- Volume 61 , Number 3
- Page: 381–8
Relationship between host histones and armadillo-derived Mycobacterium leprae
ABSTRACT
A major protein previously recognized as being primarily associated with the cell walls of Mycobacterium leprae, major wall protein (MWP), is now identified as histoprotein H2b based on N-terminal amino-acid sequencing, electrophoretic comparisons, and several other properties. An avid association between several host/armadilloderived histones and M. leprae was demonstrated. Since such armadillo-derived M. leprae are the basis of several ongoing vaccine trials, a simple procedure that permits the prompt solubilization and quantification of histones in M. leprae preparations is described. The quantity of histones associated with M. leprae is significant, ranging f rom 0.6 to 4.8 µg of histoprotein H2b per mg of bacteria.RÉSUMÉ
Une protéine majeure reconnue auparavant comme étant en premier licu associéc á la paroi cellulaire de Mycobacterium leprae la protéine majeure de la paroi (MWP), est maintenant identifiée comme une histoprotéine H2b sur base de la determination de la séquence des acides aminés de l'extrémité azotée, de comparaisons électrophorétiques et diverses autres propriétés. On a démontré l'existence d'une association étroite entre différentes histones dérivées du tatou-hôte et M. leprae. Comme de tels M. leprae obtenus à partir de tatous forment la base de différents essais de vaccination en cours, un procédé simple qui permet la solubilisation rapide et la quantification des histones dans des préparations de M. leprae est décrite. La quantité d'histones associées avec M.leprae est significative, allant de 0.6 à 4.2 µg d'histoprotéine H2b par mg de bactérie.RESUMEN
Una proteína previamente considerada como primariamente asociada a las paredes celulares del Mycobacterium leprae (la proteína MWP), ahora se identifica como la histoproteína H2b con base en su secuencia de aminoácidos N-amino terminal, su comportamiento electroforético, y varias otras propiedades. Se demostró que existe una ávida asociación entre varias histonas derivadas de armadillo y el M. leprae. Puesto que M. leprae derivado de armadillo es la base de varias vacunas experimentales, aquí se describe un procedimiento simple que permite la pronta solubilización y cuantificación de histonas en las preparaciones de M. leprae. La cantidad de histonas asociadas con M. leprae es significativa y varía de 0.6 a 4.8 µg de histoproteína H2b por mg de bacterias.Estimates of the global prevalence of leprosy have decreased dramatically over the last decade due to the introduction of multidrug therapy and the redefinition of a prevalent case as one requiring short-term multidrug therapy. The estimated number of cases in 1991 was 5.5 million as compared to the 10-12 million cases just a few years earlier (15). Nevertheless, leprosy still represents a major public health problem within the countries of Africa, Asia, and Latin America.
Despite positive trends, the development of a vaccine that would contribute to the eradication of leprosy remains a major goal of many research programs. Such a vaccine would be inexpensive compared to chemotherapy, would not involve the specter of evolving drug resistance, and would be easy to administer in geographical regions now proving much less amenable to protracted chemotherapy. However, the prospects of a vaccine based on the whole bacterium may have been undermined by the negative preliminary results from the Venezuela vaccine trials (3) (although these can be explained on purely technical grounds), and the prospects of a live recombinant vaccine (19), probably the best hope, or one based on cultivable mycobacteria, are still in the offing. We have taken the long-term tack that the protein antigens of Mycobacterium leprae still await basic molecular definition and, once defined, each one requires examination against a set of immunological correlates relevant to leprosy. A case in point is our recent molecular and immunological definition of the 10-kDa antigen of M. leprae (7,13), its single most dominant protein immunogen.
This particular strategy has been hampered, of course, by the inability to grow M. leprae in vitro. With the discovery in the 1970s that the nine-banded armadillo is susceptible to infection with M. leprae (9), tissues of infected animals have constituted the major source of the leprosy bacillus for a generation of biochemical and immunological studies. Various procedures have been developed to purify the bacteria from host tissues, combining techniques sufficiently gentle to preserve the native structure of the bacteria but powerful enough to remove host components (4 14, 17, 18, 20, 23). One widely used purification procedure, devised for production of the one M. lepraederived candidate leprosy vaccine now in trial in Venezuela, Malawi, and South India, is that of Draper (23). This protocol involves what are considered gentle separation techniques including differential and density gradient centrifugation, and partitioning in an aqueous two-phase system. Through research sponsored by the U.S.A. National Institutes of Health, we have adopted this procedure in order to provide the scientific community with both large amounts of armadillo-derived M. leprae for experimental purposes and fundamental information on its major molecular constituents (2,7).
