three groups studied, as illustrated in Figure 2. Group I (L patients) had the highest serum CRP concentrations (1.84 ± 0.39 mg/dl) (Fig. 2 A); whereas group II (T patients) had a much lower CRP concentration (0.86 ± 0.11 mg/dl) (Fig. 2 B), closer to the levels detected in group III (controls) (0.68 ± 0.03 mg/dl) (Fig. 2 C). When the results for group I are analyzed by considering only patients with ENL, CRP levels are quite elevated when compared to nonreactive L patients whose concentrations were similar to those of the control group. The difference between these values is highly significant (p < 0.00003), suggesting that the ENL-type reaction induces increased CRP production and release in L patients.
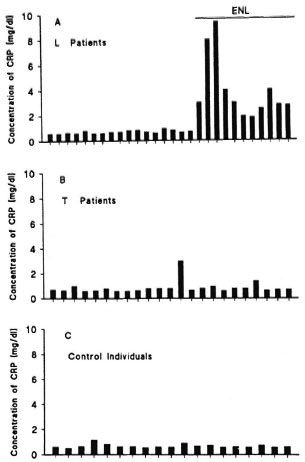
Fig. 2. CRP concentration in the serum of L (A) and T (B) leprosy patients and of control individuals (C). Each bar represents the CRP concentration expressed as mg/di of scrum for each individual of cor-responding group. Among 28 L patients, 11 presented ENL reactions (as depicted in A).
Correlation between serum TNF and CRP concentrations. The results in Figures 1 and 2 suggest the presence of a correlation between serum TNF and CRP concentrations, which can be observed by comparing the values obtained for L patients with ENL. The association was tested using the Spearman correlation coefficient (r) and a positive correlation between TNF and CR levels was found in patients with ENL (r = 0.982). The positive correlation between values indicates that when ENL patients have high TNF levels, a proportional increase in CRP concentrations also occurs.
Lymphoproliferation stimulated by PHA or PHA plus CRP. Since CRP activity involves modification of the behavior of T lymphocytes (18,44,45) and since CRP concentration is high in patients with ENL, we studied the immunomodulating effect of CRP on the lymphoproliferative response in the presence of PHA. The mitogenic action of PHA on the lymphocytes of L patients was discrete, with an SI of about 3.0 (Fig. 3A). When the results for L patients with or without ENL reaction are considered separately, it can be seen that the PHAstimulated responses were similar (M: 3.0 for both groups). Most T patients responded to PHA stimulation with an SI > 2.0 (M: 5.0) (Fig. 3B), and the proliferative lymphocyte response of control individuals was more marked, with M: 15.0 (Fig. 3C). These results suggest that lymphocytes of normal individuals proliferated more in the presence of PHA than T lymphocytes from leprosy patients.
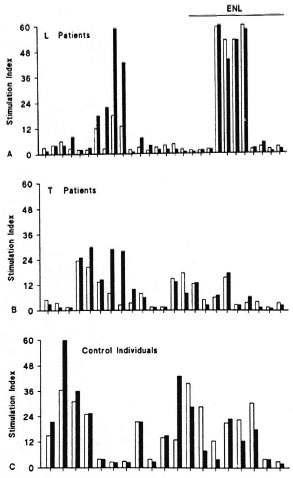
Fig. 3. Peripheral blood mononuclear cell responses of L (A) and T (B) leprosy patients and of control individuals (C) to PHA ( ) or PHA plus CRP (
) or PHA plus CRP ( ). PHA was tested at 20 µ g/ml and CRP at 5 µ g/ml in a standard lymphoproliferation assay. Results represent stimulation index (see Materials and Methods) for each individual of corresponding group. L patients with ENL reaction are depicted in A.
). PHA was tested at 20 µ g/ml and CRP at 5 µ g/ml in a standard lymphoproliferation assay. Results represent stimulation index (see Materials and Methods) for each individual of corresponding group. L patients with ENL reaction are depicted in A.
The addition of CRP to lymphocyte cultures from L or T patients and from control individuals stimulated with PHA tended to decrease the mitogenic action of PHA, as can be seen in Figure 3. However, statistical analysis revealed that the differences between the two stimuli were not significant. In patients with ENL, the addition of CRP also showed no interference with the PHA-stimulated lymphocyte response.
