- Volume 61 , Number 2
- Page: 245–9
Recognition of Mycobacterium leprae antigens with antibodies present in sera f rom patients with lepromatous leprosy
ABSTRACT
A great diversity of antigens f rom Mycobacterium lepraehavc been described. One practical approach should be to utilize them as markers to indicate when a household contact is at risk of becoming infected and then moving to an active form of leprosy. For this purpose, sonic extracts of M. leprae were fractionated in 10% SDS-PAGE under reducing conditions. The fractionated proteins were then transferred to nitrocellulose sheets and incubated with sera f rom lepromatous leprosy cases, their contacts, and normal subjects in order to reveal the frequency of antigen recognition of each set of sera. The results showed that sera f rom lepromatous leprosy patients frequently recognized two proteins, one of approximately 28 kDa and the other of approximately 65 kDa, when compared with the sera f rom normal subjects. The contacts frequently recognized an approximately 16-kDa antigenic band, while sera f rom normal subjects recognized one protein of approximately 18 kDa. According to the results, the four recognized proteins f rom M. leprae can be considered markers of the above conditions (approximately 65 kDa, approximately 28 kDa for lepromatous leprosy, approximately 16 kDa for contacts, and approximately 19 kDa for normal subjects). From these, an easy serological test, such as an ELISA, can be developed to predict if a contact is moving toward lepromatous leprosy before detection of the actual clinical signs or symptoms.RÉSUMÉ
Une grande diversité d'antigènes en provenance de Mycobacterium leprae ont été décrits. Une approche orientée vers la pratique pourrait être de les utiliser comme marqueurs pour indiquer quand un contact domiciliaire est à risque de s'infecter et ensuite de migrer vers une forme active de lèpre. Dans ce but, des extraits soniqués de M. leprae ont été fractionnés par SDS-PAGE à 10% dans des conditions réductrices. Les protéines fractionnées ont alors été transférées sur des membranes de nitrocellulose et incubées avec du serum de patients lépromateux, leurs contacts et des sujets en bonne santé, afin d'étudier la fréquence de reconnaissance de l'antigène dans chaque set de serums. Les résultats ont montré que les serums de malades lépromateux reconnaissaient fréquemment deux protéines, l'une d'environ 28 kDa et l'autre d'environ 65 kDa, par comparaison avec les serums des personnes en bonne santé. Les contacts reconnaissaient fréquemment une bande antigénique d'approximativement 16 kDa, tandis que les serums provenant d'individus sains reconnaissaient une protéine d'environ 18 kDa. D'après les résultats, les quatre protéines provenant de M. leprae qui ont été reconnues peuvent être considérées comme des marqueurs des conditions ci-dessus (environ 65 kDa et environ 28 kDa pour la lèpre lépromatcuse, environ 16 kDa pour les contacts et environ 19 kDa pour les individus en bonne santé). A partir de celles-ci, un test serologique aisé, tel qu'un ELISA, peut être développé afin de prédire, avant la détection des signes cliniques ou des symptômes, si un contact est en train de migrer vers une lèpre lépromateuse.RESUMEN
Se ha descrito una gran variedad de antígenos del Mycobacterium leprae. Una aplicación prática de ésto podria ser la utilización de estos antígenos como marcadores del riesgo que tienen los contactos de infectarse y de moverse después hacia alguna forma activa de la lepra. Con este propósito se fraccionaron extractos sonicados de M. leprae por PAGE-SDS en geles al 10% bajo condiciones de reducción. Las proteínas fraccionadas se transfirieron después a membranas de nitrocelulosa y se incubaron con sueros de casos de lepra lepromatosa, de sus contactos, y de sujetos normales, para poder revelar la frecuencia de reconocimiento antigenico de cada juego de sueros. Los resultados mostraron que los sueros de los pacientes lepromatosos reconocieron frecuentemente dos proteínas, una de aproximadamente 28 kDa y otra de aproximadamente 65 kDa, los contactos reconocieron frecuentemente una banda de aproximadamente 16 kDa, y los sueros de los individuos normales reconocieron una proteína de aproximadamente 18 kDa. De acuerdo a estos resultados, las cuatro proteínas reconocidas del M. leprae podrían considerarse como marcadores de las condiciones anteriores (aproximadamente 65 kDa y aproximadamente 28 kDa para lepra lepromatosa, aproximadamente 16 kDa para contactos, y aproximadamente 19 kDa para individuos sanos). Con base en los resultados señalados, se podría desarrollar una prueba serológica, tal como un ELISA, para predecir el desplazamiento de un contacto hacia el extremo lepromatoso de la lepra, antes de la aparición de los síntomas y signos clínicos de la enfermedad.As has been described in the literature (6), there are a great many antigens obtained from Mycobacterium leprae with different biochemical compositions that are recognized by antibodies from patients with leprosy or that inhibit the function of human T lymphocytes (2,5). One of these antigens is phenolic glycolipid-I (PGL-I) (7,18) which appears to be significant in the diagnosis of the multibacillary lepromatous form of leprosy but not of the tuberculoid form (15). Recently, SDS-PAGE and Western blot techniques have been used as methods for obtaining a marker of the disease, using the antibodies present in the sera from leprosy patients to study the recognition of M. leprae proteins (3,8,15). One practical approach to the use of these markers should be to ascertain when a household contact subject is at risk of becoming infected and then moving to the lepromatous or tuberculoid type of the disease.
