- Volume 61 , Number 3
- Page: 421–7
Immunoreactivity of mycobacterial strain ICRC and Mycobacterium leprae antigens with polyclonal and monoclonal antibodies
ABSTRACT
ICRC, a cultivable mycobacterium, is undergoing clinical trials as an antileprosy vaccine in India. In the present study, we have investigated the crossreactivity between antigens of the mycobacterial strains of ICRC and Mycobacterium leprae using polyclonal and monoclonal antibodies in a radioimmunoprecipitation assay. It was observed that polyclonal anti-ICRC and anti-M. leprae antibodies showed predominant reactivity to a 21-kDa protein of the mycobacterial strain ICRC and the 21- and 14-kDa proteins of M. leprae. Crossreactivity between the antigens of the mycobacterial strains ICRC and M. leprae was established further by using M. leprae-specific monoclonal antibody WML06 (reacting with the 14-kDa protein of M. leprae), which identified the 21- and 14-kDa proteins of the mycobacterial strain ICRC. Thus, our studies demonstrate that the 14-kDa protein of M. leprae, which is known to harbor T- and B-cell epitopes, shares crossreactive antigenic determinants with the 21 - and 14-kDa proteins of the mycobacterial strain ICRC. We believe that such proteins may provide important reagents for designing subunit vaccines and for determining skin-test reagents.RÉSUMÉ
L'ICRC, une mycobacteric cultivable, subit actuellement des essais cliniques en Inde en tant que vaccin anti-lèpre. Nous avons analysé dans la présente étude la réactivité croisée entre des antigènes des souches mycobactériennes d'ICRC et de Mycobacterium leprae à l'aide d'anticorps polyclonaux et monoclonaux dans un test de radio-immunoprécipitation. On a observe que les anticorps polyclonaux anti-ICRC et 'anti-M. leprae montraient une réactivité prédominante vis-àvis d'une protéine de 21 kDa de la souche mycobactérienne d'ICRC et des protéines de 21 et 14 kDa de M. leprae. L'existence d'une réactivité croisée entre les antigènes des souches mycobactériennes d'ICRC et M. leprae a été confirmée par l'utilisation de l'anticorps monoclonal WML06 spécifique de M. leprae (réagissant avec la protéine de 14 kDa de M. leprae); cet anticorps identifia les protéines de 21 et 14 kDa de la souche mycobactérienne ICRC. En conséquence, nos études démontrent que la protéine de 14 kDa de M. leprae, qui est connue comme hébergeant les epitopes des cellules T et B, partage des déterminants antigéniques réagissant de manière croisée avec les protéines de 21 et 14 kDa de la souche mycobactérienne d'ICRC. Nous croyons que de telles protéines pourraient fournir des réagents importants pour développer des sous-unités vaccinales et des réagents pour des tests cutanés.RESUMEN
En la India se llevan a cabo estudios de campo para valorar la eficiencia de ICRC, una nitrobacteria cultivable, como vacuna antileprosa. En este estudio, utilizando anticuerpos monoclonales y policlonales, y un ensayo de radioinmunoprecipitación, investigamos la reactividad cruzada entre los antigenos de ICRC y de Mycobacterium leprae. Observamos que los anticuerpos policlonales anti-ICRC y anti-.M. leprae mostraron una reactividad predominante contra una proteína de 21 kDa de la cepa ICRC y contra las proteínas de 21y de 14-kDa del M. leprae. La reactividad cruzada entre losantígenosde las cepas ICRC y M. leprae se confirmó usando el anticuerpo monoclonal WML06 (específico para la proteína de 14 kDa del M. leprae) el cual identificó a las proteínas de 21 -y de 14-kDa de la cepa ICRC. Así, nuestros estudios demuestran que la proteína de 14 kDa del M. leprae, la cual se sabe contiene epitopes para células T y B, comparte determinantes antigénicos con las proteínas de 21 -y 14-kDa de la cepa micobacteriana ICRC. Pensamos que tales proteínas pueden ser importantes para el diseño de vacunas a base de subunidades y para la elaboración de reactivos para pruebas en piel.The inability to cultivate Mycobacterium leprae in vitro has posed major problems in defining the M. leprae antigens which are involved in specific host immune responses. The use of murine monoclonal antibodies and recombinant DNA expression libraries have led to the identification and characterization of species-specific and crossreactive epitopes on protein and polysaccharide antigens of M. leprae (4, 5, 10, 11, 14, 20). Of these, M. leprae proteins of 70, 65, 35, 18 and 12 kDa were identified as major target antigens for humoral immune responses. Despite several efforts, it has not been possible to identify M. leprae proteins involved in protective immunity. These reports have prompted investigators to look at antigens present on other cultivable mycobacteria that may share crossreactive epitopes with M. leprae (6,9,16).
