- Volume 61 , Number 3
- Page: 439–54
Cell-mediated immunity in leprosy; an update
Editorial opinions expressed are those of the writers.
A large body of evidence suggests that in lepromatous leprosy (LL) there is a selective unresponsiveness (anergy) of the T-cell response to Mycobacterium leprae antigens and, therefore, the host is unable to mount an adequate cell-mediated immunity (CMI) which could protect the host from the infection. In this form of the disease macrophages, primarily in the peripheral nerves, skin and mucous membranes, get heavily infiltrated with the bacilli.
The disease covers wide intermediary forms of clinical manifestations with two polar types, tuberculoid and lepromatous. An accepted classification based on clinical, histological and immunological parameters which is universally followed has been described elsewhere.1 The present review will deal mainly with the various types of host cells and their products (effector molecules) which govern CMI and will try to point out the basic immunological defects that may be seen in leprosy.
CELLULAR INTERACTIONS IN CMI
The phagocytic cells (macrophages, Langerhans' cells, dendritic cells) are known to engulf and process the invading bacilli and their soluble products. Also designated as antigen-presenting cells (APC), they are capable of presentation of the processed antigens to the T cells through their receptors.2 It has been well documented that at the initial stages of the CMI response T cells cluster around the surface of the APCs before transforming into blast cells. Thereafter, production of interleukin-2 (IL-2) along with the expression of IL-2 receptors are necessary for the replication of these antigen-specific lymphocytes for clonal expansion.1 While such clonal expansion goes on, the cellular interaction further liberates a variety of other interleukins (IL-1, IL-3, IL-4 to IL-8) and lymphokines [granulocyte monocyte colony stimulating factors, interferon-gamma (IFN-γ) tumor necrosis factor-alpha (TNF-α)] which influence the morphological and functional behavior of various CMI-inducing cells. In general, all of these cells -consisting of activated mononuclear phagocytes, cytotoxic T cells, natural killer (NK) cells and lymphokine activated killer (LAK) cells-create the environment of CMI. Effector molecules such as IFN-γ and TNF-α are known to activate macrophages so that they have an enhanced production of toxic reactive oxygen intermediates (ROI), superoxide anions,4 and also reactive nitrogen intermediates (RNI)5 in order to more effectively kill both intracellular and extracellular microorganisms. Further, IL-2 and IL-6 influence T, NK and LAK cells to become cytolytic by increasing their perforin (pore forming protein) content and leukolexin.6 These interleukins are also known to modify the functions of other cells, such as endothelial cells, keratinocytes, and Langerhans' cells. All of these factors collectively influence the cellular functions in situ and also further recruit appropriate cells from the circulation, ultimately leading to the formation of an immune granuloma for the destruction of the invading microorganism.
CMI IN LEPROSY
Generalized defect
Over two decades ago, researchers often reported on a generalized defect in CMI responses in lepromatous leprosy. A majority of lepromatous leprosy patients from different geographic origins and belonging to different ethnic groups have been shown to have lowered responses to intradermal skin reactions to a variety of reagents, allergic skin sensitization to a hapten (7, 10 14-17, 19) and homograft rejection.2 0 In addition to the above, a lowered response to antigen-21-25 or mitogen-7, 9, 22-34 induced lymphocyte transformation, reductions in the number of T lymphocytes in peripheral blood,33-38 and lowered production of lymphokine9, 16 have been noted. However, from a few of the above studies it also can be noted that within a group showing an overall generalized depression in CMI many of the individuals actually did not exhibit any depression in CMI at all.16, 19 Further, in many studies the authors failed to observe any depression in general CMI in the entire lepromatous leprosy population, although these patients remained unresponsive to M. leprae antigen. 9, 10, 31, 37 In those lepromatous patients who did show some degree of depression in generalized CMI, their generalized CMI improved considerably after antileprosy treatment,15, 30 but they all still showed unresponsiveness to the lepromin reaction.38,3 9 In a recent study, while conducting skin reactions against new tuberculin and leprosin in untreated lepromatous leprosy cases, all showed a very strong tuberculin reaction, equivalent to those of controls, while being negative to lepromin.40 This indicates that the state of unresponsiveness in lepromatous leprosy cases is specific to M. leprae. They are able to respond to other mycobacterial antigens but are not able to respond to M. leprae.
The mechanism for the generalized depression in CMI, if any, in lepromatous leprosy is largely unknown. Recently, Muthukkaruppan, et al.,41, 42 using a pan T-cell marker (OKT3), found normal T-cell levels in the peripheral blood of lepromatous leprosy patients. This observation along with the recent observation of normal CD4/CD8 ratios in the peripheral blood of lepromatous leprosy patients43 strengthens the view that generalized CMI remains almost unimpaired in lepromatous leprosy. However, reports of the association of other diseases with leprosy are very difficult to interpret. The high incidences of tuberculosis,44 basal cell carcinoma,45 and lymphoma44 among lepromatous leprosy patients are very rare and may be related to some local environmental factors. Generally, whenever there is a significant generalized depression in CMI an opportunistic infection occurs, such as is being observed in HIV-1- and HIV-2-infected individuals.47-49
SPECIFIC DEFECT
The immunological basis for understanding the disease led to the establishment of a polar concept1 in leprosy. Further, the finer classification of the disease spectrum also has taken into account the CMI response of the host to M. leprae.50
CMI in leprosy always has been determined using either whole or soluble antigens of M. leprae in vivo or in vitro.
In vivo use of M. leprae. M. leprae suspensions designated as lepromin51 or soluble M. leprae antigen52,53 have been used to determine the CMI status of patients classified by the Ridley-Jopling scale.1
The lepromin reaction is an immunological skin test for leprosy first reported by Mitsuda.54 This preparation -termed Mitsuda antigen -now a whole bacillary suspension (40 million/ml) in normal saline, when injected intradermally, evokes a weak 24-48-hour skin reaction and a strong 3- 6-week skin reaction in sensitized individuals. Later, Dharmendra55 reported on a lepromin preparation consisting of a defatted bacillary suspension which recently has been standardized.56 This antigen evokes both the 24-48-hour and 3-4-week skin reactions equally well. More recently, after the successful purification of M. leprae from infected armadillo tissue,57 it was possible to sonicate a large number of bacilli, and the cell-free extract of M. leprae could be standardized by its protein content (10 µg/ml).52 This soluble antigen evokes only a 24-48-hour skin reaction.
Although a lepromin test has no diagnostic potential,51 it has considerable prognostic value1 and provides confirmatory evidence for classification of the disease. In most of the TT/BT cases the test is strongly positive, while in BL/LL cases it is negative. The negative reaction in BL/LL leprosy tends to become positive after a reversal reaction. On the other hand, the positive reaction in BT leprosy tends to become negative before the occurrence of a downgrading reaction. However, such changes do not take place in polar TT or polar LL cases.
It has been formally accepted that the 2448-hour skin reaction is an expression of preexisting delayed-type hypersensitivity (DTH) to the protein antigen(s) of M. leprae. In fact, two kinds of soluble proteins were isolated from leprosy nodules by Abe.58 However, these proteins did not induce a Mitsuda reaction. For the generation of a 3-4-weck skin reaction, the presence of whole, intact bacilli in the skin-test antigen is essential.5 9
Use of M. leprae antigen in vitro. As early as 1973, Godal and Negassi,60 while studying the lymphoproliferative response to M. leprae antigen in different categories of people, noted that individuals who were not in contact with the disease generally remained unresponsive. On the other hand, 80% of the people who were exposed to leprosy for more than 1 year responded to the antigen. This showed the sensitization of normal individuals who were subclinically infected due to their contact with patients. Myrvang, et al.61 studied the lymphoblastic response of patients along the Ridley-Jopling scale,1 and noted a gradual fall in the lymphoproliferative response from TT through LL. Various in vivo and in vitro studies carried out earlier showed that lepromatous patients exhibit a long-lasting anergy to M. leprae antigens. This has been extensively reviewed elsewhere.62, 63
Scientists have been in doubt in deciding if the lymphoproliferative response to a specific antigen is an in vivo correlate of CMI or, rather, whether it may be measuring hypersensitivity which is not a measure of resistance at all.64-6 5 Experimental proof66 in mice is available. A strain of mice susceptible to M. lepraemurium were challenged with different doses of the pathogen and infection (at a certain dose) together with a strong skin DTH reaction against the same pathogen was shown. Moreover, human lymphocyte responses to antigen have been found to vary on several occasions due to the presence of suppressive factors in the plasma of leprosy mothers67 and dapsonc in dapsone-treated patients.68 Later on it also was pointed out that variability in the lymphoproliferation and leukocyte migration inhibition could be due to the heterogeneous nature of the antigens used in these assays.64, 69, 70
A significant number of reactional tuberculoid (BT) patients (type 1 reaction) show lowered M. leprae antigen-induced lymphoproliferative response, suppressor cell generation, and lymphokinc production.71 On the other hand, the lymphocyte responses show much improvement in type 1 reaction in BB and BL cases.70 Similarly, LL patients with erythema nodosum leprosum (ENL; type 2 reaction) generally show heightened T-cell reactivity to M. leprae antigen.38, 72
STATUS OF T-CELL SUBSETS AND IN SITU PROFILE OF ACCESSORY CELLS AND CYTOKINES IN PERIPHERAL BLOOD
Bach, et al.39 and Wallach, et al.,13 while studying the pattern of distribution of T-cell subsets, noted mostly a reduction in the ratio of helper/inducer (OKT4+ or CD4 + ) and suppressor/cytotoxic (OKT8+ or CD8 + ) cells in bacilliferous leprosy patients. On the other hand, the bacillary-negative LL patients showed CD4 + and CD8 + cell numbers equivalent to those of controls. An increase in the CD4+/CD8+ ratio was also noted in ENL patients, with the CD4 + / CD8+ ratio returning to normal after subsidence of the reaction. In 1982, Mshana, et al.43 saw no change in the percentage of pan T (OKT3+ or CD3 + ) cells in tuberculoid, untreated LL, ENL patients, and controls. Similar to the above, however, there was a reduced percentage of OKT4 + cells and an increased percentage of OKT8 + cells, resulting in a reduced OKT4 + / OKT8+ ratio in untreated LL. During ENL reactions the helper/suppressor ratio increased due to an increase in the helper and a corresponding decrease in the suppressor cell percentages. However, in the same year Van Voorhis, et al.74 reported an unaltered helper/suppressor cell population in all of the disease types.