Lymphoproliferation stimulated by con a or cona plus CRP. Lymphocytes of L patients exhibited much greater blastic activity in the presence of ConA (M: 4.8) than in the presence of PHA (M: 3.0) (Fig. 4A), with a lower amplitude of variation. Nonreactive L patients had lower mitogenic responses (M: 4.0) than did patients with ENL (M: 6.8), indicating that during the leprosy reaction there is a tendency toward greater lymphoproliferative activity of lymphocytes when they are stimulated with ConA. An interesting observation was that the proliferative response of normal individuals under ConA stimulation (M: 3.0; Fig. 4C) was lower than that of group I (M: 4.8; Fig. 4A) and group II (M: 4.0; Fig. 4B) patients. These results indicate that, under ConA stimulation, the lymphocytes of the control group were less reactive than those of leprosy patients.
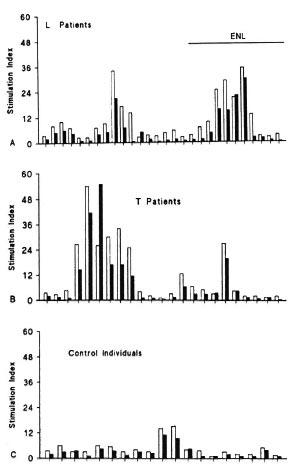
Fig. 4. Peripheral blood mononuclear cell responses of L (A) and T (B) leprosy patients and of control individuals (C) to ConA ( ) or ConA plus CRP (
) or ConA plus CRP ( ) as described in Fig. 3 legend.
) as described in Fig. 3 legend.
The addition of CRP to ConA-stimulatcd cultures reduced by 58% the blastic activity of lymphocytes from L patients and their SIs went from M: 4.8 in the absence of CRP to M: 2.0 in the presence of CRP (Fig. 4A). These results were statistically significant (p < 0.000003). The addition of CRP to lymphocyte cultures from patients with ENL caused a more marked response than that observed for nonreactive L patients (75%) whose medians changed from M: 6.8 in the presence of ConA to M: 2.0 in the presence of ConA plus CRP. This difference was statistically significant (p < 0.001). A significant decrease in the blastogenic response to Con A was also observed in T patients (30%, p < 0.0018) and for the control group (30%, p < 0.00023) after the addition of CRP (Figs. 4B and 4C).
DISCUSSION
The particularly relevant data of the present study is the positive correlation (95%) found between elevated serum TNF and CRP levels in patients with ENL. Thus, the results suggest that bacillary infection maintains a certain level of macrophage activation with discrete or minimal TNF production in lepromatous patients and that stimuli, probably triggered by bacillary components during the acute inflammatory reaction (ENL), may induce the macrophages to produce considerable amounts of TNF. Sarno, et al. (34) reported that 50% of LL patients with ENL had elevated TNF and IL-1 levels, and they concluded that these cytokines may be involved in the ENL reaction. The serum TNF levels of tuberculoid patients were much lower than those of patients with ENL, but were statistically significantly elevated when compared to those of the control group. These results corroborate a previous study in which we reported that tuberculoid patients maintain marked macrophage stimulation, with a consequent increase in TNF production, when compared with lepromatous patients and control individuals (38).
The TNF detected at high concentrations in the sera of patients with ENL may be able to bind to a variety of tissues, and hepatocytes in particular (14), by interacting with high-affinity membrane receptors (49), thus inducing production of the acute-phase protein CRP by the latter cells (1,9,32). The present results clearly demonstrate that when high TNF levels were present, high CRP concentrations also were detected.
CRP detected in high concentrations in patients with ENL may act both on macrophages or other cell types (19,28,49,50) and on damaged tissues (48) by increasing the syntheses of complement system components (20) and of TNF and other cytokines, potentiating the acute inflammatory response, causing fever, edema, erythema, neuritis, arthritis and erythematous skin nodules, among other symptoms. Furthermore, CRP, TNF and the complement system may also act synergistically, enhancing the inflammatory process and favoring increased macrophage activation and phagocytosis, thus contributing to the elimination of cells and damaged bacilli.
With respect to the lymphocyte subpopulations encountered in leprosy, it can be seen that patients with ENL are unique among lepromatous patients in that their suppressor cells are significantly decreased, whatever their bacterial load. This decrease is transient, and it is associated with an increase of T-cell responses in vitro. Now there is accumulating evidence that imbalances in T-cell subpopulations are important in the understanding of ENL (25,26,27). Thus, there must be some correlation between components released during the acute inflammatory reaction and the decrease in suppressor-T cells in these patients.
