- Volume 61 , Number 2
- Page: 259–69
Effect of recombinant interferon gamma administration on lesional monocytes/macrophages in lepromatous leprosy patients
ABSTRACT
Hydrogen peroxide (H2O2) and superoxide anion (O ) were estimated in lesional cells f rom 10 lepromatous leprosy patients injected intralesionally with recombinant interferon-gamma (rIFN-γ). Clinically similar contralateral lesions injected with excipient served as controls. Lesional esterase-positive cells (suggestive of monocytes/ macrophages) f rom rIFN-γ-injected sites of many subjects showed net increments in the H2O2 and O
) were estimated in lesional cells f rom 10 lepromatous leprosy patients injected intralesionally with recombinant interferon-gamma (rIFN-γ). Clinically similar contralateral lesions injected with excipient served as controls. Lesional esterase-positive cells (suggestive of monocytes/ macrophages) f rom rIFN-γ-injected sites of many subjects showed net increments in the H2O2 and O levels compared to controls. When these cells were exposed to Mycobacterium leprae in vitro, there was a downregulation of O
levels compared to controls. When these cells were exposed to Mycobacterium leprae in vitro, there was a downregulation of O in 4 of 5 subjects. Such inhibition was not observed in rIFN-γ-injected sites. From the present studies it was not possible to determine whether the above effects of rIFN-γ were primarily on lesional mature macrophages or on newly migrated young monocytes. Erythema and induration were observed at the cytokine-injected site but not at the control site between 24 and 72 hr. A monthly slit-skin smear examination of the former site showed a bacterial index (BI) reduction compared to the controls in 4 of 10 patients, this reduction occurring as early as 4 to 8 weeks. Histopathology of the biopsies of 6 of 10 subjects between 9 and 10 months showed a further BI decrease attributable to rIFN-γ and not to the subsequently instituted chemotherapy.
in 4 of 5 subjects. Such inhibition was not observed in rIFN-γ-injected sites. From the present studies it was not possible to determine whether the above effects of rIFN-γ were primarily on lesional mature macrophages or on newly migrated young monocytes. Erythema and induration were observed at the cytokine-injected site but not at the control site between 24 and 72 hr. A monthly slit-skin smear examination of the former site showed a bacterial index (BI) reduction compared to the controls in 4 of 10 patients, this reduction occurring as early as 4 to 8 weeks. Histopathology of the biopsies of 6 of 10 subjects between 9 and 10 months showed a further BI decrease attributable to rIFN-γ and not to the subsequently instituted chemotherapy. RÉSUMÉ
Le peroxyde d'hydrogène (H2O2) et l'anion superoxyde (O ) ont été dosés dans des cellules provenant des lésions de 10 patients lépromateux chez qui on avait injecté en intralésionnel de l'interféron-gamma recombinant (IFN-γr). Des lésion cliniquement similaires situées sur l'autre moitié du corps et à l'intérieur desquelles on avait injecté un excipient ont servi de témoins. Les cellules esterasc-positives (suggestives de monocytes/macrophages) en provenance des sites où on avait injecté de l'IFN-γr ont montré chez beaucoup de patients de nettes augmentations des taux d'H2O2 et d'O
) ont été dosés dans des cellules provenant des lésions de 10 patients lépromateux chez qui on avait injecté en intralésionnel de l'interféron-gamma recombinant (IFN-γr). Des lésion cliniquement similaires situées sur l'autre moitié du corps et à l'intérieur desquelles on avait injecté un excipient ont servi de témoins. Les cellules esterasc-positives (suggestives de monocytes/macrophages) en provenance des sites où on avait injecté de l'IFN-γr ont montré chez beaucoup de patients de nettes augmentations des taux d'H2O2 et d'O par rapport aux témoins. Quand ces cellules ont été exposées au Mycobacterium leprae in vitro, il y eut une diminution de l'O
par rapport aux témoins. Quand ces cellules ont été exposées au Mycobacterium leprae in vitro, il y eut une diminution de l'O chez 4 des 5 patients. Une telle inhibition n'a pas été observée au niveau des sites où on avait injecté de l'IFN-γr. A partir de ces analyses, il ne fut pas possible dse déterminer si les effets de l'IFN-γr ci-dessus se rapportaient premièrement à des macrophages mûrs ou à des jeunes monocytes nouvellement migres. De l'érythème et une induration ont été observés au site d'injection de cytokine mais non au site témoin entre la vingt-quatrième heure et la septante-deuxième heure. Un examen mensuel de frottis cutané du premier site a montré une diminution de l'index bactérien (IB) par rapport aux témoins pour 4 des 10 patients, cette diminution survenant de manière aussi précoce qu'entre la quatrième et la huitième semaine. L'histopathologie des biopsies de 6 des 10 patients entre 9 et 10 mois a montré une poursuite de la réduction de l'IB attribuable à l'IFN-γr et non à la chimiothérapie instaurée ultérieurement.