ICRC, a group of cultivable, leprosy-derived mycobacteria, are undergoing clinical trials as an antileprosy vaccine in India (7). The vaccine brings about persistent lepromin conversion and upgrading of skin lesions in vaccinated lepromatous leprosy (LL) patients (7). Several laboratory investigations have demonstrated antigenic relatedness between ICRC and M. leprae antigens (3,8). Recently, we have reported that sera from leprosy patients across the clinical spectrum, healthy contacts of leprosy patients, tuberculosis patients and healthy individuals were able to immunoprecipitate several common antigens of ICRC and M. leprae (2).
Using M. leprae antigens, it was not possible to differentiate among the reactivity patterns of sera from leprosy patients, contacts, or healthy individuals since all of these sera identified M. leprae antigens with molecular masses of 47, 36, 21 and 14 kDa. When the same sera were tested with ICRC antigens, it was observed that the 21-kDa protein of ICRC was precipitated exclusively by sera from all LL patients while the 14-kDa protein of ICRC was precipitated by sera from a few LL patients (5 of 26) but all of their contacts tested. These results suggest that the 21-kDa and 14-kDa proteins of ICRC may be useful in the serodiagnosis of leprosy.
In the present investigation, we have further established the crossreactivity between the antigens of ICRC and M. leprae using anti-ICRC and anti-M. leprae polyclonal antibodies as well as monoclonal antibodies specific to M. leprae in radioimmunoprecipitation assays. Our studies demonstrate that immunodominant antigens of 21 kDa and 14 kDa of ICRC share crossreactive antigenic determinants with the 14-kDa antigen of M. leprae. These studies provide an impetus to delineate and to analyze T- and B-cell epitopes present on the 21-kDa and 14-kDa proteins of ICRC. Such immunodominant proteins may provide important reagents for designing subunit vaccines and skin-test reagents.
MATERIALS AND METHODS
Antigens. ICRC bacilli, isolate C-44, were maintained in Dubos' modified medium as described by Chirmule, et al. (3). A sonicate of ICRC bacilli was prepared as previously described by Chiplunkar, et al. (2). The ICRC bacilli were sonicated at 80W on ice for 60 min at 50% duty cycle in a Branson sonifier (Branson Ultrasonics Corp., Danbury, Connecticut, U.S.A.). The bacterial extract was centrifuged at 218,200 × g × 1 hr at 4ºC. The supernatant, which contained soluble proteins, referred to as the sonicate of ICRC, was collected. After determination of the protein concentration (l7), aliquots of the sonicate were lyophilized and stored at -20ºC.
The sonicate of armadillo-derived and irradiated M. leprae (batch CD122) was kindly provided by Dr. R. J. W. Rees through the World Health Organization/Immunology of Leprosy program.
Polyclonal antibodies. Anti-M. leprae polyclonal antibodies raised in rabbits were a kind gift from Dr. P. Brennan, Colorado State University, Fort Collins, Colorado, U.S.A.