In the present study, we define a major 17-kDa cell-wall-associated protein of the bacterium as host-derived histone H2b. A procedure that permits the solubilization and quantification of histoproteins in M. leprae preparations is described, and the implication of this finding is discussed.
MATERIALS AND METHODS
Purification of major cell-wall proteins of M. leprae. M. leprae was purified from irradiated spleens and livers of infected armadillos according to Draper with minor modifications (23). M. tuberculosis Erdman strain No. 107 from the Trudeau Mycobacterial Culture Collection was grown as described (11).
Purification of the cell walls of M. leprae and solubilization of their proteins with sodium dodecyl sulfate (SDS) have been described (7). To purify the major cell wall protein (MWP), a polypeptide doublet of apparent molecular mass 17 kDa (7), the SDS extract from cell walls was initially passed through a column of Extracti-gel D (Pierce Chemical Co., Rockford, Illinois, U.S.A.) to remove the detergent. The eluate was diluted to a final concentration of 5 mg protein/ml in 10 mM Tris-HCl, pH 7.4, containing 150 mM NaCl and 6% Triton X-l 14, and submitted to phase separation as described by Bordier (1). Proteins from the detergent phase were recovered by precipitation with 4 volumes of chilled (-20ºC) acetone. The precipitate was dissolved in 10 mM piperazine, pH 5.7, containing 1% octyl-glucoside, and applied to an HPLC column of AX-300 (DEAE) (Rainin Instruments Company, Inc., Woburn, Massachusetts, U.S.A.) equilibrated with the same buffer. Proteins were eluted from the column with a gradient of 0-30% NaCl. Fractions containing the 17-kDa protein were pooled, dialyzcd against 10 mM Tris-HCl, pH 7.4, containing 6 M urea and applied to a Scphacryl S-300 gel filtration column (1 x 100 cm) equilibrated with the same buffer. Fractions containing the pure protein were dialyzcd against water and vacuumdried.
Acid extraction to remove histones. M. leprae (50 mg) and M. tuberculosis (Erdman strain) (50 mg) were submitted to an acid extraction which favors solubilization of histones (21). Briefly, the bacteria were resuspended in 1 ml of 0.25 M HC1 and sonicated with a probe sonicator (W-385 Sonicator, Ultrasonic Liquid Processor; Heat Systems-Ultrasonic, Inc., Farmingdale, New York, U.S.A.) for 2 min on ice. Cells were removed by centrifugation, and the supernatants were vacuum-dried. Whole M. leprae cells were extracted with PBS/EDTA as described (16). Histones from uninfected armadillo spleens were isolated using a standardized procedure (21). Total histones from calf thymus were purchased from Boehringer Mannheim (Indianapolis, Indiana, U.S.A.). Histones VII-S (enriched in H2b) and VI-S (enriched in H2a) from calf thymus were obtained from Sigma Chemical Co. (St. Louis, Missouri, U.S.A.). Protein was estimated by the bicinchoninic acid (BCA) protein assay reagent (Pierce) using bovine serum albumin (BSA) as standard.
Polyacrylamide gel electrophoresis. Fractions were analyzed by SDS-(10) or acetic acid-urea-PAGE (12). Acid-urea gels containing 2.5 M urea were cast in glass plates 22 x 20 cm with a 1-mm thick spacer and prerun in 0.9 M acetic acid at 25 mA until constant voltage was observed. After dissolving in 0.9 M acetic acid containing 2.5 M urea and 0.02% pyronine, the samples were loaded on the gel and a constant voltage of 200 volts was applied. Gels were stained with silver nitrate or 0.1% Coomassie brilliant blue R in 40% methanol and 10% acetic acid. The amount of histone H2b present in M. leprae acid extracts was determined by quantitative densitometry of acid-urea gels stained by Coomassie blue, using the MicroScan 1000 2-D Gel Analysis System (Technology Resources, Inc., Nashville, Tennessee, U.S.A.).
N-Group analysis of proteins. Proteins were transferred from acid-urea gels onto an Immobilon polyvinylidenedifluoride (PVDF) membrane (Millipore, Bedford, Massachusetts, U.S.A.) in 0.7% acetic acid/ 10% methanol at a constant voltage of 40 volts for 1 hr using a trans-blot unit with plate electrodes (Bio-Rad Laboratories, Richmond, California, U.S.A.). After staining with Coomassie brilliant blue (0.1% in 40% methanol and 1% acetic acid) for 2 min, the membrane was destained with 50% methanol, and the band of interest was excised. N-terminal amino-acid sequencing of proteins was performed in a 470A Gasphase Sequencer (Applied Biosystems, San Jose, California, U.S.A.) equipped for on-line bore PTH-amino acid analysis.