chez 4 des 5 patients. Une telle inhibition n'a pas été observée au niveau des sites où on avait injecté de l'IFN-γr. A partir de ces analyses, il ne fut pas possible dse déterminer si les effets de l'IFN-γr ci-dessus se rapportaient premièrement à des macrophages mûrs ou à des jeunes monocytes nouvellement migres. De l'érythème et une induration ont été observés au site d'injection de cytokine mais non au site témoin entre la vingt-quatrième heure et la septante-deuxième heure. Un examen mensuel de frottis cutané du premier site a montré une diminution de l'index bactérien (IB) par rapport aux témoins pour 4 des 10 patients, cette diminution survenant de manière aussi précoce qu'entre la quatrième et la huitième semaine. L'histopathologie des biopsies de 6 des 10 patients entre 9 et 10 mois a montré une poursuite de la réduction de l'IB attribuable à l'IFN-γr et non à la chimiothérapie instaurée ultérieurement. RESUMEN
Se estimó la cantidad de peróxido de hidrógeno (H2O2) y del anión superóxido (O ) en las células lesiónales de pacientes lepromatosos inyectados intralesionalmente con interferon gamma recombinante (rIFN-γ). Como controles se incluyeron las lesiones contralesionales inyectadas con excipiente. Comparadas con las células de las lesiones control, las células lesiónales esterasa positivas (sugestivas de monocitos/ macrófagos) de los sitios inyectados con rIFN-γ de muchos sujetos mostraron incrementos netos en sus niveles de H2O2 y O
) en las células lesiónales de pacientes lepromatosos inyectados intralesionalmente con interferon gamma recombinante (rIFN-γ). Como controles se incluyeron las lesiones contralesionales inyectadas con excipiente. Comparadas con las células de las lesiones control, las células lesiónales esterasa positivas (sugestivas de monocitos/ macrófagos) de los sitios inyectados con rIFN-γ de muchos sujetos mostraron incrementos netos en sus niveles de H2O2 y O . Cuando estas células se expusieron al Mycobacterium leprae in vitro, se observó una disminución de la producción de O
. Cuando estas células se expusieron al Mycobacterium leprae in vitro, se observó una disminución de la producción de O en 4 de 5 pacientes. Tal inhibición no se observó en los sitios inyectados con rIFN-γ. No fue posible determinar si los efectos del rIFN-γ fueron primariamente sobre los macrófagos lesiónales maduros o sobre los monocitos recién llegados a la lesión. Los sitios inyectados con citocinas mostraron eritema e induración 24 a 72 horas después. El examen mensual de los extendidos de linfa cutánea del sitio inyectado con rIFN-γ, mostró una reducción en el índice bacteriano (Bl) en 4 de 10 pacientes; esta reducción ocurrió tempranamente, entre las 4 y las 8 semanas. El estudio histopatológico realizado entre las 9 y 10 semanas después de la inyección del rIFN-γ, mostró una disminución adicional del BI en 6 de 10 pacientes. Este efecto se atribuyó al rIFN-γ y no a la quimioterapia instituida subsecuentemente.
en 4 de 5 pacientes. Tal inhibición no se observó en los sitios inyectados con rIFN-γ. No fue posible determinar si los efectos del rIFN-γ fueron primariamente sobre los macrófagos lesiónales maduros o sobre los monocitos recién llegados a la lesión. Los sitios inyectados con citocinas mostraron eritema e induración 24 a 72 horas después. El examen mensual de los extendidos de linfa cutánea del sitio inyectado con rIFN-γ, mostró una reducción en el índice bacteriano (Bl) en 4 de 10 pacientes; esta reducción ocurrió tempranamente, entre las 4 y las 8 semanas. El estudio histopatológico realizado entre las 9 y 10 semanas después de la inyección del rIFN-γ, mostró una disminución adicional del BI en 6 de 10 pacientes. Este efecto se atribuyó al rIFN-γ y no a la quimioterapia instituida subsecuentemente.
Lepromatous leprosy (BL/LL) is a disseminated disease characterized by granulomas wherein foamy macrophages are loaded with the pathogen Mycobacterium leprae. The localized tuberculoid leprosy, on the other hand, rarely shows bacilli within the epithelioid cell granulomas (31). The mechanisms by which mycobacteria are killed or resist intracellular destruction are not clear. Bactericidal activity is thought to be mediated by reactive oxygen intermediates (ROI), such as superoxide anion (O ), hydrogen peroxide (H2O2), and hydroxyl radical (OH) produced during the respiratory burst following phagocytosis (2). Macrophage activation by lymphokines, particularly interferon gamma (IFN-γ) enhances the production of these radicals both in vitro and in vivo (21,22,24). Such enhancement has been reported to be associated with the killing of various intracellular pathogens (3,6,20,24,32,33) . Lepromatous leprosy patients appeared to have defective production of IFN-γ when peripheral-bloodderived lymphocytes were stimulated with M. leprae (8,18,27). In addition, monocytes of lepromatous patients were shown to have reduced release of ROI both before and after in vitro phagocytosis of the leprosy bacilli (7,13,14). Moreover, in the experimental leprosy model using nude mice it was observed that tissue macrophages laden with M. leprae were unable to produce O
), hydrogen peroxide (H2O2), and hydroxyl radical (OH) produced during the respiratory burst following phagocytosis (2). Macrophage activation by lymphokines, particularly interferon gamma (IFN-γ) enhances the production of these radicals both in vitro and in vivo (21,22,24). Such enhancement has been reported to be associated with the killing of various intracellular pathogens (3,6,20,24,32,33) . Lepromatous leprosy patients appeared to have defective production of IFN-γ when peripheral-bloodderived lymphocytes were stimulated with M. leprae (8,18,27). In addition, monocytes of lepromatous patients were shown to have reduced release of ROI both before and after in vitro phagocytosis of the leprosy bacilli (7,13,14). Moreover, in the experimental leprosy model using nude mice it was observed that tissue macrophages laden with M. leprae were unable to produce O and were resistant to activation by IFN-γ (35,36). Subsequently, monocytes and in vitro -cultivated macrophages from peripheral blood of man also were shown to be affected negatively when M. leprae components, such as phenolic glycolipid-I (PGL-I), were used to pretreat the cells (26,39,40). Further evidence indicated that microbial glycolipids of intracellular pathogens such as M. leprae acted as scavengers of oxygen radicals, and thereby contributed to the persistence of these pathogens within the macrophages (5,26).
and were resistant to activation by IFN-γ (35,36). Subsequently, monocytes and in vitro -cultivated macrophages from peripheral blood of man also were shown to be affected negatively when M. leprae components, such as phenolic glycolipid-I (PGL-I), were used to pretreat the cells (26,39,40). Further evidence indicated that microbial glycolipids of intracellular pathogens such as M. leprae acted as scavengers of oxygen radicals, and thereby contributed to the persistence of these pathogens within the macrophages (5,26).
Of recent interest are studies wherein recombinant human IFN-γ (rIFN-γ) injections into bacilli-laden lesions of lepromatous leprosy patients showed local bacillary clearance (10). There is scant information on the functional status of macrophages constituting human granulomas with regard to reactive oxygen radical release. The ability of rIFN-γ to activate such well-differentiated, bacilliferous lesional macrophages also is not known. Since such information is required in order to design better strategies for bacterial killing, the present study was undertaken to evaluate the status of lesional macrophages in the course of the natural disease in man. Our study indicates that rIFN-γ administered into lepromatous granulomas leads to multiple effects. Many patients showed enhanced levels of H2O2 and O release as well as an increased ability of lesional macrophages to be stimulated in vitro to release ROI. In conformity with earlier studies erythema/induration and bacillary clearance were noted also at the injected sites. However, in a given individual these features were observed independently of each other, and no stringent relationship was observable between the diverse local effects of rIFN-γ.
release as well as an increased ability of lesional macrophages to be stimulated in vitro to release ROI. In conformity with earlier studies erythema/induration and bacillary clearance were noted also at the injected sites. However, in a given individual these features were observed independently of each other, and no stringent relationship was observable between the diverse local effects of rIFN-γ.
