- Volume 61 , Number 3
- Page: 398–405
Reconstitution of Mycobacterium leprae immunity in severe combined immunodeficient mice using a t-cell line
ABSTRACT
To test whether Mycobacterium lepracimmune T cells can confer protection against infection with leprosy bacilli, severe combined immunodeficient (SCID) mice were reconstituted with a BALB/c-derived, M. leprae-responsive, T-cell line. Flow cytometric analysis of spleen and peripheral blood cells confirmed reconstitution with T cells. In vitro lymphokine production and the proliferation of spleen cells f rom the reconstituted animals established that the donor cells had maintained their functional activity for the duration of the study (275 days). The transfer of immune T cells 24 hr before foot pad infection with leprosy bacilli resulted in a profound reduction in M. leprae multiplication, as compared to the nonreconstituted SCID mice. The yield of acidfast bacilli in the foot pads of SCID mice reconstituted with the M. leprae-immune T cells also was significantly lower than that found in naive BALB/c mice, and at levels previously found only in BALB/c mice that had been immunized effectively. These experiments demonstrate that M. leprae-immune T cells home effectively and control M. leprae infection in SCID mice.RÉSUMÉ
Dans le but de tester si des cellules T immunisées vis-à-vis de Mycobacterium leprae peuvent conférer une protection contre une infection par des bacilles de la lèpre, on a administré à des souris sévèrement immunodéficientes une lignée de cellules T dérivées de BALB/c et réagissant à M. leprae. Une analyse cytométrique de flux au niveau de la rate et des cellules du sang périphérique a confirmé la reconstitution des cellules T. La production de lymphokine in vitro et la prolifération des cellules spléniques chez les animaux traités ont confirmé que les cellules administrées avaient maintenu leur activité fonctionnelle pour la durée de l'étude (275 jours). Le transfert de cellules T immunes 24 h avant l'infection du coussinet plantaire par des bacilles lépreux a résulté en une diminution importante de la multiplication de M. leprae, par comparaison avec les souris non traitées. La charge en bacilles acidorésistants des coussinets plantaires des souris traitées par les cellules T immunisées vis-à-vis de M. leprae était également significativement plus faible que celle trouvée chez les souris BALB/c de souche, et seulement comparable à celle trouvée chez des souris "BALB/c effectivement immunisées. Ces expériences démontrent que les cellules T immunisées vis-à-vis de M. leprae abritent effectivement et contrôlent l'infection par M. leprae chez les souris sévèrement immunodéficientes.RESUMEN
Para investigar si las células T inmunes al Mycobacterium leprae pueden conferir protección contra la infección por el bacilo de la lepra, se reconstituyeron ratones con inmunodeficiencia combinada severa (SCID) con células T respondedoras al M. leprae derivadas de ratones BALB/c. Los análisis por citometría de flujo de las células de bazo y de sangre periférica, confirmaron el éxito de la reconstitución. La producción in vitro de linfocinas y la proliferación de las células de bazo de los animales reconstituidos indicaron que las células del donador mantuvieron su actividad funcional durante todo el tiempo del estudio (275 días). En comparación con los ratones SCID no reconstituidos, la transferencia de las células inmunes 24 horas antes de la infección de las almohadillas plantares con los bacilos de la lepra, ocasionó una profunda reducción en la multiplicación del M. leprae. La recuperación de bacilos ácido-ratones SCID reconstituidos con las células T inmunes al M. leprae también fue significativamente menor que la recuperación de bacilos a partir de los ratones BALB/c intactos, y los niveles de recuperación fueron solo similares a los encontrados en ratones BALB/c inmunizados contra el bacilo de la lepra. Estos experimentos demuestran que las células T inmunes al M. leprae se establecen de manera efectiva y controlan la infección por cl M. leprae en los ratones SCID.Unlike human lepromatous leprosy, the murine infection with Mycobacterium leprae is localized and self limiting. It is possible, however, to protect mice against foot pad infection with leprosy bacilli by vaccination with killed armadillo-derived M. leprae (23) or its subunits (8,9). In the murine model of leprosy, the restriction or prevention of mycobacterial growth in mouse foot pads is the manifestation of protective immunity.