RESULTS
In a previous study (7), cell walls were prepared from disrupted, armadillo-derived M. leprae by sucrose density centrifugation. The distinctive band at the 35%/ 45% sucrose interphase was the source of the cell wall, and the 17-kDa protein doublet, MWP, was obtained from this fraction by further SDS extraction. In the present study, pure MWP was prepared by combining detergent phase partitioning with ion exchange and gel filtration chromatography (Fig. 1). Direct N-terminal amino-acid sequencing of the purified protein readily allowed the identification of the first 26 amino acids (Fig. 2). Comparison of the aminoacid sequence of MWP with that of proteins in the National Biomedical Research Foundation protein sequence data base showed 100% homology with the corresponding N-terminal segment of human histone H2b. This finding was a surprise and, indeed, initially, a disappointment, given our earlier belief that this protein was an important specific component and immunogen of M. leprae (7).
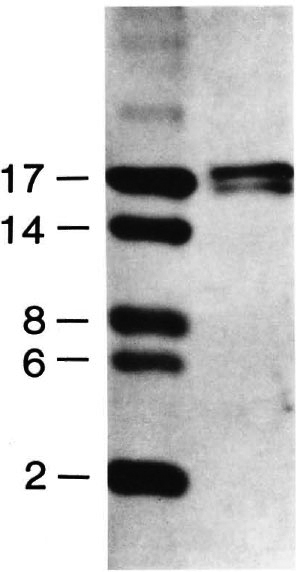
Fig. 1. Electrophoresis of the major cell-wall-associated protein (MWP) doublet (ca. 17 kDa) of M. leprae. SDS-PAGE was conducted on a 16% tricine gel and stained with silver nitrate. Molecular weight markers are shown on the left.
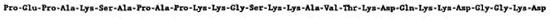
Fig. 2. The N-terminal amino-acid sequence of the MWP (17 kDa) of M. leprae
The concept of a direct association between host histoproteins and M. leprae harvested from such host tissues was further substantiated by extracting the bacteria with acid. Figure 3A shows the protein patterns on an acid-urea gel of acid extracts obtained from two M. leprae preparations and are compared with similar preparations from noninfected armadillo tissue and with calf histones. A complex mixture of bacterial proteins was observed in extracts from both M. leprae preparations. The presence of the stress proteins of Mr 71, 65, 28, 18, and 10 kDa, and the Mr 35 kDa membrane protein (major membrane protein I; MMP 17) were confirmed by Western blotting using monoclonal antibodies against these proteins produced in this laboratory or obtained through the WHO/TDR/IMMLEP program (5,8). Strong bands with electrophoretic mobility identical to HI, H2a, H2b, and H4 were also observed in an acid extract ofM. leprae purified from armadillo spleen. Incidentally, an acid extract of M. tuberculosis grown in culture did not show the presence of products indicative of histones (Fig. 3B); as demonstrated (lane 7), the M. tuberculosis extracts do contain proteins, but they are not histoproteins.
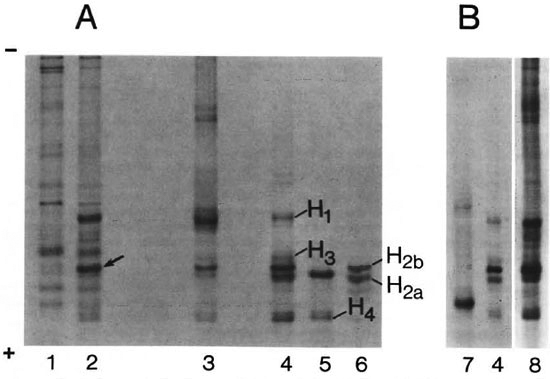
Fig. 3. Gel electrophoresis ofacid extracts ofM. leprae (A) and M. tuberculosis (B) in acetic acid-urea gels and staining with Coomassic brilliant blue. Lanes: 1, acid extract of M. leprae purified from armadillo liver; 2, acid extract of M. leprae purified from armadillo spleen; 3, armadillo histones; 4, total histones from calfthymus; 5, fraction enriched in histone H2a from calf thymus; 6, fraction enriched in histone H2b from calf thymus; 7, acid extract of M. tuberculosis; 8, acid extract of fibrous tissue plus M. leprae clumps. Arrow indicates band submitted to N-terminal amino-acid sequence analysis after transferring to PVDF membrane.