MATERIALS AND METHODS
Patients. Sixteen, newly diagnosed, untreated borderline (BL) and polar lepromatous (LL) patients attending the dermatology clinics of Safdarjung Hospital and Leprosy Mission Hospital, Shahadra, New Delhi, India, were included in the study. They were classified on the clinicopathological criteria of Ridley and Jopling (31). The bacterial index (BI) was scored on a logarithmic scale by slit-smear examination for acid-fast bacilli (AFB) from six sites (30). None of the patients had reactions at the time of examination. Multidrug therapy consisting of 600 mg rifampin monthly, 100 mg of clofazimine on alternate days, and dapsone 100 mg daily was instituted on the termination of rIFN-γ injections.
rIFN-γ injection and study design. Lyophilized rIFN-γ with a specific activity of 2 x 107 units/mg of protein was kindly provided by Dr. Rosenkaimer of Boehringer Ingelheim, West Germany. After obtaining clearance from the Institutional Ethical Committee and the Drug Controller of India and informed consent from the patients, 10 µg or 20 µg of excipient reconstituted rIFN-γ was injected intradermally into three well-characterized lepromatous lesions consecutively for 3 days. Clinically similar, neighboring or contralateral lesions used as controls were injected with the same volume of excipient. The study design was as follows.
On day 0, a biopsy for histopathological diagnosis and a slit-skin smear examination for the BI were undertaken. On days 1, 2, and 3 the diameter of the local erythma/ induration at the injected sites was recorded. On day 4, two 4-mm punch biopsies of uninjected and injected sites were taken for isolation of lesional macrophages. The BI was repeated on the same nonbiopsied injected sites on day 4 and at monthly intervals up to 1 year, except where stated otherwise. Biopsies of both types of lesions were repeated for histopathological examination between 9 and 10 months.
Isolation of peripheral blood monocytes. Venous blood (10 ml) was aseptically drawn from patients in heparinized syringes. Mononuclear cells were isolated by the density gradient method (4). After washing the cells two times in RPMI 1640 (Sigma Chemical Co., St. Louis, Missouri, U.S.A.), the cell pellet was rcsuspended in RPMI 1640 containing 10% normal AB serum at a concentration of 4 x 105 cells/well. The cells were layered in 96-well, flat-bottom plates (Nunc, Roskilde, Denmark), and incubated for 2 hr at 37ºC in a 5% CO2 incubator. The nonadherent cells were removed by washing twice, and only the adherent cells were used for functional assays.
Extraction of lesional macrophages. Punch biopsies (4-mm) from control and rIFN-γ-injected sites were washed with RPMI 1640 (Sigma) containing 10% AB serum to remove traces of blood. The epidermis was discarded, and the dermis was mechanically teased, suspended in RPMI 1640 with 0.1% collagenase (Type IV; Sigma) and incubated at 37ºC for 14-16 hr with gentle shaking. Subsequently, the tissue debris was removed by slow-speed centrifugation (Sorvall RT 6000; DuPont Company, Willmington, Delaware, U.S.A.) at 50 x g for 5 min. The supernatant consisting of lesional cells was centrifuged at 250 x g for 10 min. The pellet consisting of lesional cells was washed twice with phenol-red-free Hanks' balanced salt solution (HBSS; Sigma) and delivered into 96-well, flat-bottom plates (Nunc) at a concentration of 6 x 104 cells/well. Aliquots of lesional cells were tested for viability by trypan blue dye exclusion which ranged from 85% to 95%. Each sample also was stained for nonspecific esterase (NSE), as described below, to distinguish the monocyte/macrophage population from other cells. The yield of cells from 4-mm biopsies ranged from 5 x 105 to 1.8 x 106, of which 60%-70% consisted of NSEpositive cells. Care was taken to use phenolred-free medium to avoid interference in the H2O2 and CO2 assay.
NSE assay. The lesional cells/monocytes were tested for NSE activity (41). In brief, the cells were spun onto slides (Cytospin 2; Shandon, U.K.), fixed for 60 sec, and incubated at 37ºC for 60 min in a stain containing pararosaniline (Sigma) and alpha-naphthyl acetate as a substrate (Sigma). The slides were washed with distilled water, air dried, and scored for NSE positivity.
Extraction of M . leprae. M. leprae were extracted from the cryopreserved spleen of an infected armadillo (29) (kindly provided by Dr. R. J. W. Recs, National Institute for Medical Research, London). Care was taken to ensure sterility, and phenol-red-frce HBSS (Sigma) was used to suspend the bacilli.
Quantitation of H2O2 and O release by lesional monocytes / macrophages. H2O2 release was estimated essentially by the method of Pick and Mizel (28) based on the horscradish-pcroxidase (HRPO)-mediated oxidation of phenol red by H2O2 , which results in a compound with increased absorbance at 610 nm. Briefly, 100 µl of the reaction mixture containing 0.028 M phenol red (Sigma), 50 µg/ml of HRPO (Type II; Sigma) was added to duplicate wells. The optical density (OD) was recorded in an ELISA reader (Titertek Multiskan; Flow Labs, Scotland, U.K.) immediately and after 60 min. The differences in the absorbance values were converted into nanomoles of H2O2 released by extrapolating on a standard graph set up with each set of tests.
release by lesional monocytes / macrophages. H2O2 release was estimated essentially by the method of Pick and Mizel (28) based on the horscradish-pcroxidase (HRPO)-mediated oxidation of phenol red by H2O2 , which results in a compound with increased absorbance at 610 nm. Briefly, 100 µl of the reaction mixture containing 0.028 M phenol red (Sigma), 50 µg/ml of HRPO (Type II; Sigma) was added to duplicate wells. The optical density (OD) was recorded in an ELISA reader (Titertek Multiskan; Flow Labs, Scotland, U.K.) immediately and after 60 min. The differences in the absorbance values were converted into nanomoles of H2O2 released by extrapolating on a standard graph set up with each set of tests.