By using adoptive transfer of cells, several laboratories have assessed the role of immune responses in promoting protection against leprosy bacilli. These studies have indicated that murine resistance to infection with this mycobacterium depends on T-cellmediated immune responses (10.14). However, interpretation of data derived from studies in which cells were transferred to nonirradiated (10) or sublethally irradiated recipients (10.14) may be misleading due to the difficulty in discriminating host from donor lymphocytes.
We used severe combined immunodeficient (SCID) mice to examine the ability of adoptively transferred BALB/c-derived M. leprae-responsive T cells to confer protection against infection with leprosy bacilli. SCID mice have an autosomal recessive mutation that prevents the assembly of functional antigen receptor V region genes(21). As a result, these mice lack immunocompetent B and T cells in their peripheral lymphoid organs (3). The H-2d SCID mice are congenic to the immunocompetent C.B17 mouse strain which is allotype coisogenic to BALB/c mice (1). This study shows that adoptively transferred T cells engraft effectively, maintain their functional activity, and control M. leprae infection.
MATERIALS AND METHODS
Mice. Female C.B-17 SCID mice were bred and housed in the University of California San Francisco (UCSF) pathogen-free animal care facility. Female, 6 to 8-weekold BALB/c mice were purchased from Jackson Laboratory, Bar Harbor, Maine, U.S.A.
Leprosy bacilli. A well-characterized strain of M. leprae, initially isolated from a lepromatous patient and maintained in the laboratory by passage through mouse foot pads, was used to infect the mice. After 22 years of passage in mouse foot pads, this isolate synthesizes the M. leprae-specific phenolic glycolipid (5). Armadillo-derived, irradiation-killed, whole M. leprae were provided by Dr. P. J. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A., through contract AI-05074.
Generation of M.leprae-responsive T cells. BALB/c mice were injected intradermally in the right flank with 100 µl of 107 whole irradiation-killed M. leprae emulsified in Freund's incomplete adjuvant as described by Shepard, et al. (23). Eight days later the spleens were removed and the spleen cells were freed from the red blood cells by a brief incubation in 0.84% ammonium chloride. T cells were enriched by the passage of dispersed splenic cells through nylon wool columns after a 1 -hr incubation at 37ºC. To activate T cells in vitro, 1 × 106 M. leprae-primed, nylon-wool-nonadherent cells and 1 × 106 irradiated (2500 rad from a cesium source) unseparated spleen cells were cultured in 16-mm wells (Costar, Cambridge, Massachusetts, U.S.A.) with 5 Mg/ml sonicated M. leprae in a final volume of 1 ml of Iscove's modified Dulbecco's medium (GIBCO Laboratories, Grand Island, New York, U.S.A.) supplemented with 5% heat-inactivated fetal calf serum (FCS), 5 × 10-5 M 2-mercaptoethanol, 2 mM L-glutamine, 100 U of penicillin/ml, and 100 µg of streptomycin/ml-hereafter referred to as complete medium (CM). Cultures were maintained for 7 days at 37ºC in a humidified 10% C02 atmosphere. After 7 days, lymphoblasts were isolated by Ficoll-Hypaque gradient centrifugation. These cells were propagated in CM supplemented with 20 U recombinant IL-2/ml (a gift from Cetus Corporation, Emeryville, California, U.S.A.) in the presence of an equal number of M. leprae-pulsed antigen presenting cells (APC). APC were derived from M. leprae nonimmune BALB/c mice. Dispersed spleen cells from naive mice were incubated with 5 µg/rnl sonicated leprosy bacilli for 16 hr at 37ºC before irradiation (2500 rad) and used as APC/feeder cells. Seven days after the last restimulation, lymphoblasts (referred to as immune T cells) were again isolated from the feeder cells by using the procedures described above. These cells were then used for flow cytometric and functional analysis, as well as reconstitution of the SCID mice.
Adoptive cell transfer. SCID mice, six per group, were reconstituted with either immune T cells or freshly isolated splenic T lymphocytes (nonimmune T cells) by intraperitoneal injection. Immune T cells were isolated from the irradiated APC by Ficoll-Hypaque gradient centrifugation at the end of the growth cycle and prior to transfer. The nonimmune T cells were enriched by passage of'dispersed spleen cells derived from naive BALB/c mice through nylon wool columns. Ten million viable cells, determined by trypan blue exclusion, were resuspended in 500 \x\ phosphate buffered saline (PBS) for injection into each mouse. Twenty-four hr later reconstituted recipients, as well as nonreconstituted controls, were infected with leprosy bacilli as described.