All these above studies gave an account of the percent population of the T-cell subsets rather than their absolute values in blood. While enumerating the absolute values of these subsets in a mixture of treated and untreated lepromatous patients, Bullock, et al.75 noted a reduction in the total number of T cells and their helper and suppressor subsets. However, like previous workers, they also failed to record any change in the helper-suppressor ratio. Rea, et al.76 using the same yardsticks observed a significant cytopenia of pan T, helper, and suppressor cells when these values were compared to those of normal individuals. However, when the results were expressed as a percentage of total lymphocyte's and again compared, the differences in the groups were abolished. Even when the values of the above parameters of ENL patients were compared with their control values, no significant differences were observed.
From the above-mentioned studies, it becomes clear that when these researchers noted a reduction in CD4+ cells along with an increase in CD8+ cells, it simply indicated a state of immunosuppression.
On the other hand, workers who enumerated the absolute number of these cells could find pan-T-cell cytopenia but failed to find any abnormalities in the CD4 + / CD8+ ratio. Considering that lepromatous patients are not prone to get other infections which are known to affect immunosuppressed individuals, 77-8 2 it is more rational to assume that in lepromatous leprosy there is no biologically significant alteration in the CD4 +/CD8 + ratio in the peripheral blood.
IN LESIONS
The in situ distribution of T-cell subsets with respect to their proportion and pattern of distribution in the tissues has been widely studied74,83-86 in the granuloma itself. Van Voorhis, et al.14 noted large numbers of T cells in leprosy lesions and found the helper/ suppressor ratio to be 5.6:1 in the tuberculoid granuloma; whereas in the lepromatous lesion they noted this ratio to be 1:1.8. In addition, they observed clusters of OK.T4+ cells distributed in the form of rings in the tuberculoid granuloma. OKT6+ cells, on the other hand, remained scattered in both tuberculoid and lepromatous granuloma. In contrast to the above, Narayanan, et al.87 and Modlin, et al.86 could not account for such large numbers of T cells in the granuloma, and the helper/suppressor ratio ranged from 1.2 to 5.0 in tuberculoid and from 0.2 to 1 in lepromatous lesions.87 Moreover, Narayanan, et al.,87 unlike Van Voorhies, et al.,14 found that in tuberculoid granulomas (concentric variety) the OK.T8 + cells were scattered in a "ring-like" fashion among the lymphocytes. Conversely, OKT4+ cells showed a scattered distribution, either singly or in clusters, within the lymphocyte cuff as well as in close association with epithelioid cell aggregates. Similarly, Modlin, et al. 85 found lymphocytes expressing CD8 + cells predominantly in the mantle, while CD4+ cells were seen in large numbers within the epithelioid cell aggregates. This type of histological distribution of T-cell subsets also has been noted in the tuberculoid granuloma of sarcoidosis,85 tuberculosis,85 and DTH skin reactions.88-91 In addition, all of these studies showed un equivocally that all lymphocytes and macrophages of leprosy granulomas are la positive.
Recently, Narayanan, et al. isolated the granuloma-infiltrated cells in vitro and studied their characteristics92-9 4 and functions.95 The OKT4+/OKT8+ ratios were found to be similar to those described above in in situ situations. Functionally, the immune cells obtained from tuberculoid granulomas exhibited a high incorporation of 3H-thy midine and 14C-leucine. On the other hand, cells from lepromatous granulomas showed poor division without any impairment in their protein synthesis. A similar study96 was carried out to compare the characteristics of infiltrates in the skin and nerve granulomas of tuberculoid and lepromatous cases. Using phenotypic markers, CD4+ and CD8+ cells of nerve showed similar distributions and proportions as noted with those of skin granulomas. Moreover, in tuberculoid granulomas a higher proportion of lymphocytes of both skin and nerve were activated T cells as compared to those in the lepromatous granulomas. In both types, the granulomas were populated with macrophages which expressed HLA-DR (la) antigen. With regard to the helper/suppressor ratio in lesions of both type 1 and type 2 reactional cases, it was noted that the ratio was more than 2 in lepromatous granulomas due to the increase in the number of OKT4+ cells in the cells of the lesions.87 It was shown further that in type 1 reaction in BT leprosy, there is a decrease in the mean value of the helper/suppressor ratio when compared to that of nonreactional, untreated leprosy.
Accessory cells (Langerhans' cells, keratinocytes) other than macrophages are known to play a pivotal role in the presentation of antigens to T cells, and they also have been shown to be associated with allergic contact sensitivity and hypersensitivity reactions.97-10 0 Using phenotypic markers for T6 and la-like antigens, Langerhans' cells have been identified in leprosy lesions. While adequate numbers of these cells were noted in polar tuberculoid leprosy, the cells were virtually absent in polar lepromatous leprosy. However, la-like antigens also were found to be associated with macrophages in these lesions.101 Recent observations102,103 revealed that during type 1 and type 2 reactions there is a significant increase in Langerhans' cells in these lesions. In addition, la also was seen in all keratinocytes in type 1 reaction; whereas in ENL patients a patchy distribution of these la-positive keratinocytes was noticed. All of these events are indicative of a temporary T-cell reactivity in the lesions during manifestation of a reactional phase. Further, Cooper, et al.,104 using mRNA probes for IFN-γ in in situ hybridization in reversal reaction biopsy specimens, have elegantly shown that there was a 10-fold rise in IFN-γ -containing cells as compared to those observed in lepromatous patients who were not in reaction. When a probe for the human gene esterase (huHF), a marker for cytotoxic T cells, was used, it was noted that expression of huHF serine esterase was four times more in the reversal reaction and tuberculoid lesions than in lepromatous lesions. Their study further confirmed a selective increase of CD4+ and CD8+ cells, indicating a rise in the DTH response which may help in killing bacilli but which may result in tissue damage. On the other hand, finding a reduction in the expression of IFN-γ and human serine esterase in an atmosphere of a CD4 + rise and transient fall in CD8+ cells suggested a partial or transient boost in the CM I which may be sufficient for antibody production without any effect on bacillary clearance.
Very recently the functional parameter for cytokine production for the locally proliferated immune cells has been worked out very extensively in the tuberculoid and lepromatous groups. It was noted that while mRNAs encoding for IL-2 and IFN-γ were most evident in the tuberculoid granulomas, in lepromatous granulomas mRNAs for IL-4, IL-5 and IL-10 were noted predominantly.105 Although definite cytokine profiles have been found to be associated with resistant and susceptible types of leprosy, no definite conclusion could be made toward the role of these lymphokines in the etiopathology of such lesions in leprosy.
UNDERSTANDING THE MECHANISM F UNRESPONSIVENESS
Macrophage/antigen presenting cell
It is known that M. leprae are obligatory parasites that reside inside the macrophages/Schwann cells and produce a granuloma in the host. The earliest claim106 that macrophages from lepromatous patients are incapable of killing M. leprae in vitro was contradicted by others.107,108
While studying the capability of M. leprae antigen presentation by the macrophages of lepromatous leprosy patients, Hirschberg109 pointed out that, due to the defect in the macrophage presentation of antigen, lepromatous leprosy patients are unable to respond to M. leprae. Nath, et al.110 further confirmed this observation in HLA-Dmatched, peripheral blood leukocyte coculture experiments. Macrophages from lepromatous patients inhibited the lymphoproliferation of the M. leprae-responding individuals. They further showed that lymphocytes of lepromatous patients are capable of undergoing proliferation when macrophages from tuberculoid patients are added to the culture, thereby indicating that antigen-reactive T cells are present in the peripheral blood of lepromatous leprosy patients. Conversely, a study111 in HLA-Didentical siblings in such situations completely negated the role of the monocyte population as mentioned above. Rather, a T-cell defect accounted for the unresponsiveness. However, more data have accumulated from Nath's laboratory indicating that the adherent cells in the peripheral blood of lepromatous individuals having macrophage characteristics induce a suppressive effect on the antigen-induced lymphoproliferation in HLA-D-identical tuberculoid patients and healthy contacts. Further, it was concluded that the suppression induced by these macrophages is due to some soluble factors liberated by these cells.112 In support of the above view, Salgame, et al.113 established that lysates of lepromatous macrophages inhibit protein synthesis of normal macrophages in addition to the inhibition of lymphoproliferation.114 Recently, the soluble factor was characterized and was found to be heat-stable, indomethacin resistant, and of more than 25 kDa115
It has been shown that macrophages, after the phagocytosis of live M. leprae, downregulate their own Fc receptor expression, and biochemical and other functions."6 In addition, macrophages of lepromatous patients have been shown to have a selective depression in 3H-leucine uptake and exhibit a reduction of Fc receptors only when exposed to M. leprae.117 Mahadevan and Antia118 proposed from their experiments that, after the phagocytosis of M. leprae.macrophages lose their capacity to process antigen thereby leading to a CMI defect. At the same time, it has been noted that after the phagocytosis of M. leprae Schwann cells lose their capacity to synthesize DNA and are unable to associate themselves with axons, thereby including a defect in C-fiberfunction. Recently Mahadevan's group119 noted that macrophages from the peripheral blood of both paucibacillary and multibacillary leprosy, but not from healthy individuals, are inefficient in killing phagocytosed M. leprae due to their inability to produce superoxide 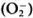 and hydroxyl radicals (OH·).
and hydroxyl radicals (OH·).