Here we report, using a sonic extract of M. leprae in SDS-PAGE and Western blot techniques, the presence of antigenic markers for lepromatous leprosy cases, for their contacts, and for normal subjects using the frequency analysis described by Larralde, et al. (10).
MATERIALS AND METHODS
Subjects (sera). Twenty-one patients (10 males, 11 females) between the ages of 20- 65 years from the Instituto Dermatológico at Guadalajara, Jalisco, México, were diagnosed as having lepromatous leprosy according to international criteria (13). All of them received an irregular treatment of 100 mg of dapsone per day. The length of treatment ranged from 2 to 15 years. Sera were obtained from the 21 patients by venipuncture. Sera were also collected from 19 contacts of the patients as a second study group (10 males and 9 females between 20 and 50 years old). The control group consisted of 20 clinically normal subjects, all matched with the two study groups for sex and age as much as possible. All sera were divided into three parts and frozen at -20ºC until used.
Antigens. Sonic extracts of M. leprae from armadillo were prepared according to the Draper protocol (17). Briefly, the spleen of armadillos infected 1 year before with a human leproma were obtained, homogenized, resuspended in DNAse buffer solution, and purified in Percoll gradients. Finally, the purified bacilli obtained were sonicated at 100 W, centrifuged at 10,000 x g, and the soluble supernatant adjusted to 1000 µg/ml of protein by the method of Lowry, et al. (12).
Western blots. The sonic extracts were fractioned in 10% SDS-PAGE under reducing conditions (9) at 150 V for 60 min using Bio-Rad minigel equipment (Bio-Rad Laboratories, Richmond, California, U.S.A.). The fractioned proteins were then transferred (14) to 0.45-µm nitrocellulose sheets (Bio-Rad) overnight at 4 mA. The sheets were reversibly dyed with Amido black to test the molecular weight of the transferred proteins, washed in phosphate buffered saline (PBS), pH 7.2, dried, cut into 3 x 65 mm wide strips, and placed in individual plastic tracks. They were blocked with 5% skim milk in PBS-0.1% Tween 20, and incubated 1.5 hr with a 1:50 dilution of each serum. They were washed three times for 10 min each with PBS-Tween 20, and incubated with a second peroxidase-conjugated goat antibody against human IgG for 1 hr, then washed again three times. The substrate (diaminobenzidine; Sigma Chemical Company, St. Louis, Missouri, U.S.A.) was added in 10 ml of PBS plus 20 µl of H2O2 and 70 µl of a 1% calcium chloride solution for the development of color. The reactions were stopped by washing the membranes with distilled water. The strips were washed, dried, and photographed. The intensity of each band was determined by two different observers and classified as highly positive (+ +) or slightly positive (+). The molecular weight of each band was calculated according to molecular markers (Sigma) previously included in the gels and transferred to the sheets.
Analysis of frequency of antigen recognition. The so-called Immunoplot method proposed by Larralde, et al. (10) was used to analyze the frequency of antigen recognition. Briefly, the recognition frequency from 0 to 1 of each individual antigen in the Western blot is plotted against each set of sera from lepromatous patients, their contacts, and normal subjects. This method allows the immediate identification of antigens reacting only with normal sera (y axis) or lepromatous sera (x axis), or antigenic bands that are recognized by both sets of sera (plane between both axes). This method considers that a band plotted on one of the axes (for example, on x or y) with a frequency above 0.5 constitutes a specific marker; bands recognized by both sera lack significance (10).
RESULTS
Western blots. Illustrative Western blots of the M. leprae antigens reacting with sera from lepromatous patients and their contacts, as well as with sera from normal subjects, are shown in Figure 1 (molecular weight markers are shown on the left). Sera from lepromatous patients recognized 21 bands of molecular weights ranging from 97-14 kDa. The sera from their contacts recognized 18 different bands ranging in the same interval (97-14 kDa). Sera from normal subjects recognized 19 bands.