M. leprae infection. SCID, as well as BALB/c mice, were infected in both hindfoot pads with 5000 live M. leprae derived from the foot pads of mice near the peak of logarithmic multiplication. Protection was evaluated at the peak of mycobacterial multiplication, when the numbers of acid-fast bacilli (AFB) from each of three naive BALB/c mice infected with the same M. leprae inoculum reached 5 × 105 per foot pad.
Assessment of protective immunity. At the peak of mycobacterial multiplication in the nonimmune BALB/c mice (7 to 9 months postinfection), blood was collected from the tail vein of each animal and peripheral blood mononuclear cells were freed from red blood cells by a brief incubation with 0.84% ammonium chloride for flow cytometric analysis. The mice were then sacrificed and their spleens and foot pads were harvested. The spleen cells were dispersed into single cell suspensions and used for a) flow cytometric analysis, b) generation of lymphokine, and c) measurement of proliferative activity. The number of AFB in both hindfoot pads were enumerated by a standard microscopic technique (22). The differences between groups were determined by comparing the geometric mean of the number of AFB in the right and left hindfoot pads by Fisher's exact median test (12).
Immunofluorescence analysis. Phycoerythrin (PE)-conjugated anti-CD4 (L3T4) monoclonal antibodies (mAb), FITC-conjugated anti-CD8 (Ly-2) mAb (Becton Dickinson, Mountain View, California, U.S.A.), and FITC-conjugated anti-CD3 mAb (PharMingen, San Diego, California, U.S.A.) were used for surface marker analysis. FITC- or PE-conjugated rat IgG2a (PharMingen) were used as negative controls. The spleen cells as well as the peripheral blood mononuclear cells were suspended in PBS containing 2% FCS and 0.01% sodium azide (staining buffer) and were incubated at 4ºC for 45 min with the FITCand/or PE-conjugated mAb. After extensive washing, the cells were fixed with 1.0% paraformaldehyde in the staining buffer and examined on a FACScan analytical flow cytometer (Bccton-Dickinson) (13). Only cells of lymphocyte size and complexity were analyzed based on forward and 90º scatter gates. Photomultiplier tube voltage and color compensation between the FITC and PE channels were determined using Becton-Dickinson CaliBRITE particles. Percent specific staining is defined as the percent staining obtained in the positive sample minus the percent staining in the negative control.
Generation of lymphokine-containing supernatants. One ml of spleen cells suspended in CM (1 × 106 cells/ml) was dispensed into 16-mm diameter wells (Costar). After 48 hr of culture in either the absence or the presence of 2.5 µg/ml Concanavalin A (ConA; Sigma Chemical Co., St. Louis, Missouri, U.S.A.), the supernatant from each culture was separated from the cells by centrifugation and stored at - 70ºC until assayed.
Proliferation assay. Freshly isolated spleen cells (5 × lO4 well) were stimulated with a previously determined optimal dose of ConA or M. leprae. All proliferation assays were performed in round-bottom microliter wells in a final volume of 200 µl of CM. The cultures were maintained for 4 to 5 days at 37ºC in a 10% C02-humidified atmosphere. [3H]Thymidine (1 µCi) was added to each well 16 hr before the cells were harvested. Immune T cells (10 × 104/ well) were cultured with 25 × 104 of irradiated (2500 rad), freshly isolated spleen cells in 200 µl of IL-2-free CM per well in the presence or absence of stimuli as described above. Following 3 days of culture, these cultures were pulsed with [3H]thymidine for 6 hr. The results of these assays are expressed as a) mean counts per minute (cpm) of quadruplicate cultures; b) Acpm, which represents cpm in cells cultured with the stimulus minus cpm in cells cultured without the stimulus; or c) stimulation index (SI) determined by the following equation: SI = cpm in cells cultured with the stimulus/cpm in cells cultured without the stimulus.
Lymphokine assay. The levels of interferon-gamma (IFN-γ) in the spleen cell culture supernatant were measured by ELISA (Genzyme, Cambridge, Massachusetts, U.S.A.) according to the manufacturer's protocol. The sensitivity of this assay is 125 pg IFN- γ/ml according to the manufacturer.