Anti-ICRC polyclonal antibodies were raised in rabbits as described by Harboe, et al. (13). Young female rabbits were obtained from the Haffkinc Institute, Bombay, India. The rabbits were immunized with ICRC sonicate (500 µg) in 0.5 ml of phosphate buffered saline (PBS; 10 mM, pH 7.5), emulsified thoroughly with an equal volume of Freund's incomplete adjuvant (Sigma Chemical Co., St. Louis, Missouri, U.S.A.). Booster immunizations consisting of the same dose were given on days 2, 16, 30, 44, 72 and 100, and thereafter every fourth week for a period of 8 months. The antigen was administered at multiple sites: a) subcutancously in the foot pad, b) intradermally in the scapular region, and c) intramuscularly in the thigh. Serum was obtained before the initial injection and at 8-10 days after the last immunization, aliquoted, and stored at -80ºC.
Monoclonal antibodies. M. leprae-specific monoclonal antibodies (MAb) WML06 (recognizing the 12-kDa protein of M. leprae), WML03, and WML04 (recognizing the 35-kDa protein of M. leprae) were a kind gift from Dr. J. Ivanyi, Medical Research Council, London, U.K.
Immunoprecipitation assay. The sonicates of ICRC and M. leprae were labeled with Na l25l (Amersham, England) by using the chloramine-T method described earlier by Chiplunkar, et al. (2). Radiolabeled mycobacterial antigens (4 × 106 cpm) in Tris-HC1 buffer (50 mM, pH 8.3) containing 0.6 M NaCl and 0.5% Triton X-100 precleared by incubating them with 10 µl of 1:10 diluted normal BALB/c mouse serum (for MAb) or normal rabbit serum (for antiICRC/anti-M. leprae polyclonal antibodies raised in rabbits), for 2 hr on ice. The nonspecific immune complexes formed were precipitated by incubation for 30 min on ice with 25µl of a 10% (w/v) suspension of Staphylococcus aureus Cowan (Sigma). This mixture was centrifuged at 10,000 × g × 5 min. The prcclearing step was repeated. The supernatant containing precleared l25I-labeled mycobacterial sonicate was incubated with 15 µl of M. leprae-specific MAb, or anti-ICRC polyclonal antibodies, or anti-M. leprae polyclonal antibodies for 2 hr on ice. The immune complexes were precipitated by incubation for 30 min on ice with 25 µl of a suspension of S. aureus Cowan. The precipitates were washed three times with Tris-HCl buffer (50 mM, pH 8.3), with a change of microtube for the last wash. Bound antigen was eluted by boiling the pellet in 30 µl of the electrophoresis sample buffer consisting of 2% sodium dodecyl sulfate (SDS), 5% 2-mercaptoethanol, 10% glycerol, and 0.0001% bromophenol blue in 62.5 mM Tris HC1, pH 6.8.
Electrophoresis and autoradiography. The immunoprccipitated antigens were separated by using 5% to 20% gradient SDSpolyacrylamide gel electrophoresis (PAGE) as described by Laemmli (15). For visualization of protein bands, the dried gels were exposed to Fuji X-ray films (Fuji Photo Film Co. Ltd., Tokyo, Japan) for 24 hr to 72 hr at -70ºC.
Molecular mass estimation. The molecular masses of proteins were determined as described by Hames (12). For all of the proteins, the relative mobility (Rr) was measured with reference to a tracking dye. Using linear regression analysis, a standard curve of the molecular mass versus the Rr values of the standard marker proteins was plotted. Molecular masses of the unknown proteins were estimated from the standard curve.
RESULTS
Immunoprecipitation of ICRC and M. leprae antigens with M. leprae-specific MAb. An autoradiogram of 125I-labeled M. leprae sonicate, separated on the gradient SDS-PAGE, revealed that proteins of molecular mass 63, 57, 36, 23, 21 and 14 kDa were radiolabeled (Fig. 1B, Lane a). Of these, the 14-kDa protein was intensely labeled. As seen in Figure 1B, Lanes b-d, MAb WML06 immunoprecipitated one protein of M. leprae of apparent molecular mass of 14-kDa at all three different dilutions tested. Although this MAb is known to recognize the 12-kDa protein of M. leprae, in our hands it showed reactivity with the 14-kDa protein of M. leprae as calculated from the Rr values.