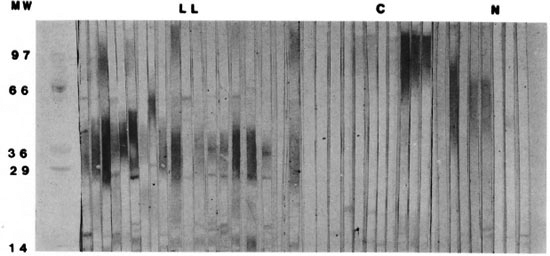
Fig. 1. Western blots of sera from patients with lepromatous leprosy, their contacts, and normal subjects reacting with sonic extract of M. leprae.
Immunoplots. The immunoplot of frequencies showed that sera from lepromatous patients frequently recognized two proteins-one of approximately 28 kDa (f = 0.63), the other of approximately 65 kDa (f = 0.68)-when compared with normal subjects' sera which did not recognize significantly any antigenic band (Fig. 2A). Several proteins from M. leprae were frequently recognized by both sets of sera (plane between the axes). Figure 2B shows the immunoplot of frequencies, comparing data from Western blots with sera from contacts (x axis) against sera from normal subjects (y axis). Here we can see that sera from contacts frequently (f = 0.57) recognized an approximately 16-kDa antigenic band, while sera from normal subjects frequently (f = 0.65) recognized one protein of approximately 18kDa molecular weight. Here, again, there were several nonsignificant antigenic bands that were recognized by both sets of sera.
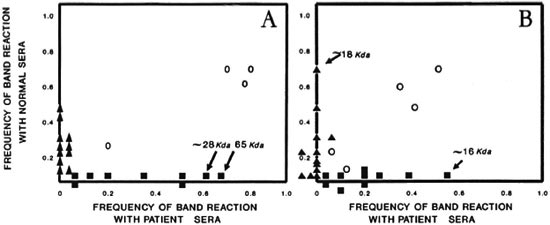
Fig. 2. Immunoplots of (A) lepromatous palicnts versus normal individuals and (B) household contacts versus normal individuals as they reacted with antigens of M. leprae. In A we sec the approximately 28-kDa and 65-kDa frequently recognized antigens as markers of lepromatous leprosy. In B we sec the approximately 16-kDa frequently recognized antigen as a marker of the contacts, and the approximately 18-kDa antigen as a possible marker of normal individuals.
DISCUSSION
Inspection of the bands recognized by the antibodies present in sera from lepromatous patients, their contacts, and normal subjects in Western blots is not an easy task. The use of immunoplots as described by Larralde, et al. (10) has made it possible to order the results from immunoelectrotransferences in such a way that complex mixtures of antigens from mycobacteria can be categorized immediately in a quantitative and specific manner. This method constitutes a useful and potent method for discriminating bands of potential biologic significance.
In our study, therefore, the four recognized proteins of approximately 65, 28, 18 and 16 kDa can be considered markers for lepromatous patients, their household contacts, or control subjects. From these M. leprae antigens, an easy serological test (such as an ELISA) can be developed in order to predict if a household contact is moving toward the lepromatous state of leprosy before the detection of clinical symptoms. It is necessary to use gradient and bidimensional SDS-PAGE and monoclonal antibodies (16) to ascertain whether the protein of approximately 16 kDa (found as a marker in contacts) and the approximately 18 kDa polypeptide (found as a marker in normal subjects) could, in fact, be the same protein. Dockrell, et al. (4) have reported that an 18kDa polypeptide from M. leprae induced the proliferation of CD4 T lymphocytes. Therefore, this 18-kDa protein frequently recognized in normal subjects and, perhaps, by contacts in our study could be involved in protective immunity. We are performing the necessary experiments to ascertain if, indeed, this is true (1,11).
Acknowledgment. This work was supported in part by the postgraduate program of the Facultad de Medicina of the Universidad Nacional Autónoma de México (UNAM) under the tutory of Dra. Cecilia Ximenez at the Departamento de Medicina Experimental.
REFERENCES
1. ABOU-ZEID, G, FILLEY, E., STEELE, J. and ROOK, G. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J. Immunol. Meth. 90(1987)5-10.
2. BRITTON, W., BELLQVIST, J., GARSIA, R. and BASTEN, A. Antigens of Mycobacterium leprae identified by immunoprecipitation with sera from leprosy and tuberculosis patients. Clin. Exp. Immunol. 71(1988)394-398.
3. CHAKRABARTY, A., MAIRE, A. and LAMBERT, P. SDS-PAGE analysis of M. leprae protein antigens reacting with antibodies from sera from lepromatous patients and infected armadillos. Clin. Exp. Immunol. 49(1982)523-531.
