- Volume 61 , Number 2
- Page: 255–8
In vivo antileprosy activity of the newly synthesized benzoxazinorifamycin, KRM-16481
ABSTRACT
The in vivo anû-Mycobacterium leprae activity of the newly synthesized benzoxazinorifamycin, KRM-1648, was studied. KRM-1648 was given orally to athymic nude mice, infected subcutaneously with M. leprae in the hindfoot pad, at doses between 0.001 and 0.01 mg of the drug/mouse/day six times per week, f rom day 31 to day 80. KRM-1648 administration markedly suppressed bacterial growth in the foot pads for 360 days.KRM-1648 given daily at the dose of 0.01 mg/mouse elicited a 2-4-log decrease in the number of acid-fast bacilli. The therapeutic effects of KRM-1648 were significantly higher than that of rifampin when both drugs were given in the same dosage. Moreover, when mice were fed a KRM 1648-containing diet (0.00004%-0.0004%), the drug displayed an even higher efficacy against M. leprae infection, causing an almost 4-log decrease in the number of leprosy bacilli in the infected foot pad compared to untreated controls.RÉSUMÉ
L'activité anti-Mycobacterium leprae in vivo de la benzoxazinorifamycine, KRM-1648, nouvellement synthétisée, a été étudiée. Le KRM-1648 a été administré oralement à des souris nues athymiques, infectées par voie sous-cutanée dans la patte arrière par du M. leprae, à des doses entre 0,001 et 0,01 mg par souris et par jour six fois par semaine, du jour 31 au jour 80. L'administration de KRM-1648 supprimait de façon marquée la croissance bactérienne dans les coussinets plantaires pour 360 jours. Le KRM-1648 administré quotidiennement à la dose de 0,01 mg par souris entraînait une réduction de 2 à 4 log du nombre de bacilles acido-résistants. Les effets thérapeutiques du KRM1648 étaient significativement plus élevés que ceux de la rifampicine quand les deux médicaments étaient donnés aux mêmes doses. De plus, quand les souris étaient nourries avec un régime contenant du KRM 1648 (0,0000 4%-0,0004º/o), le médicament montrait une efficacité encore plus élevée vis-à-vis de l'infection par M. leprae, causant une diminution de près de 4 log du nombre de bacilles lépreux dans les coussinets plantaires infectés par rapport aux témoins non traités.RESUMEN
Se estudió el efecto in vivo de la droga recién sintetizada benzoxazinorifamicina, KRM-1648, contra el Mycobacterium leprae. La droga se administró oralmente a ratones atímicos infectados subcutáneamente con M. leprae en la almohadilla plantar, a dosis entre 0.001 y 0.01 mg/ratón/día, seis veces por semana, del día 31 al dia 80. La administración de KRM-1648 suprimió marcadamente el crecimiento bacteriano en la almohadilla plantar durante 360 días. La KRM1648 administrada oralmente a la dosis de 0.01 mg/ ratón, condujo a una disminución de 2-4 log en el número de bacilos. Los efectos terapéuticos de KRM1648 fueron significativamente mayores que aquellos de la rifampina cuando ambas drogas se administraron en las mismas dosis. Además, cuando los ratones fueron alimentados con una dicta conteniendo KRM-1648 (0.00004%-0.0004%), el efecto de la droga contra el M. leprae fue todavía mayor, causando una disminución de casi 4 log en el número de bacilos en la almohadilla plantar en relación a los controles no tratados.Previously, we found that the newly synthesized benzoxazinorifamycin, KRM-1648 (Kaneka Corporation, Hyogo, Japan), had excellent in vitro antimycobacterial activity (5) and potent therapeutic efficacy against experimental murine infections with Mycobacterium avium complex (7) and with M. marinum (9). KRM-1648 also was reported to be highly efficacious against M. tuberculosis infection in mice (4). Since KRM1648 has higher antimycobacterial activity than rifampin (4,5,7,9), it could prove to be preferable to replace rifampin, currently a component of the most reliable multidrug therapy [diaminodiphenyl sulfone (DDS) + clofazimine + rifampin] used in the clinical control of leprosy patients, with KRM-1648. Thus, in this study we investigated the therapeutic efficacy of KRM-1648 against M. leprae infection induced in athymic nude mice.
MATERIALS AND METHODS
Mice. Female, BALB/c, athymic nude (nu/nu) mice were purchased from Japan Clea Co., Osaka, Japan. They were bred under sterile conditions using a vinyl isolator.
Organisms. M. leprae Thai-53 were harvested from the infected foot pads of nude mice (donated by Dr. Kohsaka, National Institute for Leprosy Research, Tokyo).