Immune responses are controlled partially by soluble factors liberated by the lymphoreticular system. IL-1, liberated by macrophages, acts upon T cells in the early Gl phase of the cell cycle, and prepares the T cells to respond to subsequent signals. An initial report by Horwitz, et al.120 of lowered production of cytokine by macrophages of lepromatous patients has been further confirmed and characterized by Watson, et al.121 while studying the IL-1 production in lipopolysaccharide-stimulated monocytes. They noted that 38.5% of BL/LL patients failed to produce IL-1, while TT/BT patients were able to produce either IL-1 in the normal range spontaneously or upon stimulation. A similar report122 also was established in a M. leprae-stimulated situation.
Tumor necrosis factor-alpha (TNF-α) is known as a secretory product of macrophages123 and activated mononuclear cells of the peripheral blood.124-125 Higher levels of TNF-α have been associated with malaria and kala azar.126 Using a bioassay, higher levels of TNF in serum127 and higher TNF production by antigen-induced mononuclear cells128 have been noted in tuberculoid patients compared to lepromatous patients. There is, however, a report of a higher level of TNF-α in the sera of lepromatous patients129 using an ELISA. This might be due to the presence of inhibitors in the sera of such patients. Such inhibitors might have resulted in relatively low levels of TNF by bioassay but relatively high values in an ELISA. The presence of such inhibitors has been reported in tuberculosis and sarcoidosis.130 Moreover, the presence of a higher number of TNF-containing cells131 and a higher concentration of TN F mRNA by in situ hybridization132 and by PCR amplification105 in tuberculoid skin lesions than in lepromatous lesions have already been reported. A protective role of TNF can be explained by the finding of its inhibitory role in mycobacterial multiplication in murine and human macrophages.133 Moreover, recently TNF has been shown to enhance the production of nitric oxide in mouse macrophages134 which, in turn, have been shown to kill M. leprae.134 Further, the finding of high levels of TNF 128 in active ENL patients could explain the clinical manifestations of fever and nerve damage which have been noted by TNF inoculation in mice135 and by in vitro experiments,136, 137 respectively.
T CELLS
The understanding of a suppressor function of a subpopulation of T cells in mice which regulates the immune response prompted researchers to work on normal and diseased states of human beings. Investigators engaged in leprosy research also generated data on their observations of T-suppressor (OKT8 + ) cells in leprosy. A preliminary study by Bjune,138 which was carried out in an Ethiopian population, indicated that M. leprae antigens generally suppressed the in vitro phytohemagglutinin (PHA)-induced lymphoproliferation in leprosy patients and their household contacts. Working with concanavalin A (ConA)-induced lymphoproliferation in leprosy patients and healthy individuals, Mehra, et al.139 noted that Dharmendra antigen suppressed the ConA-induced proliferation selectively in a majority of lepromatous and borderline patients but not in tuberculoid leprosy patients and healthy individuals. This group further pointed out that the suppression was induced due to the generation of the classical T8 + 140 and TH2 + 141 phenotype markers-bearing suppressor cells. Although the above work sufficiently indicated that suppressor-T cells were responsible for the suppression of CMI in lepromatous leprosy, Stoner, et al.142 could not establish such a role. Rather, they noted a lack of these cells in most of their Ethiopian patients. Further, from their observation on subclinically infected healthy individuals they established that there is an association of suppressor-cell activity with resistance to M. leprae infection.143 To prove the role of suppressor cells in leprosy, Nath, et al.144 have done extensive work in untreated patients from both hyperendemic and low-endemic areas in India. It was noted in a 4-day culture that ConA-induced suppressor cells selectively suppressed the autologous mitogenic responses of tuberculoid patients. Further, this ConA-stimulated lymphoproliferation was suppressed by the addition of M. leprae antigen in a majority of the tuberculoid patients but not in all of the lepromatous patients. On the contrary, many of the lepromatous patients showed an enhancement in the lymphoproliferative response. However, when the cultures were continued for 6 days the differences in suppression in the various groups were abolished and the suppressive effect was noted uniformly in all subjects,145 as noted earlier by Bjune.138 Earlier studies, when T cells bearing Fc receptors for IgG were considered to be a suppressor-T-cell subset, also indicated the presence of normal levels of these cells in tuberculoid patients146 with a reduction in their number in lepromatous patients.147
The above studies on suppressor-cell activity are very divergent in their views. The suppressor-cell activity in the tuberculoid type of leprosy gains support from only one observation wherein the suppressor-cell generation was mostly associated with a strong CMI response148 which might possibly play a role in the suppression of unwanted antibody production.149 On the other hand, with the understanding of murine TH1 /TH2 subsets and their biological function,150, 151 more and more evidence is being accumulated to understand how TH 1 lymphocyte proliferation along with IFN-γ secretion are associated with the acute stage of the disease whereas TH2 lymphocyte proliferation with an increase in IL-4 and IL-10 is associated with chronic disease.152-154 Recently, Salgame, et al.155 elegantly categorized these functional subsets of T cells in leprosy. They established that CD4+ cells cloned from tuberculoid patients produced IFN-γ whereas those obtained from lepromatous patients produced more IL-4. In addition CD8+ T-suppressor clones producing IL-4 isolated from lepromatous patients were found to be essential for the suppression of in vitro responses to antigen in these patients. However, more recently the role of contrasuppressor (Cs) cells for antagonizing the suppressor function in leprosy has been reported.156 Cs cells are known to interact with CD4+ cells and render them unresponsive to the signals of CD8+ cells.157 A Cs-like functional activity has been noted also in the CD8+ cells in leprosy.156
T-cell unresponsiveness to M. leprae stimulation in lepromatous leprosy has also been thought to be due to the lack of lymphokine production by the immune cells. 120, 158-161 It has been noted by several workers162-164 that T cells in lepromatous leprosy patients are incapable of specific antigen-stimulated proliferation due to a deficiency of IL-2. However, in spite of an exogenous supply of IL-2 in about one third of the patients, T-cell unresponsiveness could not be corrected.162 All of these studies also indicated that in the majority of lepromatous cases there is no clonal deletion of M. leprae-specific T cells, which was the view taken earlier by Godal, et al.24 This unresponsiveness may be due cither to a lack of the required number of M. leprae reactive T cells being circulated160 or to the presence of monocytes which are liberating suppressive factors165 as has been mentioned earlier. In contrast to the above findings, Mohagheghpour, et al.166 could not find any IL-2 deficiency in lepromatous patients, and they explained that the unresponsiveness was due to the lack of an IL-2 receptor on the T cells. An interesting observation16 7 has recently been made in which CD4+ cells of lepromatous leprosy patients were found to respond to M. leprae stimulation after culturing for 48 hours in medium alone. Further, they noted that the recovery of this T-cell reactivity was blocked by the presence of M. leprae in the preculture medium. These authors reasoned that the unresponsiveness was due to the persistence of antigen which renders the antigen-responsive T cells unresponsive.
Downregulation of the T-cell response, especially by M. leprae modulating the CD2 (E or SRI3C receptor), recently has been postulated by Muthukkaruppan, et al.41-42 Using OKT11 and OKT3 monoclonal antibodies, they showed that bacilliferous lepromatous leprosy patients, while exhibiting low levels of CD2 + cells, show normal levels of CD3+ cells. Further, when M. leprae (Dharmendra lepromin only) were exposed to the suspension of peripheral blood T lymphocytes obtained from normal healthy individuals, CD2+ cells were found to become reduced in number, keeping the CD3+ cell number intact. These observations indicated that M. leprae antigens, by modulating the E-receptor of T cells, might be inducing the suppression. Contrary to the above, Wong, et al.,168 while looking for the expression of CD2+ and CD3+ receptors on lymphocytes in lepromatous skin lesions and peripheral blood, found that virtually all of the CD3 + cells expressed CD2 in both situations.
OTHER FACTORS
Serum/Plasma. From time to time the presence of some unknown factors in the blood plasma/serum of leprosy patients which are capable of inhibiting the in vitro growth of autologous lymphocytes have been reported.28, 30, 169-172 Bullock and Fasal169and Nelson, and others28, 170, 173 described that some lepromatous leprosy sera are lymphocytotoxic, and these sera also suppress the lymphoproliferation induced by PHA. Later Potts, et al.171 and Kerr, et al.172 showed that the inhibition in lymphoproliferation is due to a decrease in the number of cells responding to mitogen. On the other hand, Kerr, et al.172 recently have shown that the putative inhibitory factor(s) is (are) not cytotoxic. The suppression in the proliferative response may be due to an inherent cellular defect or due to the presence of factors in the serum which inhibit lymphocyte activation. They further characterized the inhibitory factor as being resistant to heating to 60ºC for 30 minutes but which becomes denatured at 100ºC.172 A very interesting report174 indicating that lepromatous leprosy sera causes some chromosomal aberrations of normal lymphocytes in culture, leading to a lowering of the mitotic index and thus inhibiting lymphoproliferation, is also available.
Although the presence of such serum/ plasma factors have been reported by different workers in leprosy patients, these factors still need further chemical and structural characterization to substantiate the above findings.