RESULTS AND DISCUSSION
A T-cell line was established from pooled splenic T lymphocytes of BALB/c mice immunized with whole irradiation-killed leprosy bacilli. These cells, the "immune T cells," underwent DNA synthesis (56,887 ± 6531 cpm/105 cells, SI = 35) and released IFN-γ (53 ng/106 cells) in response to sonicated M. leprae.
The spleen and blood cells from the recipient SO D mice were analyzed for the expression of surface CD4 and CD8 molecules by flow cytometry to assure that both immune T cells as well as the BALB/c-derived nonimmune T cells had engrafted successfully. Although the cellularity of spleens from SCID mice reconstituted with either the immune T cells or the nonimmune T cells was lower than that of the immunocompetent BALB/c mice, the data shown in Figure 1 demonstrate that the adoptively transferred T cells have populated the recipients. An average of 12 × 106 and 5 × 106 cells were obtained from spleens of SCID mice reconstituted with the immune or nonimmune T cells, respectively. Spleens from the BALB/c mice contained about 108 cells. In individual SCID mice, a variable fraction of splenic lymphocytes expressed the CD4 orCD8 surface molecules. In five SCID mice engrafted with the immune T cells, CD4 + T cells and CD8+ T cells comprised 16.7 ± 11.6% and 5 ± 3.5% of the spleen cells, respectively. The frequency of cells expressing these surface markers in the blood of these animals was comparable to that found in the spleens (Fig. 1). A higher percentage of lymphocytes in the spleen and blood of SCID mice reconstituted with nonimmune T cells displayed CD4 and CD8 surface molecules. Although SCID mice tend to become "leaky" with age (i.e., express CD4 + and CD8+ α/β TCR+ T cells) (2), only 4.9 ± 0.9% and 1.5 ± 1.1% of splenic lymphocytes were, respectively, CD4+ and CD8 + T cells in nonreconstituted control SCID mice. The distribution of CD4 + and CD8 + T cells in the M. leprae-infected BALB/c mice was comparable to that of the agematched normal BALB/c mice and was, as expected, significantly higher than that observed in the reconstituted SCID mice (Fig. 1).
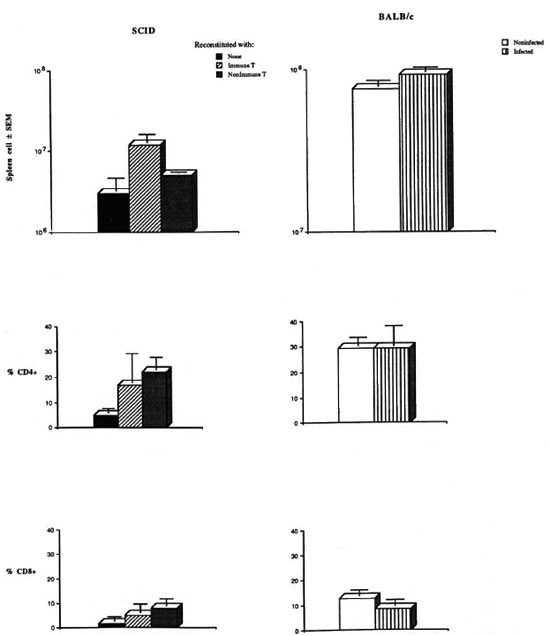
Fig. 1. CD4+ and CD8+ lymphocytes in the spleens of reconstituted SCID mice.
To determine if the donor cells had maintained their functional activity, the ability of the spleen cells from each mouse to produce IFN-γ and proliferate upon in vitro stimulation was assessed at the termination of the study. Spleen cells taken from nonreconstituted SCID mice failed to undergo proliferation when stimulated with ConA (Δcpm = 84) (Fig. 2). These data complement the findings generated by flow cytometry and rule out the possibility that the nonreconstituted SCID mice became "leaky" during the course of these studies. In contrast, spleen cells taken from animals reconstituted with the immune, as well as the nonimmune, T cells produced readily detectable levels of IFN- γ (Fig. 2).Splenic lymphocytes from animals reconstituted with nonimmune T cells demonstrated a marked increase in the uptake of 3H]thymidine when stimulated with ConA (Δcpm = 16,140, SI = 9.6) (Fig. 2). Spleen cells from mice reconstituted with the immune T cells displayed a high level of DNA synthesis even in the absence of ConA, reflecting a state of activation (Fig. 2). As expected, spleen cells taken from the M. leprae-infected, control BALB/c mice responded to ConA by lymphokine production and DNA synthesis (Fig. 2).