O release was determined by quantifying the superoxide dismutase (SOD) inhibitable reduction of the ferricytochrome cas described by Johnston (9). Briefly, 100µl of the reaction mixture containing ferricytochrome c (4 mg/ml, Sigma) alone or with SOD (100 µg/ml; Sigma) were added to duplicate wells. Absorbance changes were noted at 0 and 60 min after addition of the reaction mixture in an ELISA reader using a 550-nm filter. The difference in values was converted to nanomoles of O
release was determined by quantifying the superoxide dismutase (SOD) inhibitable reduction of the ferricytochrome cas described by Johnston (9). Briefly, 100µl of the reaction mixture containing ferricytochrome c (4 mg/ml, Sigma) alone or with SOD (100 µg/ml; Sigma) were added to duplicate wells. Absorbance changes were noted at 0 and 60 min after addition of the reaction mixture in an ELISA reader using a 550-nm filter. The difference in values was converted to nanomoles of O using the extinction coefficient of ferricytochrome c (9), All values were expressed as nanomoles/1 x 106 NSE-positive cells/60 min. NSE positivity was used instead of protein concentration to normalize the data since other lesional cells were present in the lesions. Preliminary time kinetics for H2O2 and O
using the extinction coefficient of ferricytochrome c (9), All values were expressed as nanomoles/1 x 106 NSE-positive cells/60 min. NSE positivity was used instead of protein concentration to normalize the data since other lesional cells were present in the lesions. Preliminary time kinetics for H2O2 and O over 30-120 min had indicated 60 min as the peak period.
over 30-120 min had indicated 60 min as the peak period.
Stimulants for in vitro H2O2 and O release. To evaluate the potential of lesional monocytes/macrophages to be stimulated in vitro, duplicate wells were set up as before with freshly extracted M. leprae at a ratio of 50:1 (bacilli: monocyte/macrophage). After a 1-hr incubation at 37ºC in a 95% air and 5% CO2 incubator, H2O2 and O
release. To evaluate the potential of lesional monocytes/macrophages to be stimulated in vitro, duplicate wells were set up as before with freshly extracted M. leprae at a ratio of 50:1 (bacilli: monocyte/macrophage). After a 1-hr incubation at 37ºC in a 95% air and 5% CO2 incubator, H2O2 and O were estimated.
were estimated.
Bacterial index (BI). The bacterial load in the patients was evaluated by slit-skin smears prepared from six sites and scored on a logarithmic scale for AFB (29) using Zichl-Neelsen staining prior to the start and at the termination of the study. A monthly BI was done for both control and rIFN-γ-injected sites.
Histopathology. On day 4 as well as at the termination of the study (9-10 months), the injected sites were biopsied for histopathological examination and BI scoring. Biopsies were fixed in 10% buffered formalin and stained with hematoxylin and eosin (H&E) as well as Ziehl-Neelsen stain.
Statistical analysis. The p values were calculated by the Student's t test, Wilcoxon signed rank test, and rank correlation analysis by the Spearman method (38).
RESULTS
Clinical status. Table 1 gives the clinical details of the lepromatous leprosy patients included in the study. Care was taken to select clinically similar, contralateral lesions for injections with excipient (control) and rIFN-y. Patients were injected intradermally with 100 µl of excipient or 10/20 µg of rIFN-γ into three lesions for 3 consecutive days, except where stated otherwise. No untoward systemic symptoms were reported, except for patient 12 who complained of malaise, headache and fever (38ºC) after one injection of 20 µg of rIFN-γ.
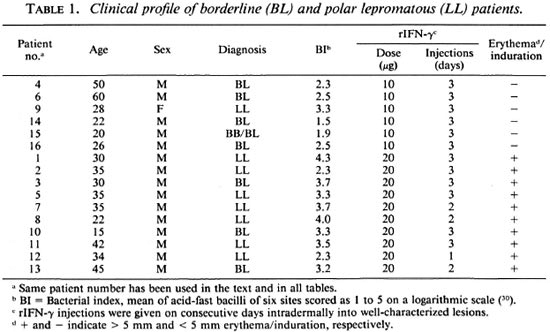
Skin reaction to rIFN-γ injections. Using a 5-mm induration diameter as the cutoff point, it was observed that none of the six patients receiving 10 µg rIFN-γ responded; whereas all responded to the higher dose. Ten of 16 borderline and polar lepromatous patients showed erythema and induration of the injected lesions, with the average diameter ranging from 5 to 25 mm (The Figure). In conformity with our earlier studies (10) maximal responses were observable by 48 hr in 6 of 10 patients; the others showed no change or a mild increase at 72 hr. However, patient 12 showed an 18-mm induration after one dose of 20 µg and was not given additional injections of rIFN-γ (The Figure).
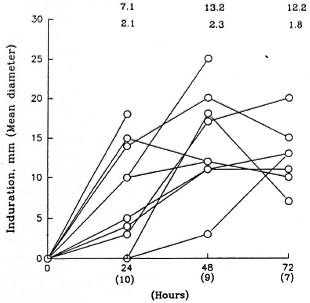
The figure. Time kinetics of local response to rIFN-γ injections into lesions of lepromatous leprosy patients who showed > 5-mm induration. Numbers above the graph at each time point depict mean diameter + S.E. of patients whose numbers are given in parentheses on the X-axis.
Twenty-four hours after the last injection (i.e., day 4), two each of control and rlFNγ-injected lesions from subjects showing erythema/induration were biopsied at previously marked sites. The extracted lesional cells were checked for nonspecific esterase (NSE) positivity, and all estimations were expressed in terms of 106 NSE-positive cells. In general, the number of lesional cells obtained from the same size biopsies of injected sites were 1.5- to twofold higher than those from control sites.