In the present study, experimental evidence was obtained indicating that CRP protein may be involved in this process. Thus, during ENL, the patients may present a defense mechanism at the cell level characterized by the action of CRP which, at high serum concentrations, may reduce the activity of suppressor/cytotoxic T lymphocytes by as much as 75% without changing PHA-induced lymphoproliferation. This would cause a relative increase in T-helpercell activity with consequent improvement of the immunologic response. Furthermore, the TNF and IL-1 released during the reactive phase of leprosy may also stimulate T-helper cells, extending the immunologic control during this phase of the disease.
The physiological mechanisms regulating the action of TNF and that of other cytokines are not fully clear. There is evidence that glucocorticoids have a recognized ability to inhibit TNF and IL-1 synthesis (4). In vivo glucocorticoid release is controlled by adrenocorticotropic hormone (ACTH) (46). Recent data have demonstrated that TNF and IL-1 act on the pituitary gland, inducing ACTH and corticosterone release (3). These data suggest that there is a negative feedback between interleukins and glucocorticoids which is mediated by the neuropeptide ACTH, thus demonstrating a connection between the immune system and the neuroendocrine system (2,5,6) at the level of regulation by TNF or by other interleukins. It has been demonstrated also that another neuropeptide hormone, melanocyte-stimulating hormone (MSH), may also be involved in the regulation of TNF and IL-1 activity by reducing fever, neutrophilia and acute-phase proteins such as CRP caused by excess cytokine in the bloodstream (8,33).
Experiments are currently underway in our laboratory to determine ACTH and glucocorticoid levels in patients with ENL in order to determine the role of these mediators in the modulation of the inflammatory process observed in ENL.
Acknowledgment. This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process nos. 300187/87-4 and 500256/91-8) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, process no. 92/3513-0). We thank Ms. M. A. Nunes Ferreira, Sebastião L. Brandão Filho and Ms. Izaira T. Brandão and for their technical support. We also thank Prof. Dr. L. M. Bechelli for the special assistance over all the steps on the development of this work.
REFERENCES
1. ANDUS, T., GEIGER, T., HIRANO T., KISHIMOTO, T. and HEINRICH, P. C. Action of recombinant human interleukin-6, interleukin-1 and tumor necrosis factor on the mRNA induction of acutephase proteins. Eur. J. Immunol. 18(1988)739-746.
2. BESEDOVSKY, H. O., DEL REY, A. E. and SORKIN, E. Immunoneuroendocrine interactions. J. Immunol. 135(1985)750s-754s.
3. BESEDOVSKY, H., DEL REY, A., SORKIN, E. and DINARELLO, C. A. Immuneregulatory feedback between interleukin-1 and glucocorticoid hormones. Science 233(1986)652-654.
4. BEUTLER, B. and CERAMI, A. Cachectin: more than a tumor necrosis factor. N. Engl. J. Med. 316(1987)379-384.
5. BLALOCK, J. E. and SMITH, E. M. A complete regulatory loop between the immune and neuroendocrine systems. Fed. Proc. 44(1985)108-111.
6. BLALOCK, J. E., HARBOUR-MCMENAMIN, D. and SMITH, E. M. Peptide hormones shared by the neuroendocrine and immunologic systems. J. Immunol. 135 Suppl. 2(1985)858s-861s.
7. BUCHTA, R., FRIDKIN, M., PONTET, M., CONTESSI, E., SCAGGIANTI, B. and ROMEO, D. Modulation of neutrophil function by C-reactive protein. Eur. J. Biochem. 163(1987)141-146.
8. DAO, T. K., BELL, R. C. and FENG, J. C-reactive protein, leukocytes and fever after central IL-1 and alpha-MSH in aged rabbits. Am. J. Physiol. 23(1988)401-408.
9. DARLINGTON, G. J., WILSON, D. R. and LACHMAN, L. B. Monocyte-conditioncd medium, intcrleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J. Cell. Biol. 103(1986)787-793.
10. DINARELLO, C. A. Intcrleukin-1 and the pathogenesis of the acute phase response. N. Engl. J. Med. 311(1984)1413-1418.
11. FIEDEL, B. A. and GEWURZ, H. Cleaved forms of C-reactive protein are associated with platelet inhibition. J. Immunol. 136(1986)2551-2555.
12. Foss, N. T., PAGNANO, P. M. G., BECHELLI, L. M. and SIMOES, A. L. Lymphocyte blastogencsis and lepromin reactivity in leprosy patients and their parents. Acta Leprol. (Geneve) 7(1990)119-128.