Mycobacterial components. In addition to the isolation of T-cell clones having suppressor functions from lepromatous lesions,175 M. leprae soluble products also have been shown to suppress lymphoproliferation to the antigen in not only lepromatous but also tuberculoid patients.176 It could also be possible that a nonspecific suppressive function by a microbial fraction may be responsible for the suppression as noted by Molloy, et al.177 Holzer, et al.178 noted that M. leprae as a whole fail to stimulate human blood monocytes, neutrophils and murine peritoneal macrophages to generate superoxide anions (respiratory burst). Vachula, et al.179 pointed out the role played by phenolic glycolipid-I (PGL-I) in blocking the respiratory burst of human macrophages. Recently, it also has been hypothesized by Parkash and Sengupta180 that the failure of the oxidative burst exhibited by macrophages which have phagocytosed M. leprae is most probably due to the complementmediated entry of M. leprae into the monocytes. Such a view has already been favored by workers studying various particulate materials including Leishmania major as a pathogen.181-184 With regard to the PGL-I- induced suppressive function by macrophages, further evidence has accumulated from the work of Neill and Klebanoff,186 who showed that both purified PGL-I and its deacylated form abolished the antimicrobial effect by blocking the xanthine oxidase system which is essential for the release of OH·. A blocking effect on the myeloperoxidase-H2O2 halide system also was observed using this lipid.
Another cell-wall-associated lipid of mycobacteria, lipoarabinomannan (LAM), which has been shown to inhibit antigen responsiveness of human peripheral blood leukocytes18 6 and antigen-induced proliferation of CD4+ T-cell clones,187 also has been found to block the activation of macrophages without any change in their capability of phagocytosis.188 The same group of workers have demonstrated further that LAM is able to block the activation of mouse macrophages induced by IFN-γ. 189
M. leprae protein components and the 120-kDa protein antigen of M. leprae have been shown by Sengupta, et al.A0 to suppress the tuberculin-induced DTH response in vivo in leprosy patients. However, such a suppression could not be reproduced by Fine, et al.190 This discrepancy may be due to the differences in the types of leprosy patients used and differences in the genetics of the ethnic groups used in these studies.
All of the above studies proved beyond doubt that there are several M. leprae components which are suppressive for cell-immune functions and these components may be playing major roles in the pathogenesis of leprosy.
Antibodies/Immune complexes. It is known that the presence of M. leprae can lead to host immune responses by the production of antibodies and by the development of CMI against the pathogen. It also is known that there is an inverse relationship between CMI and M. leprae antibody levels in leprosy patients.1,165 It might be expected that if the protective antigens would be capable of eliciting both CMI and antibody in the host, the antibodies would be able to mask the M. leprae antigens expressed on the membranes of antigen-presenting cells and this could result in the lowering of CMI. 191 Further, preliminary evidence suggesting that circulating immune complexes from leprosy patients could suppress the M. leprae-induced lymphocyte proliferation has been provided by Tyagi, et al.192
ANTIGENIC MIMICRY
Since biblical times M. leprae have infected human beings. It is quite possible that the organism during these many years might have adapted within the human host in such a way that it generally does not evoke a strong host immune response. The unresponsiveness of the host could be possible if M. leprae "share" or "mimic" some of their antigens with the host tissues. Such a possibility is not far fetched when it has been reported that human tissues possess proteins which are very similar to a mycobacterial 65-kDa heat-shock protein on the basis of their aminoacid sequences. 193,194 In addition, very recently Naafs, et al.195 have shown that eight monoclonal antibodies against M. leprae determinants (12 kDa, 18 kDa and 65 kDa) reacted with dermal determinants. If these similarities are well founded, then these M. leprae antigens would be recognized as self and, due to the presence of some minor dissimilarities in some sequences from the host proteins, they could evoke an autoimmune reaction. Already established evidence for this hypothesis is present in other diseases such as Group A streptococcal mycocarditis,196 rheumatoid arthritis in M. tuberculosis infection,197myasthenia gravis,198 and neuropathy/cardiomyopathy in Chagas' disease.-198,199
This review on cell-mediated immunity in leprosy is an effort to cover various aspects of studies concerning cellular immunology which have been carried out in order to understand the disease process. It is known that there are genes for immune responses as well as for immune suppression.200 Whether a genetic mechanism is also playing a role in modulating the host immune response has not been fully crystallized. However, a significant association of HLA-DR2 201-206- and HLA-DR3204 molecules with various types of leprosy has been claimed. A detailed review on the genetic correlation with the disease and the role played by HLA-DR antigens in modulating the immune response to M. leprae antigens requires a separate review.
- Utpal Sengupta, M.V.Sc, Ph.D.
Deputy Director
Immunology Laboratory
Central JALMA Institute for Leprosy
Agra 282001, India
Acknowledgment. 1 thank Mr. Chandra Babu and Mr. Anil Chopra for their secretarial help.
1. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr.34(1966)253-273.
2. Steinman, R. M. The dendritic cell system and its role in immunogenicity. Ann. Rev. Immunol. 9(1991)271-276.
3. Smith, K. A. Interleukin-2. Sci. Am. 262(1990)50-57.
4. Murray, H. W., Rubin, R. Y., Carricro, S. M., Harris, A. M. and JafTee, E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs. oxygen-independent activity against intracellular Toxoplasma gondii. J. Immunol. 134(1985)1982-1988.
5. Chan, J., Xing, Y., Magliozzo, R. S. and Bloom, B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175(1992)1111-1122.
6. Rappolee, D. A. and Werb, Z. Secretory products of phagocytes. Curr. Opin. Immunol. 1(1988)47-55.
7. Rea, T. H., Quismorio, F. P., Harding, B., Nies, K. M., Disara, P. J., Levan, N. E. and Frion, G. J. Immunologic responses in patients with lepromatous leprosy. Arch. Dermatol. 112(1976)791-800.
8. Guinto, R. S. Biology of mycobacterioses: skin tests in leprosy. Ann. N.Y. Acad. Sci. 154 (1968) 149-156.
9. Talwar, G. P., Krishnan, A. D., Mehra, V. L., Blum, E. A. and Pearson, J. M. H. Evaluation of cell mediated immune responses in untreated cases of leprosy. Clin. Immunol. 12(1972)195-203.
10. Convit, J., Pinardi, M. E. and Rojas, F. A. Some considerations regarding the immunology of leprosy. Int. J. Lepr. 39(1971)556-564.
11. Fernandez, J. M. M. Leprosy and tuberculosis. Arch. Dermatol. 75(1957)101-106.
12. Guinto, R. S. and Mabalay, M. C. A note on the tuberculin reaction in leprosy. Int. J. Lepr. 30(1962)278-283.
13. Buck, A. A. and Hasenclever, H. F. The influence of leprosy on delayed-type skin reactions and scrum agglutination titres to Candida albicans. Am. J. Hyg. 77(1963)305-316.
14. Waldorf, D. S., Sheagren, J. N., Trautman, J. R. and Lock, J. B. Impaired delayed hypersensitivity in patients with lepromatous leprosy. Lancet 2(1966)773-776.
15. Bullock, W. E. Studies of immune mechanisms in leprosy: I. Depression of delayed allergic responses to skin test antigens. N. Engl. J. Med. 278(1968)298-304.
16. Katz, S. I., De Betz, B. H. and Zaias, N. Production of macrophage inhibitory factor by patients with leprosy. Arch. Dermatol. 103(1971)358-361.
17. Saha, K. and Mittal, M. M. A study of cell-mediated immunity in leprosy: changing trends in the immunological spectrum of the disease. Clin. Exp. Immunol. 8(1971)901-909.
18. Mendes, E., Raphael, A. and Mota, N. G. S. Cell mediated immunity in leprosy and transfer of delayed hypersensitivity reactions. J. Allerg. Clin. Immunol. 53(1974)223-229.
19. Turk, J. L. and Waters, M. F. R. Cell-mediated immunity in patients with leprosy. Lancet 2(1969)243-246.
20. Han, S: H., Weiser, R. S. and Kau, S. T. Prolonged survival of skin allografts in leprosy patients. Int. J. Lepr. 39(1971)1-6.
21. Sengupta, S. R., Yemul, V. L. and Dhole, T. N. Lymphocyte transformation test in lepromatous leprosy patients and their healthy siblings. Lepr. India 55(1983)261-264.
22. Sheagren, J. N., Block, J. B., Trautman, J. R. and Wolff, S. M. Immunologic reactivity in patients with leprosy. Ann. Intern. Med. 70(1969)295-302.
23. Bullock, W. E. and Fasal, P. Studies of immune mechanisms in leprosy. III. The role of cellular and humoral factors in impairment of the in vitro immune response. J. Immunol. 106(1971)888-899.
24. Godal, T., Myklestad, B., Samuel, D. R. and Myrvang, B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin. Exp. Immunol. 9(1971)821-831.
25. Han, S. H., Weiscr, R. S. and Lin, Y. C. Transformation of leprous lymphocytes by lepromin, tuberculin and phytohaemagglutinin. Int. J. Lepr. 39( 1971 )789-795.
26. Dicrks, R. E. and Shepard, C. C. Effect of phytohemagglutinin and various mycobacterial antigens on lymphocyte cultures from leprosy patients. Proc. Soc. Exp. Biol. Med. 127(1968)391-395.
27. Paradisi, E. R., De Bonaparte, Y. P. and Morganfield, M. C. Blasts in lepromatous leprosy. Lancet 1(1968)308-309.
28. Nelson, D. S., Nelson, M., Thurston, J. M., Waters, M. F. R. and Pearson, J. M. H. Phytohemagglutinin-induced lymphocyte transformation in leprosy. Clin. Exp. Immunol. 9(1971)33-43.
29. Wong, P. C, Chan-Tcoh, C. H., Wu, S. and Kendall, F. H. Transformation of lymphocytes by phytohemagglutinin in leprosy sera. Int. J. Lepr. 39(1971)7-13.