In order to confirm that the bands observed in acid extracts of M. leprae were indeed histones, proteins were transferred from acid-urea gels to PVDF membranes, and N-terminal amino-acid sequencing was performed. The first ten amino acids of the M. leprae band, corresponding to H2b, showed 100% homology with human histone H2b. N-terminal amino-acid sequencing of the other histones was not completed since they proved to be blocked at the N-terminal end.
As one reflects on the history of the purification of M. leprae from host tissue, contamination with host DNA always has been a problem, probably responsible, for instance, for the interaction and clumping of bacteria with tissue components (P. Draper, personal communication). The inclusion in the Draper protocol of a step in which preparations of M. leprae are treated with DNAase was meant to obviate this problem. To determine that the histoproteins now observed in preparations of M. leprae were derived from host chromatin contamination, clumps of what are considered fibrous tissue material cum bacteria and which are a common product of the Draper purification steps (P. Draper, personal communication) were extracted with acid and analyzed by acid-urea gel electrophoresis (Fig. 3B). The similarity in protein content of one of these extracts and a standard histone preparation pointed convincingly to the chromatin nature of the fibrous tissue material long associated with M. leprae.
In order to obtain information on the frequency as well as the degree of histone content of preparations, nine different batches of M. leprae (purified from either infected armadillo livers or spleens) were extracted, and acid-urea gels of the acid extracts were analyzed by quantitative densitometry (The Table). All preparations showed the presence of histone H2b, with absolute amounts varying from 0.6 to 4.8 µg/mg of bacteria. Bacteria obtained from livers showed fewer histones than those obtained from spleens. An important point is that there was no apparent correlation between the amount of histone H2b present in a preparation and one's estimate of bacterial purity based on soluble blue staining through the Ziehl-Neelsen reagent (22).
Arising from years of experience in the purification of M. leprae from armadillo sources, we had dropped the dextran-polyethyleneglycol biphasic step of the original Draper protocol (23) and replaced it by the inclusion of at least two further Percoll density gradient centrifugation steps. This modification permitted an increase in bacterial yields without any apparent reduction in purity. To determine if the inclusion of the aqueous two-phase step could reduce the extent of histone association with M. leprae, materials prepared with and without the biphase step were compared. In two independent experiments, no significant difference was observed in the content of M. leprae prepared by the two methods. Some revelation on the tenacity of the association between M. leprae and the host histones was obtained by the observation that when bacilli were first extracted with PBS/EDTA, a step which, incidentally, solubilizes the excreted 30-31-kDa protein complex of M. leprae (16), none of the histones were removed. Subsequent application of a simplified acid extraction step to the resulting pallet whereby the bacteria were treated with acid at room temperature for 10 min with gentle agitation, omitting sonication, resulted in extracts in which histones were essentially the only proteins (Fig. 4). In two such preparations, employing M. leprae from spleens, the recovery of histones was of the order of 20 µg and 100 µg of proteins/ mg of bacteria.
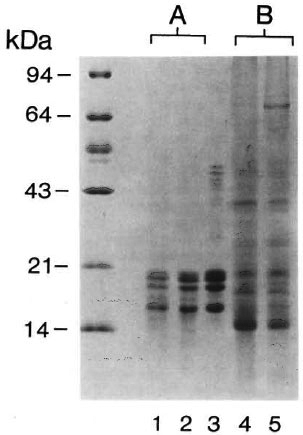
Fig. 4. Evidence for a tenacious association between histoproteins and M. leprae. Two batches of M. leprae obtained from infected spleens were extracted first with PBS/EDTA and then with acid (A) or directly with acid (B). The solubilized proteins were run on a 15% Polyacrylamide gel and stained with Coomassic blue. Lane 1, M. leprae batch No. 1; lane 2, M. leprae batch No. 2; lane 3, total histones from calf thymus. Molecular weight markers are indicated on the left.