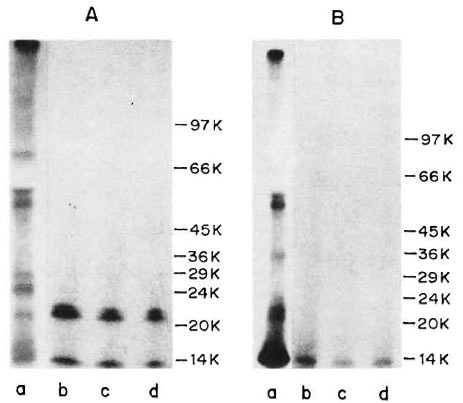
Fig. 1. Immunoprecipitation profiles of 125I-labeled ICRC (A) and M. leprae(B) antigens with MAb WML06 at various dilutions (Lane b = 1:100; Lane c = 1:500; Lane d = 1:1000). Lane a in each panel represents autoradiogram of 125M-labeled sonicate. Molecular mass markers (phosphorylase b, 97.4 kDa; bovine serum albumin. 66 kDa; egg albumin, 45 kDa; glyceraldehyde-3-phosphate dehydrogenase, 36 kDa; carbonic anhydrase, 29 kDa; phenylmethylsulfonyl fluoride-treated tripsinogen, 24 kDa; soybean trypsin inhibitor, 20.1 kDa; ot-lactalbumin, 14.2 kDa) are shown on right of each panel.
On radioiodination, the ICRC sonicate exhibited protein bands in the molecular mass range of 81, 60, 55, 29, 25, 21 and 14 kDa (Fig. 1A, Lane a). When 125I-labeled ICRC antigens were immunoprccipitated with M. leprae-specific MAb WML06, the MAb recognized the ICRC proteins of molecular mass 21 kDa and 14 kDa at all the dilutions tested (Fig. 1A, Lanes b-d).
M. leprae-specific MAb WML03 and WML04, known to react with the 35-kDa protein of M. leprae (14), showed intense reactivity with the 35-kDa protein of M. leprae and also demonstrated crossreactivity to M. leprae protein of 14 kDa to some extent (Fig. 2, Lanes b and d). When the same MAb (WML03 and WML04) were tested for their reactivity with l25I-labeled ICRC antigens, it was observed that these antibodies were unable to precipitate any ICRC antigen, (Fig. 2, Lanes a and c).
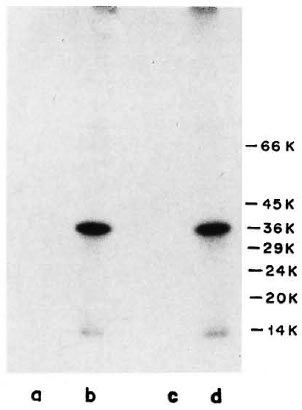
Fig. 2. Immunoprccipitation profiles of 125I-labeled ICRC (Lanes a and c) and M. leprae (Lanes b and d) with MAb WML03 (Lanes a and b) and MAb WML04 (Lanes c and d) at 1:100 dilution. Molecular mass markers are shown on right.
Immunoprecipitation of ICRC and M. leprae antigens with anti-ICRC and anti-M. leprae polvelonal antibodies. Immunoprccipitation "of l25I-labeled ICRC and M. leprae sonicates with anti-ICRC and anti-M. leprae polyclonal antibodies showed the presence of crossreactive antigens on these mycobacteria. As seen in Figure 3, Lane a, anti-ICRC polyclonal antibodies identified ICRC proteins of 66, 42, 29 and 21 kDa. Of these, the 21 -kDa ICRC protein was precipitated by anti-ICRC polyclonal antibodies with very high intensity. When the same serum was tested with 125I-labeled M. leprae antigens, it was observed that only two proteins of molecular mass 21 kDa and 14 kDa were strongly immunoprecipitated (Fig. 3, Lane b).