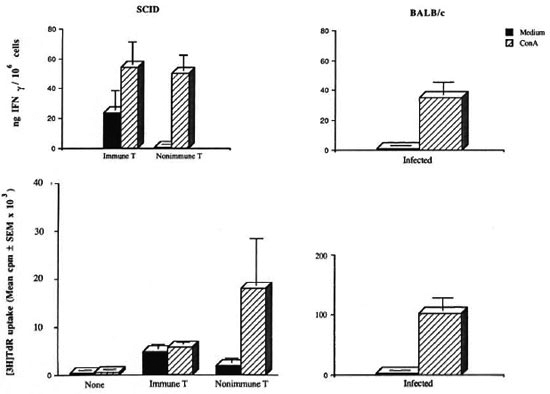
Fig. 2. ConA stimulations of splenic lymphocytes in vitro from reconstituted SCID mice.
Depending on the cell availability, the response of spleen cells from animals in each group to sonicated leprosy bacilli was also assessed. The profile of response provoked by M. leprae was similar to that induced by ConA. Uptake of [3H]thymidine (mean cpm ± S.E.M. from three animals per group) by spleen cells taken from mice reconstituted with nonimmune T cells when stimulated with sonicated M. leprae was 25,161 ± 990 cpm compared to 1109 ± 528 cpm in control cultures kept in medium alone (SI = 2.3). Spleen cells from animals reconstituted with the immune T cells showed comparable levels of DNA synthesis in the presence or absence of M. leprae (5490 ± 1509 and 5326 ± 960 cpm, respectively).
Only SCID mice reconstituted with the immune T cells 24 hr before infection with 5000 bacilli exhibited significant protection against infection with leprosy bacilli. The number of AFB per foot pad in these animals ranged from 5.49 × 104to 8.33 × 104 (mean number of 6.9 ± 1.6 × 104 per foot pad), as compared to an average of 18.92 ± 11.0 × 105 AFB per foot pad in nonreconstituted SCID mice (p < 0.03) (Fig. 3). The yield of M. leprae in the foot pads of SCID mice populated with immune T cells was also significantly lower than that found in naive BALB/c mice (mean number of 1.78 ± 0.76 × 105 AFB per foot pad, p < 0.05), and at levels previously found only in BALB/c mice that had been effectively immunized against M. leprae (8). In agreement with this observation, SCID mice populated with the immune T cells restricted the growth of M. leprae more effectively than those reconstituted with nonimmune T cells (6.9 ± 1.6 × 104 versus 2.77 ± 0.88 x 105 AFB per foot pad, p < 0.03) (Fig. 3). Although the number of M. leprae in the foot pads of SCID mice populated with nonimmune T cells was less than that found in nonreconstituted SCID mice (Fig. 3), the difference was not statistically significant.
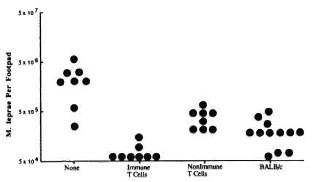
Fig. 3. Growth of M. leprae in the foot pads of reconstituted SCID mice.
