- Volume 61 , Number 2
- Page: 250–4
Therapeutic efficacy of some new quinolones and a combination of ofloxacin with rifampin against Mycobacterium leprae infection induced in athymic nude mice
ABSTRACT
Ofloxacin (OFLX), having superior antileprosy activity among the various quinolones, was studied for its combined therapeutic efficacy with rifampin (RMP) against Mycobacterium leprae infection induced in nude mice. When OFLX (3 mg/mouse) was given to infected mice in combination with RMP (0.01 mg/mouse) by gavage once daily six times per week, f rom day 31 to day 80 postinfection, a significant combined effect was observed. This study demonstrates the possibility of using OFLX in multidrug regimens for the clinical control of bacilliferous leprosy patients.RÉSUMÉ
L'olloxacinc (OFLX), qui a une activité antiléprcuse supérieure parmi les différentes quinolones, a été étudiée pour son efficacité thérapeutique en combinaison avec la rifampicine (RMP) vis-à-vis de l'infection par Mycobacterium leprae chez la souris nue. Quand l'olloxacinc (3 mg par souris) était donnée aux souris infectées en combinaison avec la rifampicine (0.01 mg par souris) par gavage une fois par jour six fois par semaine, du trente et unième au quatrevingticme jour après l'infection, on observait un effet combine significatif. Cette étude démontre la possibilité d'utiliser l'oxfloxacinc dans des régimes de polychimiothérapie pour le traitement de patients bacillifères.RESUMEN
Se estudió la eficacia terapéutica de una combinación de rifampicina (RMP) con ofioxacina (OFLX), la quinolona de mayor actividad antilcprosa, sobre la infección inducida por el Mycobacterium leprae en el ratón desnudo. Cuando se administró la OFLX (3 mg/ratón) en combinación con RMP (0.01 mg/ratón) por la via oral, una vez al día, seis veces por semana, del día 31 al día 80 postinfección, se observó un significante efecto terapéutico combinado. Este estudio demuestra la posibilidad de usar OFLX en esquemas de poliquimioterapia para el control clínico de los pacientes con lepra bacilífera.Multidrug therapy (MDT) using rifampin (RMP), clofazimine and diaminodiphenyl sulfone (DDS, dapsone) is considered to be the most effective treatment for patients with leprosy (11). However, it takes at least 6 months to 4 years or more to achieve appreciable results in multibacillary borderline lepromatous and lepromatous patients, even using this MDT regimen (3). The development of new protocols for MDT which enable more rapid therapy for leprosy patients and the use of other types of antilcprosy drugs is therefore considered urgent. Previously, we found that a new quinolone, ofloxacin (OFLX), had an appreciable in vivo activity against Mycobacterium leprae infection induced in mice (6). This finding has been confirmed by other investigators (1,4,5) Moreover, some clinical trials in lepromatous leprosy revealed the significant therapeutic efficacy of OFLX (2).
In our present study, we have evaluated the efficacy of the combination of RMP with OFLX in M. leprae infection induced in athymic nude mice. This study revealed a marked increase in the antileprosy activities of RMP in combination with OFLX, thereby suggesting the usefulness of this multidrug regimen in the chemotherapy of leprosy patients.
MATERIALS AND METHODS
Antimicrobial agents. The drugs tested were: OFLX and RMP (Daiichi Pharmaceutical Company, Tokyo, Japan); piromidie acid, pipemidic acid, and enoxacin (ENX) (Dainippon Pharmaceutical Company, Osaka, Japan); norfloxacin (NFLX) and fleroxacin (FLRX) (Kyorin Pharmaceutical Company, Tokyo); ciprofloxacin (CPFX) (Bayer Pharmaceutical Company, Osaka); dapsone (DDS) (Wako Pure Chemicals, Inc., Osaka).
Organisms. M. leprae Thai-53 were harvested from the infected foot pads of BALB/c nude mice provided by Dr. K. Kohsaka, National Institute for Leprosy Research, Tokyo, Japan. The infected foot pads were finely minced with scissors, homogenized three times in Hanks' balanced salt solution (HBSS) containing 5% fetal bovine serum (FBS), using a glass homogenizer, and then centrifuged at 1000 rmp for 5 min. The upper layer (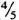 from the meniscus) was taken, re-centrifuged at 3000 rpm for 15 min, and resuspended in a small amount of FBS-HBSS. After centrifugation at 1000 rpm for 5 min, the upper layer (
from the meniscus) was taken, re-centrifuged at 3000 rpm for 15 min, and resuspended in a small amount of FBS-HBSS. After centrifugation at 1000 rpm for 5 min, the upper layer ( 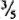 from meniscus) was taken and used as the inoculum. This method reduced the occurrence of large clumps of bacteria.
from meniscus) was taken and used as the inoculum. This method reduced the occurrence of large clumps of bacteria.