30. Mehra, V. L., Talwar, G. P., Balakrishnan, K. and Bhutani, L. K. Influence of chemotherapy and scrum factors on the mitogenic responses of peripheral leukocytes of leprosy patients to phytohemagglutinin. Clin. Exp. Immunol. 12(1972)205-213.
31. Ulrich, M., De Salas, B. and Convit, J. Lymphocyte transformation with phytomitogens in leprosy. Int. J. Lepr. 40(1972)4-9.
32. Balina, L. M., Fliess, E. L.. Bachmann, A., Cardama, J. E. and Gatti, J. C. Similar alterations of lymphoblastic dedifferentiation in lepromatous leprosy patients and their healthy lepromin-negative consanguineous offspring. Int. J. Lepr. 41(1973)7-13.
33. Dwyer, J. M., Bullock, W. E. and Fields, J. P. Disturbance of the blood T:B lymphocyte ratio in lepromatous leprosy: clinical and immunologic correlations. N. Engl. J. Med. 288(1973)1036-1039.
34. Lim, S. D., Kiszkiss, K. F., Jacobson, R. R., Choi, Y. S. and Good, R. A. Thymus-dependent lymphocytes of peripheral blood in leprosy patients. Infect. Immun. 9(1974)394-399.
35. Mendes, N. F., Kipersztych, S. and Mota, G. S. T and B lymphocytes in patients with lepromatous leprosy. Clin. Exp. Immunol. 16(1974)23-30.
36. Nath, I., Curtis, J., Bhutani, L. K. and Talwar, G. P. Reduction of a subpopulation of T lymphocytes in lepromatous leprosy. Clin. Exp. Immunol. 18(1974)81-87.
37. Faber, W. R., Leiker, D. L., Nevgerman, I. M., Zcijlemaker, W. P. and Schellekens, P. T. A. Lymphocyte transformation test in leprosy: decreased lymphocyte reactivity to Mycobacterium leprae in lepromatous leprosy, with no evidence for a generalized impairment. Infect. Immun. 22(1978)649-656.
38. Nath, I., Curtis, J., Sharma, A. K. and Talwar, G. P. Circulating T-cell numbers and their mitogenic potential in leprosy-correlation with mycobacterial load. Clin. Exp. Immunol. 29(1977)393-400.
39. Bach, M. A., Chatenoud, L., Wallach, D., PhanDinh-Tuy, F. and Cottenot, F. Studies on T cell subsets and functions in leprosy. Clin. Exp. Immunol. 44(1981)491-500.
40. Sengupta, U., Sinha, S., Ramu, G., Lamb, J. and Ivanyi, J. Suppression of delayed hypersensitivity skin reactions to tuberculin by M. leprae antigens in patients with lepromatous and tuberculoid leprosy. Clin. Exp. Immunol. 68(1987)58-64.
41. Muthukkaruppan, V. R. A possible role for E-receptor in immunosuppression. Indian J. Lepr. 58(1986)389-394.
42. Muthukkaruppan. V. R., Chakkalath, H. R. and Malarkannan, S. The classical and alternate pathways of T-cell activation are impaired in leprosy. Immunol. Lett. 19(1988)55-58.
43. Mshana, R. N., Haregewoin, A., Harboc, M. and Belehu, A. Thymus dependent lymphocytes in leprosy. T lymphocyte subpopulations defined by monoclonal antibodies. Int. J. Lepr. 50(1982)291-296.
44. Gray, H. H. and Bancroft, H. Tuberculosis at the United States Public Health Service, Carville, Louisiana. Int. J. Lepr. 20(1952)467-478.
45. Michalany, J. Malignant tumors of the skin among leprosy patients. Int. J. Lepr. 34(1966)274-286.
46. Rodriquez, E., De Bonaparte, Y. P., Morganfield, M. C. and Cabrini, R. L. Malignant lymphomas in leprosy patients: a clinical and histological study. Int. J. Lepr. 36(1968)203-212.
47. Sunderam, G., McDonald, R. J., Maniatis, T., Oleske, J., Kapila, R. and Reichman, L. B. Tuberculosis as a manifestation of the acquired immunodeficiency syndrome (AIDS). JAMA 256(1986)362-366.
48. Kovacs, J. A. and Masur, H. Pneumocystis carimi pneumonia: therapy and prophylaxis. J. Infect. Dis. 158(1988)254-259.
49. Chuck, S. L. and Sande, M. A. Infections with Crvplococcus neoformans in the acquired immunodeficiency syndrome. N. Engl. J. Med. 321(1989)794-799.
50. Rea, T. H. and Levan, N. E. Current concepts in the immunology of leprosy. Arch. Dermatol. 113(1977)345-352.
51. Rees, R. J. W. The significance of lepromin reaction in man. Progr. Allergy 8(1964)224-258.
52. Smelt, A. H. M., Rees, R. J. W. and Liew, F. Y. Induction of delayed hypersensitivity to Mycobacterium leprae in healthy individuals. Clin. Exp. Immunol. 44(1981)501-506.
53. Smelt, A. H. M., Rees, R. J. W. and Liew, F. Y. Failure to induce delayed type hypersensitivity to Mycobacterium leprae in long-term treated Lepromatous leprosy patients. Clin. Exp. Immunol. 44(1981)507-511.
54. Mitsuda, K. On the value of a skin reaction to suspension of leprous nodules. Hifuka Hingoka Zasshi 19(1919)697-708. Reprinted in Int. J. Lepr. 2(1953)347-358.
55. Dharmendra. Studies of the lepromin test (5). The active principle of lepromin is a protein antigen of the bacillus. Lepr. India 13(1941)89-103.
56. Sengupta, U., Ramu, G. and Desikan, K. V. Assessment of Dharmendra antigen II. Standardisation of the antigen. Lepr. India 51(1979)316-322.
57. World Health Organization. Report of second IMMLEP task force meeting, 1-5 December, 1975. Reprinted in Lepr. Rev. 47(1976)313-332.
58. Abe, M. Studies on the antigenic specificity of Mycobacterium leprae. I. Demonstration of soluble antigens in leprosy nodules by immunodiffusion. Int. J. Lepr. 38(1970)113-125.
59. Sinha, S., Sengupta, U., Ramu, G. and Desikan, K. V. Assessment of Dharmendra antigen. III. Comparative study with Mitsuda antigen. Lepr. India 51(1979)323-329.
60. Godal, T. and Nagassi, K. Subclinical infection in leprosy. Br. Med. J. 3(1973)557-559.
61. Myrvang, B., Godal, T., Ridley, D. S., Froland, S. S. and Song, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum ofleprosy. Clin. Exp. Immunol. 14(1973)541-553.
62. Turk, J. L. and Bryceson, A. D. M. Immunological phenomena in leprosy and related diseases. Adv. Immunol. 13(1971)209-266.
63. Godal, T. Immunological aspects of leprosy-present status. Prog. Allergy 25(1978)211-242.
64. Bjune, G., Barnetson, R. St. C, Ridley, D. S. and Kronvall, G. Lymphocyte transformation test in leprosy: correlation of the response with inflammation of lesions. Clin. Exp. Immunol. 25(1976)85-94.
65. Barnetson, R. St. C, Bjune, G., Pearson, J. M. H. and Kronvall, G. Antigenic heterogeneity in patients with reactions in borderline leprosy. Br. Med. J. 4(1975)435-437.
66. Lovik, M. and Closs, O. Repeated delayed-type hypersensitivity reactions against Mycobacterium lepremurium antigens at the infection site do not affect bacillary multiplication in C3H mice. Infect. Immun. 36(1982)768-774.
67. Bjune, G., Duncan, E., Barnetson, R. St. C. and Melsom, R. in vitro modulation of lymphocyte responses to PHA by plasma in mother and baby at the time of birth. Increased lymphocyte responses in babies of mothers with lepromatous leprosy. Clin. Exp. Immunol. 32(1978)517-522.
68. Ghei, S. K., Sengupta, U. and Ramu, G. PHA induced transformation of peripheral blood lymphocytes in leprosy patients. Lepr. India 52 (1980) 223-228.
69. Bjune, G. Comparison of various preparations of Mycobacterium leprae and other mycobacteria by lymphocyte stimulation. Int. J. Lepr. 46(1978)386-393.
70. Bjune, G. Variation of in vitro lymphocyte responses to ,M. leprae antigen in borderline tuberculoid leprosy patients. Int. J. Lepr. 48(1980)30-40.
71. Laal, S., Mishra, R. S. and Nath, I. Type 1 reactions in leprosy-heterogeneity in T-cell functions related to the background leprosy type. Int. J. Lepr. 55(1987)481-496.
72. Laal, S., Bhutani, L. K. and Nath, I. Natural emergence of antigen-reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect. Immun. 50(1985)887-892.
73. Wallach, D., Cottenot, F. and Bach, M. A. Imbalances in T cell subpopulations in lepromatous leprosy. Int. J. Lepr. 50(1982)282-290.
74. Van Voorhis, W. C, Kaplan, G., Sarno, E. N., Horwitz, M. A., Steinman, R. M., Levis, W. R., Nogueira. N.. Hair, L. S.. Gattas, C. R.. Arrick, B. A. and Cohn, Z. A. The cutaneous infiltrates of leprosy; cellular characteristics and the predominant T cell phenotypes. N. Engl. J. Med. 307(1982)1593-1597.
75. Bullock, W. E., Watson, S., Nelson, K. E., Schauf, V., Makonkawkeyoon. S. and Jacobson, R. R. Aberrant immunoregulatory control of B lymphocyte function in lepromatous leprosy. Clin. Exp. Immunol. 49(1982)105-114.