Antimicrobial agents. The drugs tested were: KRM-1648 (Kaneka) and rifampin (Daiichi Pharmaceutical Company, Tokyo, Japan).
Experimental infection. Female, BALB/c nude mice (5 weeks old) were infected subcutaneously with 1 x 106 M. leprae in the left hindfoot pad. KRM-1648 and rifampin were emulsified in 0.1 ml of 2.5% gum arabic-0.2% Tween 80 solution and given to the mice by gavage once daily, six times per week, for 50 days from day 31 to day 80 after infection. All mice were observed for swelling in the left hindfoot pad. On day 360 the mice were killed, and the number of acid-fast bacilli (AFB) in the left hindfoot pad were measured according to a modified method of Shepard (6). Foot pad thickness was measured using dial-gauge calipers (Kagaku Kyoeisha Co., Tokyo, Japan).
RESULTS
Table 1 compares the in vivo antileprosy activity of KRM-1648 with rifampin. The infected mice were given each drug at the dose of 0.1 mg/mousc/day. Log decreases of 3.9 and 1.2 were recorded compared to untreated control animals in the number of organisms recovered from the infected foot pads at day 360 in mice given KRM-1648 and rifampin, respectively. There was a statistically significant difference between the value for KRM-1648 and that for rifampin (p < 0.01). Therefore, when given in the same doses the therapeutic activity of KRM1648 against leprosy is higher than rifampin.
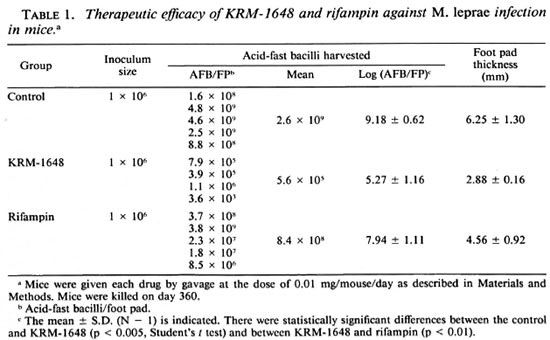
Table 2 shows the dose-dependent therapeutic effect of KRM-1648. Even at the lowest dose, 0.001 mg/mouse/day, KRM1648 significantly decreased (ca 0.6-log) the number of AFB recovered from the infected foot pad on day 360 (p < 0.05). The other doses, 0.005 and 0.01 mg/mouse/day, caused a 1.0- and a 2.2-log decrease, respectively. Thus, KRM-1648 exhibited a dose-dependent antileprosy action. It also markedly inhibited foot pad swelling due to the infection in a dose-dependent manner.
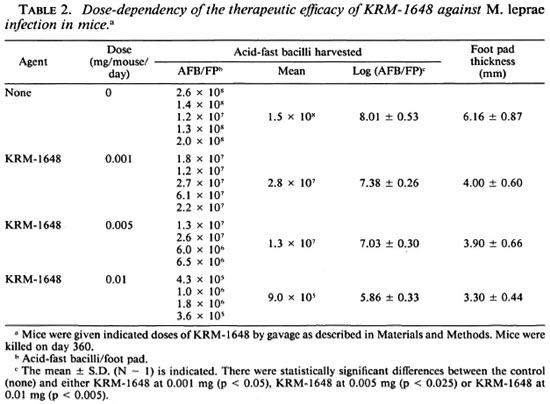
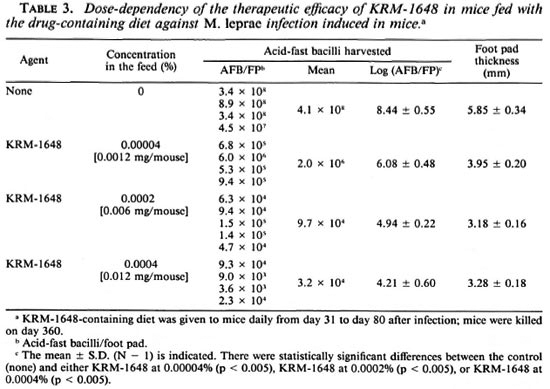
Table 3 indicates the therapeutic efficacy of KRM-1648 in mice treated with a drugcontaining diet prepared by Japan Clea. When mice were fed a diet containing 0.00004%-0.0004% of KRM-1648 (equivalent to doses of 0.0012 mg-0.012 mg/ mouse/day) from day 31 to day 80, 2.4- to 4.2-log decreases were seen in the number of AFB in the infected foot pad on day 360. Thus, the therapeutic efficacy of KRM-1648 may be increased by feeding mice the drugcontaining diet (as compared to the gavage schedule of once daily, six times per week). It is difficult to make a definite conclusion on this point because there were differences in conditions between the two experiments in Tables 2 and 3.