Experimental infection. Female, BALB/c nude mice (5 weeks old) were infected with 1 x 106 M. leprae subcutaneously into the left hindfoot pad. The drug, dissolved or emulsified in 0.1 ml of distilled water, was given to mice by gavage once daily, six times per week, from day 31 to day 80. At day 350 or 365 after infection, the mice were killed and the number of acid-fast bacilli (AFB) in the left hindfoot pad was enumerated according to a modification of the method of Shepard (8), unless otherwise specified. Swelling in the left hindfoot pad was measured using a dial-gauge caliper (Kagaku Kyoeisha Co., Tokyo). Solute control mice were given distilled water under the same protocol as in the drug-treated imals, as determined by the reduction in the mice.
RESULTS AND DISCUSSION
Table 1 compares the therapeutic efficacies of some new quinolones against M. leprae infection induced in nude mice. Among the test agents given at the dose of 3 mg/mouse/day, OFLX was effective in inhibiting the growth of leprosy bacilli in the animals, as determined by the reduction in the number of AFB in the infected foot pads at day 365 (about 1.1-log decrease compared to the control mice) and the suppression ofswelling in the infected foot pads (2.5 mm less than controls). FLRX exhibited similar levels of therapeutic efficacy (decrease in the number of AFB by about 1.8-log units); NFLX and CPFX failed to show any anti- M. leprae action. Pipemidic acid, piromidic acid, and ENX were not effective against M. leprae infection in mice (data not shown).
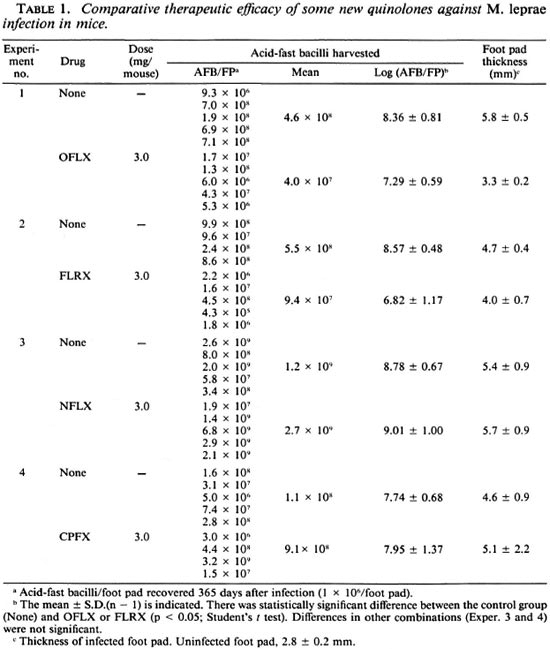
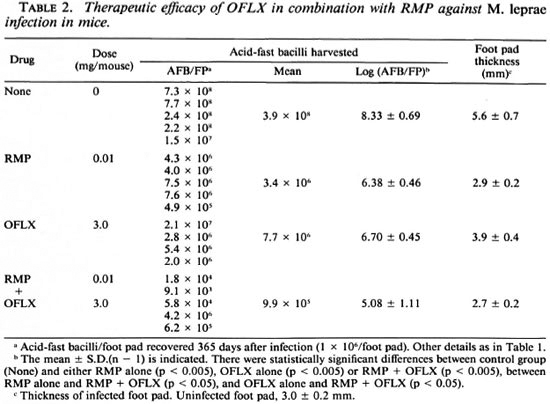
Table 2 shows the antileprosy activity of RMP (0.01 mg/mouse/day) and OFLX (3 mg/mouse/day) alone or in combination. RMP alone at this dose caused a 2.0-log decrease in the number of AFB recovered from the infected foot pad on day 365 postinfection compared to the control mice. The relatively weak reactivity of RMP may be due to its being given in a much smaller dosage in the mice compared to routine clinical dosage in humans. OFLX alone also caused a 1.6-log decrease in the AFB 365 days postinoculation. The combined use of OFLX with RMP gave a 3.4-log decrease in the number of AFB as compared to controls. Thus, there was a combined effect between OFLX and RMP. These findings were corroborated by foot pad swelling in M. leprae -infected animals. Foot pad swelling induced by infection was almost completely inhibited by RMP treatment with or without OFLX. OFLX alone caused a 1.7-mm reduction in foot pad swelling compared to the control values.