76. Rea, T. H. Suppressor cell activity and phenotypes in the blood or tissues of patients with leprosy. Clin. Exp. Immunol. 54 (1983)298-304.
77. Whelan, W. L.. Kirsch, D. R., Kwon-Chung, K. J., Wahl, S. M. and Smith, P. D. Candida albicans in patients with the acquired immunodeficiency syndrome: absence of a novel or hypervirulent strain. J. Infect. Dis. 162(1990)513-518.
78. Sunderam, G., McDonald, R. J., Maniatis, T., Oleske, J., Kapila, R. and Reichman, L. B. Tuberculosis as a manifestation of the acquired immunodeficiency syndrome (AIDS). JAMA 256(1986)362-366.
79. American Thoracic Society. Mycobacterioscs and the acquired immunodeficiency svndrome. Am. Rev. Respir. Dis. 136(1987)492-496.
80. Denning. D. W., Follansbec, S. E., Scolaro, M., Norris, S., Edelstein. H. and Stevens, D. A. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 324(1991)654-662.
81. Chatterjec, S. N., Fiala. M., Weincr. J.. Stewart. J. A., Stacey, B. and Warmer, N. Primary cytomegalovirus and opportunistic infections: incidence in renal transplant recipients. JAMA 240(1978)2446-2449.
82. Meyers, J. D., Fluornoy, N. and Thomas, E. D. Risk factors for cytomegalovirus infection after human marrow transplantation. J. Infect. Dis. 153(1986)478-488.
83. Modlin, R. L., Hofman, F. M., Taylor, C. R. and Rea, T. H. In situ characterization of T lymphocyte subsets in Leprosy granulomas. (Letter) Int. J. Lepr. 50(1982)361-362.
84. Modlin, R. L.. Hofman, F. M., Taylor, C. R. and Rea, T. H. T lymphocyte subsets in the skin lesions of patients with Leprosy. J. Am. Acad. Dermatol. 8(1983)182-189.
85. Modlin, R. L., Hofman, F. M., Meyer, P. R.. Sharma, O. P., Taylor, C. R. and Rea, T. H. in situ demonstration of T lymphocyte subsets in granulomatous inflammation: leprosy, rhinoscleroma and sarcoidosis. Clin. Exp. Immunol. 51(1983)430-438.
86. Modlin, R. L., Gebhard, J. F., Taylor, C. R. and Rea, T. H. In situ characterisation of T lymphocyte subsets in the reactional states of leprosy. Clin. Exp. Immunol. 53(1983)17-24.
87. Narayanan, R. B., Laal, S., Sharma, A. K., Bhutani, L. K. and Nath, I. Differences in predominant T cell phenotypes and distribution pattern in reactional lesions of tuberculoid and lepromatous leprosy. Clin. Exp. Immunol. 55(1984)623-628.
88. Narayanan, R. B., Ramu, G., Sinha, S., Sengupta. U., Malaviya, G. N. and Desikan, K. V. In situ characterisation of cells in the dermal infiltrates of lepromin reaction using monoclonal antibodies. Indian J. Lepr. 57(1985)265-272.
89. Poulter, L. W.. Seymour, G. J., Dube, O.. Janossy, G. and Panayi, G. Immunohistological analysis of delayed type hypersensitivity in man. Cell. Immunol. 74(1982)358-367.
90. Narayanan, R. B., Ramu, G., Malaviya, G. N., Sinha, S. and Sengupta, U. Immunohistological analysis of skin reaction to MY1 derived from Mycobacterium leprae. Int. J. Lepr. 54(1986)46-51. '" Narayanan, R. B., Ramu, G., Sinha, S., Sengupta,
91. U. and Gupta. C. M. Immunohistologic comparison between armadillo-derived leprosin and standard lepromin skin tests in leprosy patients. Int. Arch. Allergv Appl. Immunol. 82(1987)202-207.
92. Narayanan, R. B., Bhutani, L. K., Sharma, A. K. and Nath, I. T cell subsets in leprosy lesions: in situ characterisation using monoclonal antibodies. Clin. Exp. Immunol. 51(1983)421-429.
93. Narayanan, R. B., Girdhar, B. K., Sengupta, U. and Desikan, K. V. in vitro studies on dermal granulomas of human leprosy-cellular characteristics. Int. J. Lepr. 53(1985)39-44.
94. Narayanan, R. B. Immunopathology of leprosy granulomas; current status: a review. Lepr. Rev. 59(1988)75-82.
95. Narayanan. R. B. and Girdhar, B. K. in vitro studies on dermal leprosy granulomas: assessment of division and protein synthesis of cells. Acta Leprol. 7(1989)13-17.
96. Kumar. V., Narayanan, R. B. and Girdhar, B. K. Comparison of the characteristics of infiltrates in skin and nerve granulomas of leprosy. Acta Leprol. 7(1989)19-24.
97. StingI, G., Katz, S. I., Clement, L., Greene, I. and Shevach, E. M. Immunological functions of la bearing epidermal Langerhans cells. J. Immunol. 121(1978)2005-2013.
98. StingI, G, Tamaki, K. and Katz, S. I. Origin and function of epidermal Langerhans cells. Immunol. Rev. 53(1980)149-174.
99. Silberg, I., Baer, R. L., Rosenthal, S. A., Thorbecke, G. J. and Berezowsky, V. Dermal and intravascular Langerhans cells at sites of passively induced allergic contact sensitivity. Cell. Immunol. 18(1975)435-453.
100. Kaplan, G., Nursat, A., Witmer, M. D., Nath, I. and Cohn, Z. A. Distribution and turnover of Langerhans' cells during delayed immune responses in human skin. J. Exp. Med. 165(1987)763-776.
101. Narayanan, R. B., Bhutani, L. K., Sharma, A. K. and Nath, I. Normal numbers of T6 positive epidermal Langerhans cells across the leprosy spectrum. Lepr. Rev. 55(1984)301-309.
102. Rea, T. H., Shen, J.-Y. and Modlin, R. L. Epidermal keratinocyte la expression, Langerhans cell hyperplasia and lymphocytic infiltration in skin lesions of leprosy. Clin. Exp. Immunol. 65(1986)253-259.
103. Thangaraj, H., Laal, S., Thangaraj, I. and Nath, I. Epidermal changes in reactional leprosy: keratinocyte la expression as an indicator of cell mediated immune responses. Int. J. Lepr. 56(1988)401-407.
104. Cooper, C. L., Meuller, C, Sinchaisri, T.-A., Pirmez, C, Chan, J., Kaplan, G., Young, S. M. M., Weissman, I. L., Bloom, B. R., Rea, T. H. and Modlin, R. L. Analysis of naturally occurring delayed-type hypersensitivity reaction in leprosy by in situ hybridization. J. Exp. Med. 169(1989)1565-1581.
105. Yamamura, M., Uyemura, K., Deans, R. J., Weinberg, K., Rea, T. H., Bloom, B. R. and Modlin, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254 (1991) 277-279.
106. Beiguelman, B. Leprosy and genetics; a review of past research with remarks concerning future investigations. Bull. WHO 37 (1967) 461-476.
107. Godal, T. and Rces, R. J. W. Fate of M. leprae in macrophages of patients with lepromatous or tuberculoid leprosy. Int. J. Lepr. 38(1970)439-441.
108. Samuel, D. R., Godal, T., Myrvang, B. and Song, Y. K. Behaviour of M. leprae in human macrophages in vitro. Infect. Immun. 8(1973)446-449.
109. Hirschberg, H. The role of macrophages in the lymphoproliferative response to M. leprae in vitro. Clin. Exp. Immunol. 34(1978)46-51.
110. Nath, I., Van Rood, J. J., Mehra, N. K. and Vaidya, M. C. Natural suppressor cells in human leprosy: the role of HLA-D-identical peripheral lymphocytes and macrophages in the in vitro modulation of lymphoproliferative responses. Clin. Exp. Immunol. 42(1980)203-210.
111. Stoner, G. L., Mshana, R. N., Touw, J. and Belchu, A. Studies on the defect in CMI in lepromatous leprosy using HLA-D-identical siblings. Absence of circulating suppressor cells and evidence that the defect is in the T-lymphocyte rather than the monocyte population. Scand. J. Immunol. 15(1982)33-48
112. Nath, I. Mechanisms underlying unresponsiveness to M. leprae: the role of suppressor T cells and adherent cells in human leprosy. 6th IMMLEP Scientific Working Group Meeting. Geneva: World Health Organization, 1982.
113. Salgame, P. R., Birdi, T. J., Mahadevan, P. R., and Antia, N. H. Role of macrophages in defective CMI in lepromatous leprosy. I. Factors afiecting protein synthesis and lymphocyte transformation. Int. J. Lepr. 48(1980)171-177.
114. Salgame, P. R., Mahadevan, P. R. and Antia, N. H. Mechanism of immunosuppression in leprosy: Presence of suppressor factor(s) from macrophages of lepromatous patients. Infect. Immun. 40(1983)1119-1126.
115. Sathish, M., Bhutani, L. K., Sharma, A. K. and Nath, I. Monocyte-derived soluble suppressor factor(s) in patients with lepromatous leprosy. Infect. Immun. 42(1983)890-899.
116. Birdi, T. J., Salgame, P. R. and Antia, N. H. The role of macrophages in defective CMI in lepromatous leprosy. II. Macrophage and lymphocyte interaction. Int. J. Lepr. 48(1980)178-182.
117. Salgame, P. R., Birdi, T. J., Mahadevan, P. R. and Antia, N. H. Role of macrophages in defective cell mediated immunity in lepromatous leprosy. I. Factors) from macrophages affecting protein synthesis and lymphocyte transformation. Int. J. Lepr.48(1980)172-177.