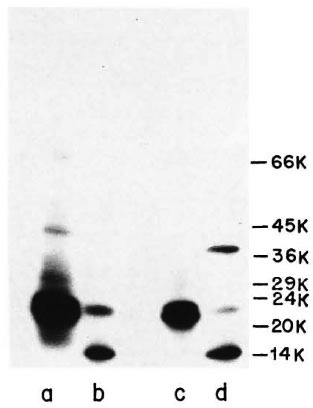
Fig. 3. Immunoprecipitation profiles of 125I-labeled ICRC (Lanes a and c) and M. leprae (Lanes b and d) with polyclonal anti-ICRC (Lanes a and b) and anti-M. leprae (Lanes c and d) antibodies at 1:100 dilution. Molecular mass markers are shown on right.
A similar pattern of reactivity was observed when 125I-labeled ICRC and M. leprae were tested with anti-M. leprae polyclonal antibodies (Fig. 3, Lanes c and d). Three major proteins of M. leprae Of molecular mass 36, 21 and 14 kDa were identified by anti-M. leprae polyclonal antibodies, of which the 14-kDa protein showed intense reactivity (Fig. 3, Lane d). The crossreactivity between the antigens of ICRC and M. leprae was confirmed further when anti- M. leprae polyclonal antibodies immunoprecipitated the 21-kDa ICRC protein with an intensity comparable to that observed with anti-ICRC polyclonal antibodies (Fig. 3, Lanes a and c).
DISCUSSION
The importance of studying antigens present on cultivable mycobacteria has emerged from studies by several workers (1, 18). They suggest that protective antigens of M. leprae are those that are shared by mycobacterial species rather than speciesspecific antigens.
In the present investigations, we have analyzed antigens present on a cultivable mycobacterium, ICRC, used as an antileprosy vaccine in India. Our earlier studies using crossed-immunoelectrophoresis have demonstrated antigenic relatedness between ICRC and M. leprae (3). The present data confirm and extend these findings, using a more-sensitive technique and specific probes such as anti-ICRC and anti-M. leprae polyclonal antibodies and M. leprae-specific monoclonal antibodies.
Our results demonstrate that the polyclonal anti-ICRC sera immunoprecipitated ICRC antigens of 66, 42, 29 and 21 kDa. Of these, the 21-kDa protein of ICRC was precipitated with very high intensity. Anti-M. leprae polyclonal antibodies also precipitated this antigen with equal intensity, thereby establishing the immunodominance of the 21-kDa ICRC protein and the presence of crossreactive epitopes on this protein. Exclusive reactivity of the 21-kDa protein of ICRC with sera of LL patients and LL patients in ENL reaction has been reported earlier (2). In the present study, we observed that MAb WML06, known to recognize the 12-kDa protein of M. leprae (12), immunoprecipitated the 14-kDa protein of M. leprae when tested in our laboratory using a 5%-20% gradient SDS-PAGE. The difference in the molecular mass of the protein recognized by MAb WML06 could be due to personal variations in experiments performed. We wish to emphasize that the 12-kDa protein of M. leprae reported by others and the 14-kDa protein of M. leprae identified by us are identical. We observed that the M. leprae-specific MAb WML06 reacted with the 21-kDa and 14-kDa proteins of ICRC. We have earlier shown that the 14-kDa protein of ICRC was identified by sera from all contacts of leprosy patients (2).
Crossreactivity of M. leprae-specific MAb with other cultivable mycobacteria such as M. habana (16) and Mycobacterium w(9) has also been reported. Lamb, et al. (16) demonstrated that the 18-kDa antigen of M. habana shares crossreactive epitopes with a M. leprae protein of a similar mass when analyzed with M. leprae-specific MAb L5. Ganju, et al. (9) demonstrated that M. leprae-specific MAb WML06 reacted with the 10-, 23- and 35-kDa proteins of Mycobacterium iv when analyzed using Western blotting.