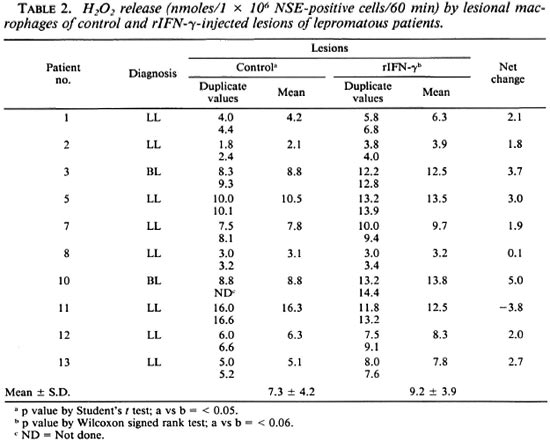
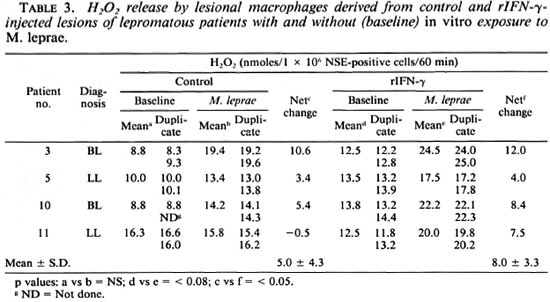
Effect of rIFN-γ on ROI release by lesional macrophages. H2O2. Lesional NSEpositive cells from 10 individuals were evaluated for baseline H2O2 release in control and rIFN-γ-injected sites (Table 2). There was individual variation in both sites in the mean nmoles of H2O2 released per 106 lesional cells. However, when the two sites of the same individual were compared, the cytokine-injected site showed a net increment/change of H2O2 release in 8 of 10 subjects. When the two groups of control and test sites were compared by Student's t test, this increment was of significance (p < 0.05). However, when the nonparametric Wilcoxon signed rank test was used, no statistical significance was obtained. To ascertain whether the lesional macrophages could be induced further to release H2O2 , aliquots of cells from control and rIFN-γ-injected sites were exposed in vitro to freshly extracted M. leprae (Table 3). Cells from both control and rIFN-γ-injected lesions of 3 of 4 patients showed a negligible-to-low increase in H2O2 after treatment with bacilli. Patient 11, however, showed marked improvement after three injections of 20 µg rIFN-y.
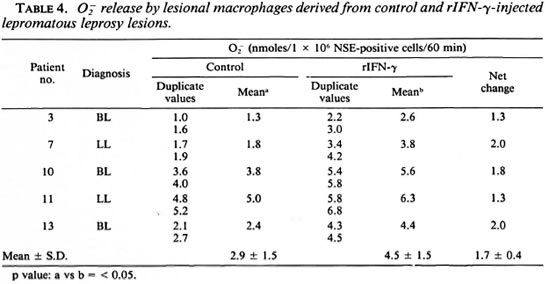
O  . Due to the paucity of cells only five patients were tested for O
. Due to the paucity of cells only five patients were tested for O release. In general, O
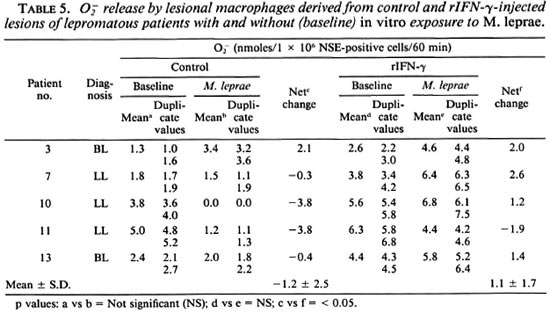
release. In general, O release was low in lesional macrophages. However, cells from rIFN-y sites showed higher levels (p < 0.05) as compared to control lesional cells (Table 4). In vitro exposure to M. leprae showed a decrease in O
release was low in lesional macrophages. However, cells from rIFN-y sites showed higher levels (p < 0.05) as compared to control lesional cells (Table 4). In vitro exposure to M. leprae showed a decrease in O release which was reversed in 4 of 5 rIFN-γ-injected lesions (Table 5).
release which was reversed in 4 of 5 rIFN-γ-injected lesions (Table 5).
Peripheral blood monocytes. Monocytes isolated from blood before and after the termination of rIFN-γ injections showed no statistically significant increases in H2O2 or O either at the basal level or after in vitro exposure to M. leprae. H2O2 released (mean ± S.D. nmoles/106 NSE-positive cells) by NSE-positive monocytes from 10 patients before and after three rIFN-γ injections was 16.5 ± 6.5 and 20.5 ± 10.2, respectively. After in vitro exposure to M. leprae, the levels of H2O2 showed mild but insignificant differences before (14.6 ± 5.3) and after (18.5 ± 8.9) intralesional cytokine administration. Similar results were obtained for Oj, where mean S.D. nmoles/106 NSE-positive cells before and after treatment were 4.4 ± 1.3 and 4.8 ± 2.8, respectively. After in vitro M. leprae exposure, the respective values were 3.4 ± 0.6 and 2.7 ± 1.0 nmoles/ 106 NSE-positive cells.
either at the basal level or after in vitro exposure to M. leprae. H2O2 released (mean ± S.D. nmoles/106 NSE-positive cells) by NSE-positive monocytes from 10 patients before and after three rIFN-γ injections was 16.5 ± 6.5 and 20.5 ± 10.2, respectively. After in vitro exposure to M. leprae, the levels of H2O2 showed mild but insignificant differences before (14.6 ± 5.3) and after (18.5 ± 8.9) intralesional cytokine administration. Similar results were obtained for Oj, where mean S.D. nmoles/106 NSE-positive cells before and after treatment were 4.4 ± 1.3 and 4.8 ± 2.8, respectively. After in vitro M. leprae exposure, the respective values were 3.4 ± 0.6 and 2.7 ± 1.0 nmoles/ 106 NSE-positive cells.
Local clearance of M. leprae and granuloma histology. The patients' Bis were taken from the same predetermined lesions injected or not injected with rIFN-γ at monthly intervals for 1 year (Table 6). In addition, the BI was taken from histopathological examinations of the biopsies on day 4 and 9 to 10 months after injections. Table 6 gives the mean BI of two lesions each. Using the criteria of change in the BI of at least 2 consecutive months and also comparing the control values with the cytokine-treated sites, it was observed that only 4 of 9 subjects showed a decrease in their Bis attributable to rIFN-γ. This decrease occurred as early as 4 to 8 weeks and ranged from 0.5 to 3 logs. Histopathological examination of the biopsied site at 9 months revealed two additional patients with a higher bacillary clearance at the cytokine-administered site which was missed by slit-skin smear examination. Thus, in total, 6 of 10 patients showed bacillary clearance. The Bis of those patients who had received the lower dose of the cytokine did not show any change (data not shown).