These findings confirm earlier observations implicating the central role of T cells in providing protection in the murine model of leprosy (10,14). We used SCID mice because data generated in this leprosy model are more convincing than those derived from sublethally irradiated recipients (14). Considering the long duration of this infection (6 to 9 months), the possibility that endogenous cells may restore the host's immunity in the irradiated recipients cannot be ruled out. We elected to use an unseparated T-cell line in these initial studies because CD8 + T cells appear to play a pivotal role in the control of mycobacterial infections (4,6,11,20)-and because highly purified CD8+ T cells do not engraft successfully into SCID mice (19). These studies in SCID mice demonstrate that after the initial selection in vitro, immune T cells homed effectively (Fig. 1) and were able to exert their anti-M. leprae activity (Fig. 3). The ability of CD4 + T cells to confer protection against leprosy bacilli in the absence of CD8+ remains to be studied. A role for CD4+ (L3T4 + ), but not CD8+ (Lyt-2+), T cells in the protective immune responses against M. bovis has been suggested by adoptive transfer studies in thymectomized mice treated with either anti-L3T4 or anti-Lyt-2 monoclonal antibody (6). The ability of immune T cells to control the growth of leprosy bacilli in the recipient SCID mice may be caused, in part, by the activation of infected macrophages by elaboration of IFN-7. Such an antimycobacterial function has been ascribed to INF-γ (15,17,18)Additional studies on the effect of treating reconstituted SCID mice with anti-IFN-γ neutralizing antibody should provide support for this possibility. Alternatively, immune T cells were able to reduce the M. leprae load by direct interaction with the infected macrophages (4). Consistent with this possibility is our unpublished observation that these immune T cells were able to lyse BALB/c-derived peritoneal macrophages pulsed with killed M. leprae, as revealed by the release of 5lCr]sodium chromate (data not shown).
This study demonstrates that transferred immune T cells populated SCID mice and maintained their functional activities for the duration of this study (275 days). This study further suggests that these cells are able to migrate to the sites of infection and upon reactivation in vivo to reduce the mycobacterial burden of macrophages.
Acknowledgment. The authors thank Patricia Siu and Mabel Tsang for their excellent technical assistance and Dr. Luiz E. Bermudez, Kuzell Institute for Arthritis and Infectious Diseases, for performing the cytotoxicity assays. This work was supported in part by grants AI22653 and AI26918 from the National Institute of Allergy and Infectious Diseases, a contract with the GWL Hansen's Disease Center, and the Burroughs Wellcome Fund.
REFERENCES
1. ANSELL, J. D. and BANCROFT, J. The biology of the scid mutation. Immunol. Today 10(1989)322-325.
2. BOSMA, G. C, FRIED, M., CUSTER, R. P., CARROLL, A. M., GIBSON, D. M. and BOSMA, J. J. Evidence of functional lymphocytes in some (leaky) scid mice. J. Exp. Med. 167(1988)1016-1033.
3. BOSMA, J. M. and C ARROLL, A.M. The scid mouse mutant; definition, characterization, and potential uses. Ann. Rev. Immunol. 9(1991)323-350.
4. CHIFLUNKAR, S., DE LIBERO, G. and K AUFMANN, S. H. E. Mycobacterium leprae-specific Lyt-2+ T lymphocytes with cytolytic activity. Infect. Immun. 54(1986)793-797.
5. CHO, S.-N., KIM, J. D., GELBER, R. H. and BRENNAN, P. J. Comparison of PGL-L level with AFB numbers in foot pad suspension. (Letter) Int. J. Lepr. 60(1992)96-98.
6. DE LIBERO, G., FLESCHL. I. and KAUFMANN, S. H. E. Mycobacleria-reactive Lyt+ T cell lines. Eur. J. Immunol. 18(1988)59-66.
7. FLYNN, J. L., GOLDSTEIN, M. M., TRIEBOLD, K. J., KOLLER, B. and BLOOM, B. R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U.S.A. 89(1992)12013-12017.
8. GELBER, R. H., BRENNAN, P. J., HUNTER, S. W., MUNN, M. W., MONSON, J. M., MURRAY, L. P., Siu, P., TSANG, M., ENGELMAN, E. G. and MOHAGHEGHPOUR, N. Effective vaccination of mice against leprosy bacilli with subunits of Mycobacterium leprae. Infect. Immun. 58(1990)711-718.
9. GELBER, R. H., MURRAY, L., SIU, P. and TSANG, M. Vaccination of mice with a soluble protein fraction of Mycobacterium leprae provides consistent and long-term protection against M. leprae infection. Infect. Immun. 60(1992)1840-1844.
10. GRAHAM, L., JR. and NAVALKAR, R. G. Evaluation of Mycobacterium leprae immunogenicity via adoptive transfer studies. Infect. Immun. 43(1984)79-83.
11. KALEAB, B., O TTENHOFF, T., CONVERSE, P., HALA-PI, E., TADESSE, G., ROTTENBERG, M. and KIESSLING, R. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur. J. Immunol. 20(1990)2651-2659.