In a separate experiment, we evaluated the antileprosy activity of DDS (0.2 mg/ mouse/day) or OFLX (3 mg/mouse/day) alone or in combination. DDS alone, OFLX alone, and DDS + OFLX caused a 0.8-, 0.9-, and 2.0-log decrease, respectively, in the number of organisms recovered from the infected foot pads of host mice at 365 days postinfection compared to control mice (log AFB/FP = 8.48 ± 0.61). In this case, although a significant combined effect was observed, the therapeutic effect of OFLX alone observed in this experiment was not significant. This differs from the results in Tables 1 and 2.
In this study, we evaluated the combined therapeutic effect of OFLX with RMP against M. leprae infection induced in nude mice. The combination of these drugs results in a significant increase in their efficacies, although it is slightly less than what one would expect by a simple additive effect. In this study, the dosage of RMP in the mice was relatively much smaller than dosages used clinically in the treatment of leprosy patients. Therefore, further studies using drugs at the dose equivalent to routine dosages in humans are needed to evaluate the clinical relevance of this finding. Moreover, it seems that the present administration protocol for the drugs in a short period (day 31 to day 80) resulted in relatively weak efficacy of the test drugs. A study of the therapeutic efficacy of these drugs given in different protocols is also needed. In any case, the inclusion of OFLX in multidrug regimens for leprosy using RMP, DDS and clofazimine (11) deserves clinical investigation.
In this study the other quinolines, ENX, NFLX and CPFX, exhibited no in vivo anti- M. leprae action, while FLRX showed antileprosy activity similar to that of OFLX. Recent studies by Tsutsumi and Gidoh (10) and Grosset, el al. (2) have demonstrated the potent efficacy of sparfloxacin and pefloxin, respectively. It would therefore be interesting to elucidate more clearly the combined effects of these new quinolones with RMP. We recently found more potent anti- M. leprae activity in a newly synthesized rifamycin derivative, benzoxazinorifamycin (KRM-1648), which has excellent in vitro and in vivo antimycobacterial activity (7,9), than in RMP (unpublished observation). Studies of the combined efficacies of KRM-1648 and some new quinolones with potent antileprosy activity presently are under way.
Acknowledgment. This study was supported in part by the Sasakawa Memorial Health Foundation and in part by the U.S.-Japan Cooperative Medical Science Program. Wc are grateful to Dr. K. Kohsaka for donating M. leprae -infected foot pads and to Daiichi Pharmaceutical Company for providing rifampin and ofloxacin.
REFERENCES
1. GROSSET, J. H., GUELPA-LAURAS, C. C, PERANI, E. G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
2. GROSSET, J. H., Ji, B., GUELPA-LAURAS, C. C, PERANI, E. G. and N'DELI, L. N. Clinical trial of pcfioxacin and ofloxacin in the treatment of lepromatousleprosy. Int.J. Lepr. 58(1990)281-295.
3. KATOCH, K., RAMU, G., RAMANATHAN, U., SENGUPTA, U., SREEVASTA, SIIARMA, V. D., Sni-VANNAVAR, C. T. and KATOCH, V. M. Results of a modified WHO regimen in highly bacilliferous BL/LL patients. Int. J. Lepr. 57(1989)451-457.
4. KOHSAKA, K. and ITO, T. Effect of ofloxacin and minocycline on experimental leprosy. (Abstract) Int. J. Lepr. 56(1988)678.
5. PATTYN, S. R. Activity of ofloxacin and pelloxacin against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 31(1987)671-672.
6. SAITO, H., TOMIOKA, H. and NAGASHIMA, K. In vitro and in vivo activities of ofloxacin against Mycobacterium leprae infection induced in mice. Int. J. Lepr. 54(1986)560-562.
7. SAITO, H., TOMIOKA, H., SATO, K., EMORI, M., YAMANE, T., YAMASHITA, K. and HIDAKA, T. In vitro antimycobacterial activities of newly synthesized benzoxazinorifamycins. Antimicrob. Agents Chemother. 35(1991)542-547.
8. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112(1960)445-454.
9. TOMIOKA, H., SAITO, H., SATO, K., YAMANE, T., YAMASHITA, K., HOSOE, K., FUJII, K. and HIDAKA, T. Chemothcrapcutic efficacy of a newly synthesized benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex infection induced in mice. Antimicrob. Agents Chemother. 36(1992)387-393.
10. TSUTSUMI, S. and GIDOH, M. Studies on the development of novel antilcprous chemotherapcutics using nude mice with special reference to a new quinolonc carboxylic acid, AT-4140. Jpn. J. Lepr. 58(1989)250-258.
11. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. Ph.D., Associate Professor; Department of Microbiology and Immunology, Shimane Medical University, Izumo 693, Japan.
2. M.D., Ph.D., Professor, Department of Microbiology and Immunology, Shimane Medical University, Izumo 693, Japan.
Received for publication on 12 August 1992.
Accepted for publication in revised form on 5 February 1993.