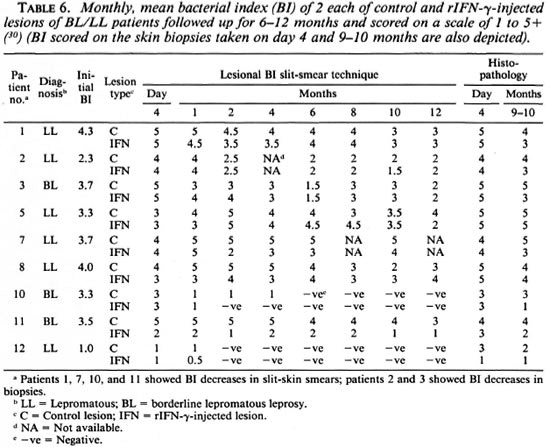
In addition, granuloma size was moderately reduced at the rIFN-γ sites in five subjects; one showed an ill-formed granuloma with epithelioid cells and another had perivascular cuffing and increased cellularity with mononuclear cells.
DISCUSSION
The reversibility of immune defects in disease states has become possible with the recent availability of recombinant lymphokines. Interleukin-2 and IFN-γ are of particular interest in leprosy since they are required for T-cell proliferation (19,37) and macrophage activation (1,23), respectively, and have, moreover, been shown to be low in lepromatous leprosy (8,27). Earlier studies on Indian patients (10) had shown that local injection of rIFN-γ led to an influx of CD4-positive T cells and monocytes as well as bacillary clearance (10,17). The present study on a different ethnic population in India confirms the development of lymphokineinduced dermal reaction similar to a delayed-type reaction in bacilliferous lesions of lepromatous leprosy patients.
To evaluate the effect of rIFN-γ on oxygen intermediates produced by lesional bacilli-laden macrophages, the H2O2 and O release were studied. Of interest was the enhanced induction of H2O2 in lesional cells of rIFN-γ-injected sites as compared to control sites in 8 of 10 patients. Although there was considerable variability in H2O2 production in the two sites among the lepromatous leprosy patients, the increment was significant when the control and rIFN-γ-injected lesions of the same individual were compared with each other. Although the O
release were studied. Of interest was the enhanced induction of H2O2 in lesional cells of rIFN-γ-injected sites as compared to control sites in 8 of 10 patients. Although there was considerable variability in H2O2 production in the two sites among the lepromatous leprosy patients, the increment was significant when the control and rIFN-γ-injected lesions of the same individual were compared with each other. Although the O levels were generally low in lesional cells, there was a consistent increase in rIFN-γ-injected sites compared to the control contralateral lesions. On in vitro exposure to M. leprae, lesional cells from both sites showed statistically insignificant and low increases in H2O2 as compared to the equivalent unexposed cells. The rIFN-γ-injected sites did not show any further change. O
levels were generally low in lesional cells, there was a consistent increase in rIFN-γ-injected sites compared to the control contralateral lesions. On in vitro exposure to M. leprae, lesional cells from both sites showed statistically insignificant and low increases in H2O2 as compared to the equivalent unexposed cells. The rIFN-γ-injected sites did not show any further change. O levels were inhibited by the in vitro addition of M. leprae in 3 of 4 subjects. This inhibition was not observed in rIFN-γ-injected lesions of the same individual.
levels were inhibited by the in vitro addition of M. leprae in 3 of 4 subjects. This inhibition was not observed in rIFN-γ-injected lesions of the same individual.
Previously reported studies on granulomas of experimental models (35,36) and blood-derived monocytes and macrophages (14,39,40) showed that O production not only was low but also unresponsive to the activating effects of rIFN-γ. Using pure products of M. leprae, such as phenolic glycolipid and lipoarabinomannan, inhibition of O
production not only was low but also unresponsive to the activating effects of rIFN-γ. Using pure products of M. leprae, such as phenolic glycolipid and lipoarabinomannan, inhibition of O generation was observed (38,39). IFN-γ has been reported to reverse the loss of functional activity that occurs in aged human monocytes cultivated in vitro (1). Our studies indicate that cells from rIFN-γ-injected lesions are able to withstand the inhibitory effects of M. leprae in vitro. Although, histologically, the granulomas in our studies consisted mainly of well-differentiated macrophages, the role of newly migrating monocytes (10,11,22,34) in contributing to the oxygen radical release cannot be stringently excluded. As far as we are aware, studies similar to ours on macrophages of human granulomas have not been reported.
generation was observed (38,39). IFN-γ has been reported to reverse the loss of functional activity that occurs in aged human monocytes cultivated in vitro (1). Our studies indicate that cells from rIFN-γ-injected lesions are able to withstand the inhibitory effects of M. leprae in vitro. Although, histologically, the granulomas in our studies consisted mainly of well-differentiated macrophages, the role of newly migrating monocytes (10,11,22,34) in contributing to the oxygen radical release cannot be stringently excluded. As far as we are aware, studies similar to ours on macrophages of human granulomas have not been reported.
In the present study, peripheral blood monocytes showed a mild but not statistically significant increase in ROI production after intralesional rIFN-γ. No consistent pattern was discernable since individuals within the lepromatous group showed variable levels of ROI both before and after cytokine administration. Using a different methodology, Nathan, et al. (22) earlier had reported augmentation of H2O2 levels after a single intradermal rIFN-γ injection.