We observed that anti-M. leprae polyclonal antibodies immuno-precipitated the 36-, 21-, and 14-kDa antigens of M. leprae. Of these, the 21-kDa and 14-kDa proteins of M. leprae also were recognized by polyclonal anti-ICRC antibodies. These data again indicate that the 21-kDa and 14-kDa proteins of M. leprae share crossreactive epitopes with antigens of ICRC.
Another interesting observation was that ICRC antigens could not be immunoprecipitated with other M. leprae-specific MAb, such as WML03 and WML04 which bind to an epitope expressed by the 35-kDa protein of M. leprae and have similar paratope specificities. On the other hand, we observed that MAb WML06, which recognizes the 14-kDa protein of M. leprae, showed reactivity with the 21-and 14-kDa proteins of ICRC. These results indicate that the epitopes present on the 21-and 14-kDa proteins of ICRC are shared only with the 14kDa protein of M. leprae and not with the 35-kDa protein of M. leprae.
Ottenhoff, et al. (19) have reported that the 12-kDa and 36-kDa proteins of M. leprae, recognized by MAb WML06 and WML03, respectively, were able to stimulate T cells of TT patients. Using SDS-PAGE separated nitrocellulose-blotted antigens of ICRC, we were able to demonstrate that the 21-kDa and 14-kDa proteins of ICRC stimulated T-cell responses in tuberculoid (TT) leprosy patients (unpublished observations), indicating that these proteins may harbor T-cell epitopes.
In conclusion, the present studies provide an impetus to explore further T- and B-cell epitopes present on the 21 -kDa and 14-kDa antigens of ICRC. It would be interesting to dissect the T-cell reactivity patterns of these proteins using lymphocytes from patients at the polar ends of the leprosy spectrum. We believe that the epitope mapping and sequencing of the 21-kDa and 14-kDa proteins of ICRC would reveal important immunogenic domains of these proteins that may be useful for designing diagnostic reagents and, possibly, for immunotherapy.
Acknowledgment. This work was supported by the Indian Council of Medical Research, New Delhi, India, under the project Immunoprophylaxis Trials of ICRC Anti-Leprosy Vaccine and by the European Economic Community, Brussels, Belgium, under the project Immunology and Immunoprophylaxis of Leprosy. Jyoti L. Kudalkar received financial support from the Council of Scientific and Industrial Research, New Delhi, India.
REFERENCES
1. BRITTON, W. J., HELI.QVIST, L., GARSIA, R. J. and BASTEN, A. Dominant cell wall proteins of Mycobacterium leprae recognized by monoclonal antibodies. Clin. Exp. Immunol. 67(1987)31-42.
2. CHIPLUNKAR, S. V., KUDALKAR, J. L., BUTLIN, R., SAMSON, P. D., DEO, M. G. and GANGAL, S. G. Major proteins of mycobacterial strain ICRC and Mycobacterium leprae identified by antibodies in sera from leprosy patients and their contacts. J. Clin. Microbiol. 30(1992)336-341.
3. CHIRMULE, N. B., MULHERKAR, R. and DEO, M. G. Antigenic profile of ICRC bacilli with special reference to isolation of immunogenic subunit. Int. Arch. Allergy Appl. Immunol. 86(1988)19-27.
4. CLARK-CURTISS, J. E., JACOBS, W. R., DOCHERTY, M.A., RITCHIE, L. R. and CURTISS, R., III. Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J. Bacteriol. 161(1985)1093-1 102.
5. CLARK-CURTISS, J. E., THOLE, J. E. R., SATHISH, M., BOSECKER, B. A., SELA, S., DECARVALHO, E. F. and ESSER, R. E. Protein antigens of Mycobacterium leprae. Res. Microbiol. 141(1990)859-871.
6. DAS, P. K., RAMBUKKANA, A., BASS, J. G., GROOT-HUIS, D. G. and HALPERIN, M. Enzyme-linked immunosorbent assay for distinguishing serological responses oflepromatousand tuberculoid leprosies to the 29/33 kilodalton doublet and 64 kD antigens of Mycobacterium tuberculosis. J. Clin. Microbiol. 28(1990)379-382.