12. LEHMANN, E. L. Nonparamelric Statistical Methods Based on Ranks. San Francisco: Holden-Day, Inc.. 1975, pp. 5-13.
13. LIFSON, J. a, SASAKI, D. T. and ENGLEMAN, E. G.Utility of formaldehyde fixation for flow cytometry and inactivation of AIDS associated retroviruses. J. Immun. Meth. 86(1986)143-149.
14. LOWE, C., BRETT, S. J. and REES, R. J. W. Adoptive cell transfer of resistance to Mycobacterium leprae infections in mice. Clin. Exp. Immunol. 61(1985)336-342.
15. NATHAN, C. F., KAPLAN, G., LEVIS, W. R., NUSRAT,A., WITMER, M. D., SHERWIN, S. A., JOB, C. K.,HOROWITZ, C. R., STEINMAN, R. M. and COHN, Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepro-matous leprosy. N. Engl. J. Med. 315(1986)6-15.
16. PEDRAllINI, T. and Louis, J. A. Functional analysis in vitro and in vivo of Mycobacterium bovis strain BCG-specific T cell clones. J. Immunol. 136(1986)1828-1834.
17. PEDRAllINI, T., HuG, K. and Louis, J. A. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette-Guerin in mice; assessment by elimination of T cell subsets in vivo. J. Immunol. 139(1987) 2032-2037.
18. ROOK, G. A. W., CHAMPION, B. R., STEELE, J., VAREY, A. M. and STANFORD, J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin. Exp. Immunol. 59(1985)414-420.
19. RUDOLPHI, A., SPIESS, S., CONRADT, P., CLAESSON, M. H. and REIMANN, J. CD3+ T cells in severe combined immune deficiency (scid) mice. I. Transferred purified CD4+ T cells, but not CD8+T cells are engrafted in the spleen of congenic scid mice. Eur. J. Immunol. 21(1991)523-533.
20. SASIAIN, NI. D., DELA BARRERA, S., MINNUCCI, F., VALDEZ, R., DE BRACCO, M. M. D. and BALINA, L. NI. T-cell-mediated cytotoxicity against mycobacterium antigen-pulsed autologous macrophages in leprosy patients. Infect. Immun. 60(1992)3389-3395.
21. SCHULER, W., WELLER, I. J., SCHULER, A., PHILLIPS, R. A., ROSENBERG, N., MAK, T. W., KEARNEY, J. F., PERRY, R. P. and BOSMAN, M. J. Rearrangement of antigen receptor genes is defective in micewith severe combined immune deficiency. Cell 46(1986) 963-972.
22. SIIEPARD, C. C. and McRAE, D. H. A method forcounting acid-fast bacteria. Int. J. Lepr. 36(1968)78-82.
23. SHEPARD, C. C., VAN LANDINGHAM, R. M. and WALKER, L. L. Immunity to Mycobacterium leprae infection in mice stimulated by M. leprae, BCG,and graft-versus-host reactions. Infect. Immun. 14(1976) 919-928.
1. D.V.M.; Kuzell Institute for Arthritis and Infectious Diseases, 2200 Webster Street, San Francisco, California 94115-1896,U.S.A.
2. M.D.; Kuzell Institute for Arthritis and Infectious Diseases, 2200 Webster Street, San Francisco, California 94115-1896,U.S.A.
3. M.S.; Kuzell Institute for Arthritis and Infectious Diseases, 2200 Webster Street, San Francisco, California 94115-1896,U.S.A.
4. Kuzell Institute for Arthritis and Infectious Diseases, 2200 Webster Street, San Francisco, California 94115-1896,U.S.A.
5. Ph.D., Kuzell Institute for Arthritis and Infectious Diseases, 2200 Webster Street, San Francisco, California 94115-1896,U.S.A.
6. M.D., GWL Hansen's Disease Center, Carville, Louisiana 70721, U.S.A. D. T. Sasaki, M.A., SYSTEMIX Inc., Palo Alto, California 94304, U.S.A.
7. M.D., Department of Medicine and Microbiology/Immunology, University of California San Francisco, San Francisco, California 94143, U.S.A.
Received for publication on 27 January 1993.
Accepted for publication in revised form on 12 May 1993.