13. GANAPATHI, M. K., MAY, L. T., SCHULTZ, D., BRA-BENEC, A., WENSTEIN, J., SEHGAL, P. B. and KUSHNER, I. Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem. Biophys. Res. Commun. 157(1988)271-277.
14. GAUDIE, J., RICHARDS, C, NORTHEMANN, W., FEY, G. and BAUMANN, H. IFNB2/BSF2/IL6 is the monocyte-derived HSF that regulates receptorspecific acute phase gene regulation in hepatocytes. Ann. N. Y. Acad. Sci. 557(1989)46-59.
15. GODAL, T. Leprosy immunology -some aspects of the role of the immune system in the pathogenesis of disease. Lepr. Rev. 55(1984)407-414.
16. GOTSCHLICH, E. C-reactive protein; a historical overview. Ann. N. Y. Acad. Sci. 557(1989)9-18.
17. HEINRICH, P. C, CASTELL, J. V. and ANDUS, T. Interleukin-6 and acute phase response. Biochem. J. 265(1990)621-636.
18. JAMES, K., BAUM, L. L., VETTER, M. L. and GEWÜRZ, H. Interactions of C-reactive protein with lymphoid cells. Ann. N. Y. Acad. Sci. 389(1982)274-285.
19. JAMES, K., HANSEN, B. and GEWÜRZ, H. Binding of C-reactive protein to human lymphocyte. I. Requirement for a binding specificity. J. Immunol. 127(1981)2539-2544.
20. KAPLAN, M. H . and VOLANAKIS, J. E. Interaction of of C-reactive protein complexes with complement system. I. Consumption of human complement associated with pneumococcal C-polysaccharide and with choline phosphatides, lecithin and sphingomyelin. J. Immunol. 112(1974)2135-2147.
21. KUSHNER, I. The phenomenon of the acute phase response. Ann. N. Y. Acad. Sci. 389(1982)39-48.
22. KUSHNER, I. and FELDMANN, G. Control of the acute phase response: demonstration of C-reactive protein synthesis and secretion by hepatocytes during acute inflammation in the rabbit. J. Exp. Med. 148(1978)466-477.
23. KUSHNER, I., GANAPATHI, M. and SCHULTZ, D. The acute phase response is mediated by heterogeneous mechanisms. Ann. N. Y. Acad. Sci. 557(1989)19-30.
24. KUSHNER, I., VOLANAKIS, J. E. and GEWÜRZ, H. C-reactive protein and the plasma protein response to tissue injury'. Ann. N. Y. Acad. Sci. 389(1982) 39-48.
25. MEURA, V., CONVIT, J., RUBINSTEIN, A. and BLOOM, B. R. Activated suppressor T-cells in leprosy. J. Immunol. 129(1982)1946-1951.
26. MEURA, V., MASSON, L. H., FIELDS, J. P. and BLOOM, B. R. Lepromin induced suppressor cells in patients with leprosy. J. Immunol. 123(1979)1813-1817.
27. MEURA, V., MASSON, L. H., REINHERZ, E. L., SCHLOSSMAN, S. F. and BLOOM, B. R. Delineation of a human T-cell subset responsible for lepromininduced suppression in leprosy patients. J. Im munol. 125(1980)1183-1188.
28. MULLEX, I. L. and FEHR, J. Binding of C-reactive protein to human polymorphonuclear leukocytes; evidence for association of binding sites with Fc receptors. J. Immunol. 136(1986)2202-2207.
29. OLIVEIRA, E. B., GOTSCHLICH, E. C. and Liu, T. Y. Primary structure of human C-reactive pro tein. Proc. Natl. Acad. Sci. U.S.A. 74(1979)3148-3151.
30. PEARSON, J. M. H. Reactions in leprosy. In: Essentials of Leprosy. 4th edn. Würzburg: German Leprosy Relief Association, 1986.
31. PEPYS, M. B. and BALTZ, M. L. Acute-phase pro teins with special reference to C-reactive protein and related proteins (pentaxins) and serum amy loid A protein. Adv. Immunol. 34(1983)141-212.
32. PERLMUTTER, D. H., DLNARELLO, C. A., PLUNSAL, P. I. and COLTEN, H. R. Cachcctin/tumor necrosis factor regulates hepatic acute phase gene expression. J. Clin. Invest. 78(1986)1349-1354.