118. Mahadevan, P. R. and Antia, N. H. Biochemical alterations in cells following phagocytosis of M. leprae-the consequence-a basic concept. Int. J. Lepr. 48(1980)167-171.
119. Marolia, J., Robinson, P. and Mahadevan, P. R. A complex component modulating immune-deficient cells in leprosy patients leading to loss of viability of Mycobacterium leprae-a. possible vaccine. Clin. Exp. Immunol. 79(1990)7-14.
120. Horwitz, M. A., Levis, W. R. and Cohn, Z. A. Defective production of monocyte-activating cytokines in lepromatous leprosy. J. Exp. Med. 159(1984)666-678.
121. Watson, S., Bullock, W., Nelson, K., Schauf, V., Gclber, R. and Jacobson, R. Interleukin 1 production by peripheral blood mononuclear cells from leprosy patients. Infect. Immun. 45(1984)787-789.
122. Ridel, P.R., Jamet, P., Robin, Y. and Bach, M.- A. Interleukin-1 released by blood monocyte-derived macrophages from patients with leprosy. Infect. Immun. 52(1986)303-308.
123. Beutler, B. and Cerami, A. Cachectin and tumor necrosis factor as two sides of the same biological coin. Nature 320(1986)584-588 (74 refs.).
124. Peters, P. M., Ortaldo, J. R., Shalaby, M. R., Svedersky, L. P., Nedwin, G. E., Bringman, T. S., Hass, P. E., Aggarwal, B. B., Heberman, R. B., Goeddel, D. Y. and Palladino, M. A. Natural killer-sensitive targets stimulate production of TNF-alpha but not TNF-beta (lymphotoxin) by highly purified human peripheral blood large granular lymphocytes. J. Immunol. 137(1986)2592-2598.
125. Culturi, M. C, Murphy, M., Costa-Giomi, M. P., Weinmann, R., Perussia, B. and Trinchieri, G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J. Exp. Med. 165(1987)1581-1594.
126. Scuderi, P., Lam, K. S., Ryan, K. J., Petersen, E., Sterling, K. E., Finley, P. R., Ray, C. G., Slymen, D. J. and Salmon, S. E. Raised serum levels of tumor necrosis factor in parasitic infections. Lancet 2(1986)1364-1365.
127. Silva, C. L. and Foss, N. T. Tumor necrosis factor in leprosy patients. J. Infect. Dis. 159(1989)787-790.
128. Barnes, P. F., Chatterjee, D., Brennan, P. J., Rea, T. H. and Modlin, R. L. Tumor necrosis factor production in patients with leprosy. Infect. Immun. 60(1992)1441-1446.
129. Pisa, P., Gennene, M., Soder, O., OttenhofF, T., Hansson, M. and Kiessling, R. Serum tumor necrosis factor levels and disease dissemination in leprosy and leishmaniasis. J. Infect. Dis. 161(1990)988-991.
130. Foley, N., Lambert, C, McNicol, M., Johnson, N. and Rook, G. A. W. An inhibitor of the toxicity of tumor necrosis factor in the serum of patients with sarcoidosis, tuberculosis and Crohn's disease. Clin. Exp. Immunol. 80(1990)395-399.
131. Arnoldi, J., Gerdes, J. and Flad, H. D. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am. J. Pathol. 137(1990)749-753.
132. Sullivan, L., Sano, S., Pirmez, C, Salgame, P., Mueller, C, Hofman, F., Uyemura, K., Rea, T. H., Bloom, B. R. and Modlin, R. L. Expression of adhesian molecules in leprosy lesions. Infect. Immun. 59(1991)4154-4160.
133. Bermudez, L. E. M. and Young, L. S. Tumour necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J. Immunol. 140(1988)3006-3013.
134. Adams, L. B., Franzblau, S. G., Vavrin, Z., Hibbs, J. B. and Krahenbuhl, J. L. L-Arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J. Immunol. 147(1991)1642-1646.
135. Beutler, B. and Cerami, A. Cachectin: more than a tumor necrosis factor. N. Engl. J. Med. 316(1987)379-385.
136. Selmaj, K. W. and Raine, C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann. Neurol. 23(1988)339-346.
137. Thomson, B. M., Mundy, G. R. and Chambers, T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J. Immunol. 138(1987)775-779.
138. Bjune, G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohemagglutinin. Clin. Exp. Immunol. 36(1979)479-487.
139. Mehra, V., Mason, L. H., Fields, J. P. and Bloom, B. R. Lepromin-induced suppressor cells in patients with leprosy. J. Immunol. 123(1979)1813-1817.
140. Mehra, V., Convit, J., Rubinstein, A. and Bloom, B. R. Activated suppressor T cells in leprosy. J. Immunol. 129(1982)1946-1951.
141. Mehra, V., Mason, L. H., Rothman, W., Reinherz, E., Schlossman, S. F. and Bloom, B. R. Delineation of a human T-cell subset responsible for lepromin-induced suppression in leprosy patients. J. Immunol. 125(1980)1183-1188.
142. Stoner, G. L., Mshana, R. N., Touw, J. and Belehu, A. Studies on the defect in CMI in lepromatous leprosy using HLA-D-identical siblings. Absence of circulating suppressor cells and evidence that the defect is in the T-lymphocyte rather than the monocyte population. Scand. J. Immunol. 15(1982)33-48.
143. Stoner, G. L., Atlaw, T., Touw, J. and Belchu, A. Antigen-specific suppressor cells in subclinical leprosy infection. Lancet 2(1981)1372-1377.
144. Nath, I., Narayanan, R. B., Mehra, N. K., Sharma, A. K. and Gupta, M. D. Concanavalin A-induced suppressor activity in human leprosy. J. Clin. Lab. Immunol. 2(1979)319-324.
145. Nath, I. and Singh, R. The suppressive effect of M. leprae on the in vitro proliferative responses of lymphocytes from patients with leprosy. Clin. Exp. Immunol. 41(1980)406-414.
146. Moretta, L., Webb, S. R., Grossi, C. E., Lydyard, P. M. and Cooper, M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM and IgG. J. Exp. Med. 146(1977)184-200.
147. Singh, S. and Nath, I. Reduction of a subset of T cells bearing Fc receptors for IgG in lepromatous leprosy. Int. Arch. Allergy Appl. Immunol. 62( 1980)81-85.
148. Scheper, R. J., Parker, D., Noble, B. and Turk, J. L. The relation of immune depression and B-cell stimulation during the development of delayed type hypersensitivity to soluble antigens. Immunology 32(1977)365-372.
149. Nath, I. Immunology of human leprosy-current status. Lep. Rev. Special Issue (1983)31S-45S.
150. Mosman, T. R. and Coffman, R. L. TH 1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7(1989)145-173.
151. Mosman, T. R. and Moore, K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today 12(1991)A49-A53.
152. Boom, W. H., Leibster, L., Abbas, A. K. and Titus, R. G. Patterns of cytokine secretion in murine leishmaniasis: correlation with disease progression or resolution. Infect. Immun. 58 (1990)3863-3870.
153. Scott, P. IFN-gamma modulates the early development Thl and Th2 responses in a murine model of cutaneous leishmaniasis. J. Immunol. 147(1991) 3149-3155.
154. Sher, A., Fiorentino, D., Cospar, P., Pearce, E. and Mosmann, T. Production of IL-10 by CD4 T lymphocytesin helminth infection. J. Immunol. 147(1991) 2713-2716.
155. Salgame. P., Abrams, J. S., Clayberger, C, Goldstein, H., Convit, J., Modlin, R. L. and Bloom, B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254(1991)279-282.
156. Gonzalez-Amaro, R., Salazar-Gonzalez, J. F., Baranda, L., Abud-Mendoza, C, Moneada, R., Garcia, R. and Alcocer-Várela, J. Evidence of cell-mediated immune contrasuppression in lepromatous leprosy: modulation of a putative T contrasuppressor cell-subset. Clin. Exp. Immunol. 71(1988)399-404.
157. Green, D. R., Earley, D. D., Kimura, A., Murphy, D. B., Yamauchi, K. and Gershon, R. K. Immunoregulatory circuits which modulate responsiveness to suppressor cell signals: characterization of an effector cell in the contrasuppressor circuit. Eur. J. Immunol. 11(1981)973-980.
158. Haregewoin, A., Godal, T., Mustafa, A. S., Belehu, A. and Yemaneberhan, T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
159. Nogueira, N., Kaplan, G., Levy, E., Sarno, E. N., Kushner, P., Granclli-Piperno, A., Vicira. L., Gould, V. C, Levis, W., Steinman, R., Yip, Y. K. and Colin. Z. A. Defective gamma interferon production in leprosy. Reversal with antigen and interleukin 2. J. Exp. Med. 158(1983)2165-2170.
160. Modlin, R. L., Hofman, F. M., Horwitz, D. A.. Husmann, L. A., Gillis, S., Taylor, C. R. and Rea, T. H. in situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin 2 and its receptor. J. Immunol. 132(1984)3085-3090.
161. Nathan, C. F., Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M. D., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systemic effects of intradermal recombinant interferon in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
162. Nath, I., Sathish, M., Jayraman, T., Bhutani, L. K. and Sharma, A. K. Evidence for the presence of M. leprae reactive T lymphocytes in patients with lepromatous leprosy. Clin. Exp. Immunol. 58(1984)522-530.
163. Kaplan, G., Weinstein, D., Steinman, R. M., Levis, W. R., Elvers, U., Patarroyo, M. E. and Cohn, Z. A. An analysis of;/! vitro T cell responsiveness in lepromatous leprosy. J. Exp. Med. 162(1985)917-929.