7. DEO, M. G., BAPAT, C. V., BHALERAO, V., CHATURVEDI, R. M., CHULAWALA, R. G. and BHATKI, W. S. Antileprosy potential of the ICRC vaccine; a study in patients and healthy volunteers. Int. J. Lepr. 51(1983)540-549.
8. EMMRICH, F. and KAUFMANN, S. H. E. Human T-cell clones with reactivity to Mycobacterium leprae as tools for the characterization of potential vaccines against leprosy. Infect. Immun. 51(1986)879-883.
9. GANJU, L., MUKHERJEE, R., BATRA, H. V. and TALWAR, G. P. Immunoblot analysis of antigens of Mycobacterium w. a candidate anti-leprosy vaccine using monoclonal antibodies and patient sera. Int. J. Med. Microbiol. 273(1990)378-385.
10. GARSIA, R. J., HELLQVIST, L., BOOTH, R. J., RADFORD, A. J., BRITTON, W. J., ASTBURY, L., TRENT, R. J. and B ASTEN, A. Homology of the 70-kilo dalton antigens of Mycobacterium leprae and Mycobacterium bovis with the Mycobacterium tuberculosis 71-kilodalton antigen and with the conserved heat shock protein 70 of eucaryotes. Infect. Immun. 57(1989)204-212.
11. GILLIS, T. P. and BUCHANAN, T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect. Immun. 37(1982)172-178.
12. HAMES, B. D. An introduction to Polyacrylamide gel electrophoresis. In: Gel Electrophoresis of Proteins. 4th edn. Hames, B. D. and Rickwood, D., eds. Oxford: IRL Press, 1985, pp. 1-91.
13. HARBOE, M., CLOSS, O., BJORVATN, B., KRONVALL, G. and AXELSEN, N. H. Antibody response in rabbits to immunization with Mycobacterium leprae. Infect. Immun. 18(1977)792-805.
14. IVANYI, J. S., SINHA, S., ASTON, R., CUSSELL, D., KEEN, M. and SENGUPTA, U. Definition of species-specific and cross-reactive antigenic determinants of Mycobacterium leprae using monoclonal antibodies. Clin. Exp. Immunol. 52(1983)528-536.
15. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227(1990)680-685.
16. LAMB, F. I., S INGH, N. B. and COLSTON, M. J. The specific 18-kilodaltons antigen of Mycobacteriumleprae is present in Mycobacterium habana and functions as a heat-shock protein. J. Immunol. 144(1990)1922-1925.
17. LOWRY, O. H., ROSEBROUGH, N. J., F ARR, A. L. and RANDALL, R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193(1951)265-275.
18. MOHAGHEGHPOUR, N, M UNN, M. W., G ELBER, R. H. and ENGLEMAN, E. G. Identification of an immunostimulating protein from Mycobacterium leprae. Infect. Immun. 58(1990)703-710.
19. OTTENHOFF, T. H. M., KLATSER, P. R., IVANYI, J., ELFERINK, D. G., DEWIT, M. Y. L. and DEVRIES, R. R. P. Mycobacterium feprae-specific protein antigens defined by cloned human helper T cells. Nature (London) 319(1986)66-68.
20. YOUNG, R. A., MEHRA, V., SWEESTER, D., BUCHANAN, T., CLARK-CURTISS, J., DAVIS, R. W. and BLOOM, B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature (London) 316(1985)450-452.
1. Ph.D.; Immunology Division, Cancer Research Institute, Tata Memorial Center, Parol, Bombay 400012, India.
2. Ph.D.; Immunology Division, Cancer Research Institute, Tata Memorial Center, Parol, Bombay 400012, India.
3. M.D., Ph.D., Immunology Division, Cancer Research Institute, Tata Memorial Center, Parol, Bombay 400012, India.
Received for publication on 9 December 1992.
Accepted for publication in revised form on 24 May 1993.