4. DOCKRELL, H., STOKER, N., LEE, S., JACKSON, M., GRANT, K., JOUY, N., LUCAS, S., HASAN, R., HUSSAIN, R. and MCADAM, K. T cell recognition of the 18-kDa antigens of Mycobacterium leprae Infect. Immun. 57(1989)1979-1983.
5. FOURNIE, J., ADAMS, E., MULLINS, R. and BASTEN, A. Inhibition of human lymphoprolifcrativc responses by mycobacterial phenol glycolipid. Infect. Immun. 57(1989)3653-3659.
6. HASTINGS, R., GILLIS, T., KRAHENBUHL, J. and FRANZBLAU, S. Leprosy. Clin. Microbiol. Rev. 1(1988)330-348.
7. HUNTER, S. and BRENNAN, P. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bactcriol. 147(1981)728-735.
8. KLATSER, P., VAN RENS, M. and EGGELTE, T. Immunochemical characterization of Mycobacterium leprae antigens by SDS-polyacrylamyde gel electrophoresis immunopcroxidase technique (SGIP) using patients' sera. Clin. Exp. Immunol. 56(1984)537-544.
9. LAEMMLI, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(1970)680-685.
10. LARRALDE, C, MONTOYA, R., SCIUTTO, E., DIAZ, M., GOVEZENSKY, T. and COLTORI, E. Deciphering Western blots of tapeworm antigens (Taenia solium, Equinococcus granulosus, and Taenia crassiseps) reacting with sera from neurocisticercosis and hydatidic disease patients. Am. J. Trop. Med. Hyg. 40(1989)282-290.
11. LEE, S., STOKER, N., GRANT, K., HANDZEL, Z., HUSSAIN, R., MCADAM, K. and DOCKRELL, H. Cellular immune response of leprosy contacts to faclionatcd Mycobacterium leprae antigens. Infect. Immun. 57(1989)2475-2480.
12. LOWRY, O. H., ROSEBROUGH, N. J., FARR, A. L. and RANDALL, R. J. Protein measurements with the folin-Phenol reagent. J. Biol. Chem. 193(1951)265-270.
13. RIDLEY, D. and JOPLING, W. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
14. TOWBIN, H., STAEBELIN, T. and GORDON, J. Electrophorctic transfer of proteins from Polyacrylamide gels to nitrocellulsoe sheets: procedure and some applications. Proc. Natl. Acad. Sei. U.S.A. 76(1979)4350-4354.
15. VEGA-LOPEZ, F., STOKER, N., LOCNISKAR, M., DOCKRELL, H., GRANT, K. and MCADAM, K. Recognition of mycobacterial antigens by sera from patients with leprosy. J. Clin. Microbiol. 26(1988)2474-2479.
16. WATSON, J. Leprosy: understanding protective immunity. Immunol. Today 10(1989)218-221.
17. WORLD HEALTH ORGANIZATION. Report on the fifth meeting of the Scientific Working Group on the Immunology of Leprosy (IMMLEP). Geneva; World Health Organization, 1990, Annex 4, p. 3. TDR/IMM-LEP-SWG (5) 80.3.
18. YOUNG, D. and BUCHANAN, T. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. M.Sc.; Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadalajara/Instituto Dermatológico de Guadalajara (SSBS), Apartado Postal 51-189, Colonia Las Águilas, 45080 Guadalajara, Jalisco, México.
2. M.T.; Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadalajara/Instituto Dermatológico de Guadalajara (SSBS), Apartado Postal 51-189, Colonia Las Águilas, 45080 Guadalajara, Jalisco, México.
3. M.Sc; Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadalajara/Instituto Dermatológico de Guadalajara (SSBS), Apartado Postal 51-189, Colonia Las Águilas, 45080 Guadalajara, Jalisco, México.
4. M.D.; Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadalajara/Instituto Dermatológico de Guadalajara (SSBS), Apartado Postal 51-189, Colonia Las Águilas, 45080 Guadalajara, Jalisco, México.
5. M.D., Centro de Investigación en Inmunología y Dermatologia, Universidad de Guadalajara/Instituto Dermatológico de Guadalajara (SSBS), Apartado Postal 51-189, Colonia Las Águilas, 45080 Guadalajara, Jalisco, México.
6. M.Sc, Dirección de Desarrollo Académico, Universidad de Guadalajara, Guadalajara, Jalisco, México.
7. Ph.D., Laboratorio de Inmunología Molecular II del Departamento de Investigación de la Escuela de Ciencias Biológicas del Instituto Politécnico Nacional, México, D.F., México.
Reprint requests to A. E. Islas-Rodríguez.
Received for publication on 7 July 1992.
Accepted for publication in revised form on 20 January 1993.