DISCUSSION
Although H2b seems to be the predominant histone associated with M. leprae cell walls, bands with electrophoretic mobility identical to that of other nucleosome histones, as well as to histone HI, were also observed. In our view, two lines of evidence indicate that the histoproteins of M. leprae preparations are host-derived and are not the products of horizontal gene transfer between host and parasite (6), a transfer which apparently accounts for the presence of histone-like proteins in other organisms. Firstly, from present and previous evidence (7), it is obvious that these proteins are preferentially associated with the surface of M. leprae, unlike the histones and histone-like proteins of other eukaryotes and prokaryotes which are clearly cytosolic. Secondly, present experiments established that chromatin is the major source of histoprotein contamination of M. leprae preparations. The fact that histones were recovered in the detergent phase from the Triton phase separation suggests that they are present in their denatured forms and probably attach to the cell envelope of M. leprae by hydrophobic interaction. On the other hand, histoproteins are highly cationic with a pi range of 11 to 12 because of the predominance of lysine and arginine residues, and are not readily displaced by chelating agents, all pointing to ionic interaction. What might be the nature of the corresponding negative charge on the surface of M. leprae is not known; perhaps it is lipoarabinorhannan which does carry an overall negative charge.
The Draper protocol has been used for several years for the preparation of vaccinegrade M. leprae, largely on the basis of the protective efficacy of the product against foot pad infection in mice (18). The essence of the protocol is the application of relatively gentle separation methods, obviating, for instance, the drastic alkaline and protease treatments which are part of other protocols. Thus, the presence of host-derived components in preparations of M. leprae obtained in this way is not unexpected and, in fact, has already been demonstrated (P. Draper, personal communication). The present study clearly establishes the composition of some of these contaminants and shows that they are not trivial. The consequences of the injection of host components such as histones into humans in the context of the present vaccine trials are probably not harmful. The procedures described in this present study allow for a quantitation of the histone content of leprosy vaccine preparations and a determination of what effects they might have on potency.
Acknowledgment. This work was supported by a contract (NOl AI-05074) from the National Institute of Allergy and Infectious Diseases, Division of Microbial and Infectious Diseases, National Institutes of Health. M.C.V.P. was supported by a Post-Doctoral Fellowship from the Heiser Program for Research in Leprosy and Tuberculosis. We thank Drs. Eleanor E. Storrs and Darrel D. Gwinn for ensuring a steady supply of armadillo-derived products.
REFERENCES
1. BORDIER, C. Phase separation of integral proteins in triton X-114 solution. J. Biol. Chem. 256( 1981 )1604-1607.
2. BRENNAN, P. J. Structures of mycobacteria: recent developments in defining cell wall carbohydrates and proteins. Rev. Infect. Dis. 11 Suppl. 2(1989)S420-S430.
3. CONVIT, J., SAMPSON, C, ZUNUGA, M., SMITH, P. G., PLATA, J., SILVA, J., MOLINA, J., PINARDI, M. E., BLOOM, B. R. and SALGADO, A. Immunoprophylactic trial with combined Mycobacterium leprae/BCG vaccine against leprosy: preliminary results. Lancet 339(1992)446-450.
4. DAS, P. K. and TULP, A. Use of a unit gravity sedimentation chamber for the purification of Mycobacterium leprae. Ann. Microbiol. (Paris) 133B(1982)389-400.
5. ENGERS, H. D., HOUBA, V., BENNEDSEN, J., BUCHANAN, T. M., CHAPARAS, S. D., KADIVAL, G., CLOSS, O., DAVID, J. R., VAN EMBDEN, J. D. A., GODAL, T., MUSTAFA, S. A., IVANYI, J., YOUNG, D. B., KAUFMANN, S. H. E., KHOMENKO, A. G., KOLK, A. H. J., KUBIN, M., LOUIS, J. A., MINDEN, P., SHINNICK, T. M., TRNKA, L. and YOUNG, R. A. Results of a World Health Organization-sponsored workshop to characterize antigens recognized by mycobacteria-specific monoclonal antibodies. Infect. Immun. 51(1986)718-720.
6. HOUCK, M. A., CLARK, J. B., PETERSON, K. R. and Ki DWELL, M. G. Possible horizontal transfer of Drosophila genes by the mite Proctolaelaps regalis. Science 253(1991)1125-1129.
7. HUNTER, S. W., RIVOIRE, B., MEHRA, V., BLOOM, B. R. and BRENNAN, P. J. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265(1990)14065-14068.
8. KHANOLKAR-YOUNG, S., KOLK, A. H. J., ANDERSEN, A. B., BENNEDSEN, J., BRENNAN, P. J., RIVOIRE, B., KUIJPER, S., MCADAM, K. P. W. J., ABE, C, BATRA, H. V., CHAPARAS, S. D., DAMIANI, G., SINGH, M. and ENGERS, H. D. Results of the third immunology of leprosy/immunology of tuberculosis antimycobacterial monoclonal antibody workshop. Infect. Immun. 60(1992)3925-3927.