That rIFN-γ can alter lesional macrophages in lepromatous leprosy is supported further by the early but persistent clearance of bacilli in many lepromatous patients. Monthly conventional slit-skin smear examination of the injected site showed a 0.5to 3-log decrease in the BI compared to clinically similar lesions in the same patients. In conformity with previous studies (10), this decrease was observed by 4 to 8 weeks. Chemotherapy had been instituted on day 4, i.e., after the completion of rIFN-γ administration. Such therapy is known to show only a 1-log reduction of bacilli as late as 12 months. Although the intracellular removal and clearance of M. leprae from the dermal macrophages is hastened in many patients, in the absence of viability studies it is not possible for us to comment on whether this clearance reflects microbicidal activity or the removal of previously killed bacilli. Nor can we stringently rule out the migration of bacilli-containing cells, altering the BI status of the lesions. We earlier had reported that patients with nodular lesions showed lower clearance of bacilli (10), and the present study had included mostly such patients. Further supporting this observation is patient 5, who had histoid leprosy and failed to clear bacilli from the injected nodule.
Although oxygen radicals have been implicated in bacterial killing (16), the possibility of other microbicidal pathways playing a role in the killing of M. leprae cannot be ruled out (12,15), particularly since strict correlation was not observed between bacillary clearance and the levels of H2O2, or O released by the cells of the same lesion. A stringent relationship also could not be established in individual cases between the initial bacterial load of the patient, BI of injected sites, erythema/induration and bacillary clearance. Taken together, the observations in the present study indicate that multiple events may be initiated by rIFN-γ in the bacilli-laden granulomas of human lepromatous leprosy. Some of these relate to direct activation events in lesional macrophages such as increased H2O2 and O
released by the cells of the same lesion. A stringent relationship also could not be established in individual cases between the initial bacterial load of the patient, BI of injected sites, erythema/induration and bacillary clearance. Taken together, the observations in the present study indicate that multiple events may be initiated by rIFN-γ in the bacilli-laden granulomas of human lepromatous leprosy. Some of these relate to direct activation events in lesional macrophages such as increased H2O2 and O levels and clearance of intracellular M. leprae; whereas others, such as erythema and induration of the skin, are more complex and may involve recruitment of other cells and cell products. At the individual level, all of the effects are not observed concomitantly.
levels and clearance of intracellular M. leprae; whereas others, such as erythema and induration of the skin, are more complex and may involve recruitment of other cells and cell products. At the individual level, all of the effects are not observed concomitantly.
Recent data on systemic administration of rIFN-γ have shown encouraging results (25). rIFN-y may, therefore, have therapeutic value in the early clearance of bacilli from the skin of lepromatous patients and may act as an adjunct to chemotherapy, particularly in drug-resistant and recalcitrant leprosy.
Acknowledgment. This study was supported by a grant from the Indian Council for Medical Research. We thank Drs. Z. A. Cohn and G. Kaplan for helpful discussions, Dr. Rosenkaimer for recombinant human interfcron-gamma, and Dr. R. J. W. Rees for providing M. leprae- infected armadillo spleens through the IMMLEP component of the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases.
REFERENCES
1. ARENZANA-SEISDEDOS, F., VIRELIZIER, J. L. and FIERS, W. Interferons as macrophage-activating factors: III. preferential effects of interfcron-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J. Immunol. 134(1985)2444-2448.
2. BABIOR, B. M. Oxygen dependent microbial killing by phagocytes. N. Engl. J. Med. 298(1978)659-668,721-725.
3. BHARDWAJ, N., NASH, T. W. and HORWITZ, M. A. Interfcron-gamma activated human monocytes inhibit the intracellular multiplication of Legionella pneumophilia. J. Immunol. 137(1986)2662-2669.
4. BOYUM, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 21(1968)77-89.
5. CHAN, J., FUJIWARA, T., BRENNAN, P. J., MCNEIL, M., TURCO, S. J., SIBILLE, J., SNAPPER, M., AISEN, P. and BLOOM, B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc. Natl. Acad. Sci. U.S.A. 86( 1989)2453-2457.
6. DENIS, M. Interferon-gamma treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive intermediates. Cell. Immunol. 132(1991)150-157.
7. HOLZER, T. J., NELSON, K. E., SCHAUF, V., CRISPEN, R. G. and ANDERSEN, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51(1986)514-520.
8. HORWITZ, M. A., LEVIS, W. R. and COHN, Z. A. Defective production of monocyte activating cytokines in lepromatous leprosy. J. Exp. Med. 159(1984)666-678.
9. JOHNSTON, R. B. Secretion in superoxide anion. In: Methods for Studying Mononuclear Phagocytes. Adams, D. O., Edelson, P. J. and Koren, H., eds. New York: Academic Press, Inc.. 1981 , pp. 489-497.
10. KAPLAN, G., MATHUR, N. K., JOB, C. K., NATH, I. and COHN, Z. A. Effect of multiple interfcrongamma injections on the disposal of Mycobacterium leprae. Proc. Natl. Acad. Sci. U.S.A. 86(1989)8073-8077.
11. KAPLAN, G., NURSAT, A., SARNO, E. N., JOB, C. K., MCELRATH, J., PORTO, J. A., NATHAN, C. F. and COHN, Z. A. Cellular responses to the intradermal injection of recombinant human gammainterfcron in lepromatous leprosy patients. Am. J. Pathol. 128(1987)345-353.
12. KLEBANOFF, S. J. and SHEPARD, C. C. Toxic effect of the peroxidase-hydrogen pcroxide-halide antimicrobial system on Mycobacterium leprae. Infect. Immun. 44(1984)534-536.
13. LAUNOIS, P., MAILLERE, B., DIEYE, A., SARTHOU, J. L. and BACH, M.-A. Human phagocyte oxidative burst activation by BCG, Mycobacterium leprae and atypical mycobacteria; defective activation by Mycobacterium leprae is not reversed by interferon gamma. Cell. Immunol. 124(1989)168-174.
14. MAROLIA, J. and MAHADEVAN, P. R. Superoxide production from macrophages of leprosy patients after stimulation with Mycobacterium leprae. J. Biosci. 12(1987)273-279.
15. MAROLIA, J. and MAHADEVAN, P. R. Mycobacterium leprae mediated stimulation of macrophages from leprosy patients and hydrogen peroxide production. J. Biosci. 13(1988)295-303 .
16. MAROLIA, J. and MAHADEVAN, P. R. Reactive oxygen intermediates inactivate Mycobacterium leprae in the phagocytes from human peripheral blood. Int. J. Lepr. 57(1989)483-491.