164. Nath, I., Jayraman, J., Sathish, M., Bhutani, L. K. and Sharma, A. K. Inhibition of interleukin-2 production by adherent cell factors from lepromatous leprosy patients. Clin. Exp. Immunol. 58(1984)531-538.
165. Kaplan, G. and Cohn, Z. A. The immunobiology of leprosy. Int. Rev. Exp. Pathol. 28(1986)45-78(124 ref).
166. Mohagheghpour, N., Gelber, R. H., Larrick, J. W., Sasaki, D. T., Brennan, P. J. and Engleman, E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J. Immunol. 135(1985)1443-1449.
167. Mohagheghpour, N., Gelber. R. H. and Engleman, E. G. T cell defect in lepromatous leprosy is reversible in vitro in the absence of exogenous growth factors. J. Immunol. 138(1987)570-574.
168. Wong. L., Salgame, P., Torigian, V. K., Fu, T. H., Rea, T. H. and Modlin, R. L. CD2 expression and function in lepromatous leprosy. Infect. Immun. 57(1989)2815-2819.
169. Bullock, W. E. and Fasal, P. Studies of immune mechanisms in leprosy: III. The role of cellular and humoral factors in impairment of the in vitro immune response. J. Immunol. 106(1971)888-899.
170. Nelson, D. S., Penrose, J. M., Waters, M. F. R., Pearson, J. M. H. and Nelson, M. Depressive effect of serum from patients with leprosy on mixed lymphocyte reactions', influence of antileprosy treatment. Clin. Exp. Immunol. 22(1975)385-392.
171. Potts, R. C, Sheriff, M. M., Robertson, A. J., Gibbs, J. H.. Brown. R. A. and Beck. J. S. Serum inhibitory factor in lepromatous leprosy, its effect on the pre-S phase cell cycle kinetics of mitogen stimulated normal human lymphocytes. Immunology 14(1981)269-280.
172. Kerr, M. A., Hussein, Y. N., Potts, R. C, Beck, J. S. and Sheriff, M. M. Characterisation of a factor in leprosy serum that inhibits the growth of mitogen stimulated normal human lymphocytes. Immunology 61(1987)117-123.
173. Naik, S., Kumar, B.. Kaur, S. and Sehgal, S. Cold reactive lymphocytotoxic antibodies in patients with tuberculoid and lepromatous leprosy. Int. J. Lepr. 55(1987)273-276.
174. D'Souza, D., Das, B. C. and Thomas, I. M. Effects of lepromatous leprosy (LL) serum factor(s) on normal blood lymphocytes. Int. J. Lepr. 58(1990)666-673.
175. Modlin, R. L.. Kato, H., Mehra. V., Nelson, E. E., Fan, X., Rea, T. H., Pattengale, P. K. and Bloom, B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. (Letter) Nature 322(1986)459-461.
176. Kaplan, G.. Gandhi, R. R., Weinstein, D. E., Levis, W. R., Patarroyo, M. E., Brennan, P. J. and Cohn, Z. A. Mycobacterium leprae antigen induced suppression of T cell proliferation in vitro. J. Immunol. 138(1987)3028-3034.
177. Molloy, A., Gaudernack, G., Levis, W. R., Cohn, Z. A. and Kaplan, G. Suppression of T-cell proliferation by Mycobacterium leprae and its products: the role of lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. 87(1990)973-977.
178. Holzer, T. J., Nelson, K. E., Schauf, V., Crispen, R. G. and Andersen, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51(1986)514-520.
179. Vachula, M., Holzer, T. J. and Andersen, B. R. Suppression of monocyte oxidative response by phenolic glycolipid I of Mycobacterium leprae. J. Immunol. 142(1989)1696-1701.
180. Parkash, O. and Sengupta, U. Survival of Mycobacterium leprae in mononuclear phagocytes: a possible role of complement system. Acta Leprol. 7(1991)375-377.
181. Neuberger, P. E., Pagano, J. S., Greenberger, J. S., Karpas, A. and Cohen, H. J. Dissociation of opsonised particle phagocytosis and respiratory burst activity in an Epstein-Barr virus-infected myeloid cell line. J. Cell Biol. 85(1980)549-554.
182. Wright, S. D. and Silverstein, S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 158(1983)2016-2023.
183. Yamamoto, K. and Johnston, R. B., Jr. Dissociation of phagocytosis from stimulation of the oxidative metabolic burst in macrophages. J. Exp. Med. 159(1984)405-416.
184. Mosser, D. M. and Edelson, P. J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 135(1985)2785-2789.
185. Mosser, D. M. and Edelson, P. J. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature 327 (1987) 329-331.
186. Neill, M. A. and KlebanolT, S. The ef Tect of phenolic glycolipid-I from Mycobacterium leprae on the antimicrobial activity of human macrophages. J. Exp. Med. 167(1988)30-42.
187. Moreno, C, Mchlert, A. and Lamb, J. The inhibitory effects of mycobacterial lipoarabinomannan and polysaccharides upon polyclonal and monoclonal human T cell proliferation. Clin. Exp. Immunol. 74(1988)206-210.
188. Sibley, L. D., Hunter, S. W., Brennan, P. J. and Krahenbuhl, J. L. Mycobacterial lipoarabinomannan inhibits gamma interferon-mediated activation of macrophages. Infect. Immun. 56(1988)1232-1236.
189. Sibley, L. D., Adams, L. B. and Krahenbuhl, J. L. Inhibition of interferon-gamma-mediated activation in mouse macrophages treated with lipoarabinomannan. Clin. Exp. Immunol. 80(1990)141-148.
190. Fine, P. E. M., Gruer, P. J. K., Maine, N., Ponnighaus, J. M., Rees, R. J. W. and Stanford, J. L. Failure of Mycobacterium leprae soluble antigens to suppress delayed-type hypersensitivity reaction to tuberculin. Clin. Exp. Immunol. 77(1989)226-229.
191. Parkash, O. and Sengupta, U. Possible roles of anti-Mycobacterium leprae antibodies in suppression of cell-mediated immune response against M. leprae. (Letter) Immunol. Today 13(1992)513.
192. Tyagi, P., Patil, S. A., Girdhar, B. K., Katoch, K. and Sengupta, U. Suppressive effect of circulating immune complexes from leprosy patients on the lymphocyte proliferation induced by M. leprae antigens in healthy responders. Int. J. Lepr. 60(1992)562-569.
193. Thole, J. E. R.,Hindersson, P., de Bruyn, J., Cremers, F., van der Zee, J., de Cock, H., Tommassen, J., van Eden, W. and van Embden, J. D. A. Antigenic relatednces of a strongly immunogenic 65-kDa mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb. Pathog. 4(1988)71-83.
194. Colston, M. J. and Lamb, F. I. Molecular biology of the mycobacteria. Lepr. Rev. 60(1989)93-98.
195. Naafs, B., Kolk, A. H. J., Chin-A-Lien, R. A. M., Faber, W. R., VanDijk, G., Kuijper, S., Stolz, E. and Van Joost, T. Anti-Mycobacterium leprae monoclonal antibodies cross-react with human skin: an alternative explanation for the immune responses in leprosy. J. Invest. Dermatol. 94(1990)685-688.
196. Cunningham, M. W., Hall, N. K., Krisher, K. K. and Spanier, A. M. A study of anti-group A streptococcal monoclonal antibodies cross-reactive with myosin. J. Immunol. 136(1986)293-298.
197. Holoshitz, J., Klajman, A., Drucker, I., Lapidol, Z., Yarctzky, A., Frenkal, A., van Eden, W. and Cohen, I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet 2(1986)305-309.
198. Stefansson, K., Dieperink, M. E., Richman, D. P., Gomez, C. M. and Marton, L. S. Sharing of antigenic determinants between the nicotinic acetylcholine receptor and proteins in Escherichia coli, Proteus vulgaris, and Klebsiella pneumoniae. N. Engl. J. Med. 312(1985)221-225.
199. Wood, J. N., Hudson, L., Jessell, T. M. and Yamamoto, M. A monoclonal antibody defining antigenic determinants on subpopulations of mammalian neurones and Trypanosoma cruzi parasites. Nature 296(1982)34-38.
200. OttenholT, T. H. M. and de Vries. R. R. P. HLA class II immune response and suppression genes in leprosy. Int. J. Lepr. 55(1987)521-534.
201. de Vries, R. R. P., Mehra, N. K., Vaidya, M. C, Gupte, M. D., Khan, P. M. and Van Rood, J. J. HLAlinked control of susceptibility to tuberculoid leprosy and association with HLA-DR types. Tissue Antigens 16(1980)294-304.
202. Miyanaga, K., Juji, T., Maeda, H., Nakajima, S. and Kobayashi, S. Tuberculoid leprosy and HLA in Japanese. Tissue Antigens 18(1981)331-334.
203. Izumi, S., Sugiyama, K., Matsumolo, Y. and Ohkawa, S. Analysis of the immunogenetic background of Japanese leprosy patients by the HLA system. Vox Sang. 42(1982)243-247.
204. Van Eden, W., de Vries, R. R. P., D'Amaro, J., Schreuder, I., Leiker, D. L. and Van Rood, J. J. HLA-DR associated genetic control of the type of leprosy in a population from Surinam. Hum. Immunol. 4(1982)383-350.
205. Todd, J. R., West, B. C. and McDonald, J. C. Human leukocyte antigen and leprosy: study in northern Louisiana and review. Rev. Infect. Dis. 12(1990)63-74.
206. Ram, R., Zaheer, S. A. and Mukherjee, R. Do human leukocyte antigens have a role to play in differential manifestation of multibacillary leprosy: a study on multibacillary' leprosy patients from north India. Tissue Antigens 40(1992)124-127.