DISCUSSION
A newly developed benzoxazinorifamycin, KRM-1648, exhibited excellent in vivo antileprosy activity. The therapeutic efficacy of KRM-1648 was significantly higher on an equal weight basis compared to that of rifampin. The higher activity of KRM1648 seems mainly to be due to a higher inherent antimicrobial activity against M. leprae, since its pharmacokinetics are similar to rifampin in that they both attained similar blood levels (7). Furthermore, it should be emphasized that the toxicity of KRM-1648 is similar to or lower than that of rifampin (7). Therefore, it may be advantageous to use KRM-1648 instead or rifampin in the multidrug therapy of leprosy along with dapsone and clofazimine and other agents having potent antileprosy activity, including clarithromycin (1,2,8) . However, more research related to the pharmacokinetics and toxicity of KRM-1648 is needed before large-scale clinical application can be recommended.
When mice were fed with a KRM-1468containing diet, the drug exhibited a higher efficacy against leprosy than when given once daily by gavage. Although the precise reason is unknown, this mode of administration might considerably improve the pharmokinetics (absorption, tissue distribution and retention) of KRM-1648. Since the results of Tables 2 and 3 were derived from different experiments, it is difficult to arrive at a conclusion on this point. More detailed studies are needed with respect to administration protocols of this drug, which may be superior to rifampin in leprosy multidrug therapy if the toxicity and cost are comparable to rifampin.
Acknowledgment. This study received financial support from the Sasakawa Memorial Health Foundation and the U.S.-Japan Cooperative Medical Science Program for which are are deeply grateful. We thank Dr. Hidaka, Keneka Corporation, for reading this manuscript and Dr. Kohsaka for donating the M. /cyjrae-infected foot pads. We also thank the Kaneka Corporation and Daiichi Pharmaceutical Company for providing KRM-1648 and rifampin, respectively.
REFERENCES
1. FERNANDES, P. B., HARDY, D. J., MCDANIEL, D., HANSON, C. W. and SWANSON, R. N. vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob. Agents Chemother. 33(1989)1531-1534.
2. HARDY, D. J., GUAY, D. R. P. and JONES, R. N. Clarithromycin, a unique macrolidc; a pharmacokinetics, microbiological, and clinical overview. Diagn. Microbiol. Infect. Dis. 15(1992)39.
3. KATOCH, K., RAMU, G., RAMANATHAN, U., SEN-GUPTA, U., SREEVASTA, SHARMA, V. D., SHIVANNAV-AR, C. T. and KATOCH, V. M. Results of a modified WHO regimen in highly bacillifcrous BL/LL patients. Int. J. Lepr. 57(1989)451-457.
4. KUZE, F., YAMAMOTO, T., AMITANI, R. and SUZUKI, K. //; vivo activities of new rifamycin derivatives against mycobacteria. Kekkaku 66(1990)7-12.
5. SAITO, H., TOMIOKA, H., SATO, K., EMORI, M., YA-MANE, T., YAMASHITA, K. and HIDAKA, T. In vitro antimycobactcrial activities of newly synthesized benzoxazinorifamycins. Antimicrob. Agents Chemother. 35(1991)542-547.
6. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112(1960)445-454.
7. TOMIOKA, H., SAITO, H., SATO, K., YAMANE, T., YAMASHITA, K., HOSOE, K., FUJII, K. and HIDAKA, T. Chemotherapeutic efficacy of a newly synthesized benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex infection induced in mice. Antimicrob. Agents Chemother. 36(1992)387-393.
8. TOMIOKA, H., SATO, K. and SAITO, H. Antimycobactcrial activity of clarithromycin. Kekkaku 67(1992)289-291.
9. HAMAMOTO, Y., SAITO, H., TOMIOKA, H., SATO, K., YAMANE, T., YAMASHITA, K., HOSOE, K. and HIDAKA, T. In vitro and in vivo activities of KRM1648, a newly synthesized benzoxazinorifamycin, against Mycobacterium marinum. Zentralbl. Bakteriol. 554(1992)625-631.
1. Ph.D., Associate Professor; Department of Microbiology and Immunology, Shimane Medical University, Izumo 693, Japan.
2. M.D., Ph.D., Professor, Department of Microbiology and Immunology, Shimane Medical University, Izumo 693, Japan.
Received for publication on 27 July 1992.
Accepted for publication in revised form on 2 December 1992.