9. KIRCHHEIMER, W. F. and STORRS, E. E. Attempts to establish the armadillo (Dasypus noxemcinetus Linn.) as a model for the study of leprosy. I. Report of Iepromatoid leprosy in an experimentally infected armadillo. Int. J. Lepr. 39(1971)693-702.
10. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227(1970)680-685.
11. LEE, B.-Y.,HEFTA,S. A. and BRENNAN, P.J. Characterization of the major membrane protein of virulent Mycobacterium tuberculosis. Infect. Immun. 60(1992)2066-2074.
12. LENNOX, R. W. and COHEN, L. H. Analysis of histone subtypes and their modified forms by polyacrylamide gel electrophoresis. Meth. Enzymol. 170(1989)532-571.
13. MEHRA, V., BLOOM, B. R., BAJARDI, A. C, GRISSO, C. L., SIELING, P. A., ALLAND, D., CONVIT, J., FAN, X., HUNTER, S. W., BRENNAN, P. J., REA, T. H. and MODLIN, R. L. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock cognate protein. J. Exp. Med. 175(1992)275-284.
14. MORI, T., MIYATA, Y., YONEDA, K. and ITO, T. Collection method for Mycobacterium leprae from infected armadillo liver. Int. J. Lepr. 52(1984)41-43.
15. NOORDEEN, S. K., BRAVO, L. L. and SUNDARESAN, T. K. Estimated number of leprosy cases in the world. Bull. WHO 70(1992)7-10.
16. PESSOLANI, M. C. V. and BRENNAN, P. J. Mycobacterium leprae produces extracellular homologs of the antigen 85 complex. Infect. Immun. 60(1992)4452-4459.
17. PRABHAKARAN, K., HARRIS, E. B. and KIRCHHEIMER, W. F. Binding of 14C-labeled DOPA by Mycobacterium leprae in vitro. Int. J. Lepr. 44(1976)58-64.
18. SHEPARD, C. C, DRAPER, P., REES, R. J. W. and LOWE, C. Effect of purification steps on the immunogenicity of Mycobacterium leprae. Br. J. Exp. Pathol. 61(1980)376-379.
19. STOVER, C. K., DE LA CRUZ, V. F., FUERST, T. R., BURLEIN, J. E., BENSON, L. A., BENNETT, L. T., BANSAL, G. P., YOUNG, J. F., LEE, M. H., HATFULL, G. F., SNAPPER, S.. BARLETTA, R. G., JACOBS, W. R., JR. and BLOOM, B. R. New use of BCG for recombinant vaccines. Nature 351(1991)456-460.
20. UNDP/WORLD BANK/WHO SPECIAL PROGRAMME for RESEARCH and TRAINING in TROPICAL DISEASES. Sixth programme report. (Abstract) Indian J. Lepr. 56(1984)118-147.
21. VON HOLT, C., BRANDT, W. F., GREYLING, H. J., LINDSEY, G. G., RETIE'', J. D., RODRIGUES, J. D., SCHWAGER, S. A. and SEWELL, B. T. Isolation and characterization of histones. Methods Enzymol. 170(1989)431-523.
22. WHEELER, P. R. and DRAPER, P. Soluble blue as a counterstain in the Zichl-Neelsen procedure; a brief communication. Int. J. Lepr. 48(1980)15-17.
23. WORLD HEALTH ORGANIZATION. Purification of M. leprae. Annex 4 of Report of the Fifth Meeting of the Scientific Working Group on the Immunology of Leprosy (I M M LEP), Geneva, 24-26 June1980. WHO Document TDR/IMMLEP-SWG(5)/80.3.
1. Ph.D., Heiser Postdoctoral Research Fellow; Department of Microbiology, Colorado State University, Fort Collins, Colorado 80523, U.S.A.
2. Ph.D., Associate Professor; Department of Microbiology, Colorado State University, Fort Collins, Colorado 80523, U.S.A.
3. Ph.D., Professor, Department of Microbiology, Colorado State University, Fort Collins, Colorado 80523, U.S.A.
Dr. Pessolani's permanent address: Setor Hanseniase, Fundacao Oswaldo Cruz, Rio de Janeiro. Brasil.
Dr. Hunter's present address: Paravax, Inc., 2301 Research Blvd., Suite 1 10, Fort Collins. Colorado 80526. U.S.A.
Received for publication on 2 February 1993.
Accepted for publication in revised form on 1 1 May 1993.