33. ROBERTSON, B., DOSTAL, K. and DAYNES, R. A. Neuropeptide regulation of inflammatory and immunologic responses. J. Immunol. 140(1988)4300-4307.
34. SARNO, E. N., GRAU, G. E., VIEIRA, L. M. and 44.NERY, J. A. Serum levels of tumor necrosis factoralpha and interleukin-1 beta during leprosy reactional states. Clin. Exp. Immunol. 84(1991)103-108.
35. SCUDERI, P., LAM, K. S., RYAN, K. J., PETERSEN, E., STERLING, K. E., FINLEY, P. R., RAY, C. G., SLYMEN, D. J. and SALMON, S. E. Raised serum level of tumor necrosis factor in parasitic infections. Lancet 2(1986)1364-1365.
36. SILVA, C. L., FACCIOLI, L. H. and ROCHA, G. M. The role of cachectin/TNF in the pathogenesis of tuberculosis. Braz. J. Med. Biol. Res. 21(1988)489-492.
37. SILVA, C. L. and FIGUEIREIXJ, F. Tumor necrosis factor in paracoccidioidomycosis patients. J. Infect. Dis. 164(1991)1033-1034.
38. SILVA, C. L. and Foss, N. T. Tumor necrosis factor in leprosy patients. J. Infect. Dis. 159(1989)787-790.
39. TAYLOR, A. W., Ku, N. O. and MORTENSEN, R. F. Both human IL1 and IL6 induce synthesis of C-reactive protein (CRP) by PLC/PRF/5 hepatoma cell line. Ann. N. Y. Acad. Sci. 557(1989)532-533.
40. TILLETT, W. S. and FRANCIS, T., JR. Serological reactions in pneumonia with a non-protein somatic fraction of pncumococcus. J. Exp. Med. 52(1930)561-585.
41. TRACEY, K. J., LOWRY, S. F. and CERAMI, A. Cachectin; hormone that triggers acute shock and chronic cachexia, J. Infect. Dis. 157(1988)413-420.
42. WAAGE, A., HALSTENSEN, A. and ESPEVIK, T. Association between tumor necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet 1(1987)355-357.
43. WEMAMBU, S. N. C, TURK, J. L., WATERS, M. F. R. and REES, R. J. W. Erythema nodosum leprosum; a clinical manifestation of the Arthus phenomenon. Lancet 2(1969)933-935.
44. WHISLER, R. L., NEWHOUSE, Y. G. and MORTENSEN, R. F. Modulation of human B lymphocyte colony formation by C-reactive protein. J. Immunol. 130(1983)248-253.
45. WILLIAMS, R. C, JR. C-reactive protein binding to lymphocyte subpopulations in human disease states. Ann. N. Y. Acad. Sci. 389(1982)395-405.
46. WOLOSKI, B. M. R. N. J., SMITH, E. M., MEYER, W. J., FULLER, G. M. and BLALOCK, J. E. Corticotropin-releasing activity of monokines. Science 230(1985)1035-1037.
47. WORLD HEALTH ORGANIZATION. Guia para la lucha anti-leprosa. Ginebra: Organization Mundial de la Salud, 1980.
48. YAMADA, Y., KIMBALL, K., OKUSAWA, S., VACHINO. G., MARGOLIS, N., SOHN, J., Li, J. J., WAKABAYASHI, G., MCADAM, K., BURKE, J. F., DINARELLO, C. A. and GELFAND, J. A. Cytokines, acute phase proteins, and tissue injury; C-reactive protein opsonizes dead cells for debridement and stimulates cytokine production. Ann. N. Y. Acad. Sci. 587(1990)351-361.
49. ZAHEDI, K., TEBO, J. M., SIRIPONT, J., KLIMO, G. K. and MORTENSEN, R. F. Binding of human C-reactive protein to mouse macrophage is mediated by distinct receptors. J. Immunol. 142(1989)2384-2392.
50. ZELLER, J. M., KUBAK, B. M. and GEWURZ, H. Binding sites for C-reactive protein on human monocytes are distinct from IgGFc receptors. Immunology 67(1989)51-55.
1. M.D., Ph.D., Associate Professor of Medicine;
2. Ph.D., Professor of Biochemistry of Medicine;
3. Ph.D., Associate Professor of Microbiology of Medicine, School of Medicine of Ribeirão Preto, University of São Paulo, 3900 Avenida Bandeirantes, 14048-900 Ribeirão Preto, SP, Brazil.
Received for publication on 21 October 1992.
Accepted for publication in revised form on 6 January 1993.