17. MATHUR, N. K., MITTAL, A., MATHUR, D. and JAIN, S. K. Long term follow up of lepromatous leprosy patients receiving intralesional recombinant gamma-interferon. Int. J. Lepr. 60(1992)98-100.
18. MITTAL, A., MISRA, R. S. and NATH, I. Accessory cell heterogeneity in lepromatous leprosy; dendritic cells and not monocytes support T cell response. Clin. Exp. Immunol. 76(1989)233-239.
19. MORGAN, D. A., RUSCETTI, F. W. and GALLO, R. A. Selective in vitro growth of T-lymphocytes from normal human bone marrows. Science 193(1976)1007-1008.
20. MURRAY, H. W., RUBIN, B. Y. and ROTHERMEL, C. D. Killing of intracellular Leishmania donovani by lymphokine stimulated human mononuclear phagocytes. J. Clin. Invest. 72(1983)1506-1510.
21. MURRAY, H. W., SPITALNY, G. L. and NATHAN, C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J. Immunol. 134(1985)1619-1622.
22. NATHAN, C. F., KAPLAN, G., LEVIS, W. R., NUSRAT, A., WITMER, M. O., SHERWIN, S. A., JOB, C. K., HOROWITZ, C. R., STEINMAN, R. M. and COHN, Z. A. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
23. NATHAN, C. F., MURRAY, H. W., WIEBE, M. E. and RUBIN, B. Y. Identification of interferon-gamma as the lymphokinc that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158(1983)670-689.
24. NATHAN, C. F., NOGUEIRA, N., JUANGBHANICH, C, ELLIS, J. and COHN, Z. A. Activation of macrophages in vivo and in vitro: correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J. Exp. Med. 149(1979)1056-1068.
25. NATHAN, C. F., SQUIRES, K., GRIFFO, W., LEVIS, W., VARGHESE, M., JOB, C. K., NUSRAT, A., SHER-WIN, S., RAPPOPORT, S., SANCHEZ, E., BURKHARDT, R. A. and KAPLAN, G. Widespread intradermal accumulation of mononuclear leukocytes in lepromatous leprosy patients treated systemically with recombinant interferon-gamma. J. Exp. Med. 172(1990)1509-1512.
26. NEILL, M. A. and KLEBANOFF, S. J. The effect of phenolic glycolipid-I from Mycobacterium leprae on the antimicrobicidal activity of human macrophages. J. Exp. Med. 167(1988)30-42.
27. NOGUEIRA, N., KAPLAN, G., LEVY, E., SARNO, E. N., KUSHNER, P., GRANELLI-PIPERNO, A., VIERA, L., GOULD, V., LEVIS, W., STEINMAN, R., YIP, Y. K. and COHN, Z. A. Defective gamma-interferon production in leprosy. Reversal with antigen and interleukin-2. J. Exp. Med. 158(1983)2165-2170.
28. PICK, E. and MIZEL, D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in cultures using an automatic enzyme immuno assay reader. J. Immunol. Methods 46(1981)211-226.
29. PRASAD, H. K. and NATH, I. Factors influencing the incorporation of 'H-thymidine in Mycobacterium leprae. J. Med. Microbiol. 14(1981)279-293.
30. RIDLEY, D. S. A logarithmic index of bacilli in biopsies 2. Evaluation. Int. J. Lepr. 35(1967)187-193.
31. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
32. ROOK, G. A., STEELE. W. J., AINSWORTH, M. and CHAMPION, B. R. Activation of macrophages to inhibit proliferation of Mycobacterium tuberculosis: comparison of the effects of recombinant gamma-interferon on human monocytes and murine peritoneal macrophages. Immunology 59(1986)333-338.
33. ROTHERMEL, C. D., RUBIN, B. Y. and MURRAY, H. W. Gamma-interferon is the factor in lymphokinc that activates human monocytes to inhibit intracellular Chlamydia psittaci replication. J. Immunol. 131(1983)2542-2544.
34. SAMUEL, N. M., GRANGE, J. M., SAMUEL, S., LUCAS, S., OWILLI, O. M., ADALLA, S., LEIGH, I. M. and NAVARRETTE, C. A study of the effects of intradermal administration of recombinant gamma-interferon in lepromatous leprosy patients. Lepr. Rev. 58(1987)389-400.
35. SIBLEY, L. D. and KRAHENBUHL, J. L. Mycobacterium leprae burdened macrophages are refractory to activation by gamma-interferon. Infect. Immun. 55(1987)446-450.
36. SIBLEY, L. D. and KRAHENBUHL, J. L. Defective activation of granuloma macrophages from Mycobacterium leprae infected nude mice. J. leukoc. Biol. 43(1988)60-66.
37. SMITH, K. A. Interleukin-2; inception, impact, and implications. Science 240(1988)1169-1176.
38. SNEDECOR, G. W. and COCHRAN, W. G. Wilcoxon signed rank test and Spearman rank correlation. In: Statistical Methods. New Delhi: IBH, 1967, pp. 130-131, 193-194.
39. VACHULA, M., HOLZER, T. J. and ANDERSEN, B. R. Suppression of monocyte oxidative response by phenolic glycolipid-I of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.
40. VACHULA, M., WOROBEC, S. and ANDERSEN, B. R. A comparison of monocyte oxidative responses in leprosy patients and healthy subjects as influenced by mycobacterial lipid treatment. Int. J. Lepr. 58(1990)534-539.
41. YAM, L. T., LI, C. Y. and CROSBY, W. H. Cytochemical identification of monocytes and granulocytes. Am. J. Clin. Pathol. 55(1971)283-290.
1. M.Sc, Research Fellow; Department of Biotechnology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
2. Ph.D., Associate Professor; Department of Biotechnology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
3. M.D., F.R.C.Path., F.N.A., F.A.Sc, F.N.A.Sc, F.A.M.S., Professor and Head, Department of Biotechnology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110029, India.
4. M.D., Senior Dermatologist; Department of Dermatology, Safdarjung Hospital, New Delhi 110029, India.
5. M.D., Consultant, Department of Dermatology, Safdarjung Hospital, New Delhi 110029, India.
6. M.D., Medical Superintendent, Leprosy Mission Hospital, Shahadra, India.
Reprint requests to Dr. Nath.
Received for publication on 28 October 1992.
Accepted for publication in revised form on 5 January 1993.