- Volume 60 , Number 1
- Page: 84–7
Evaluation of MLPA test for the serodiagnosis of leprosy
To the Editor:
An enzyme-linked immunosorbent assay (ELISA) using Mycobacterium leprae cell wall phenolic glycolipid-I (PGL-I)(1,3,12) and a serum antibody competition test (SACT) based on the 35-kDa(10) and 36kDa(6) protein antigens of M. leprae may be some of the most reliable serological tests available today for the detection of M. leprae infection. ELISA has been used widely in serological studies probably due to the easy availability of PGL-I antigen through the World Health Organization (WHO) in a semisynthetic disaccharide octyl bovine serum albumin (ND-O-BSA) form. However, ELISA has little field application since it can be performed only in a laboratory with expensive equipment such as an ELISA reader. Attempts to simplify this technique for field use as the dot ELISA (13) or stick ELISA(7) have been of little success. Nevertheless, a serological test called the M. leprae particle agglutination (MLPA) test that is applicable to the field has recently been described by Izumi and his colleagues(5). In order to assess the usefulness of the MLPA test, we compared this test with the PGL-I ELISA and SACT using monoclonal antibody to the 35-kDa protein antigen. Serum samples for this study were collected from 188 leprosy patients, 34 healthy occupational contacts, and 48 healthy noncontacts. The patients were classified according to Ridley and Jopling (8), and the numbers of serum samples in each patient group are given in Table 1. At the time of blood collection they were undergoing multidrug therapy (MDT) for periods ranging from 6 months to 1 year but had skin lesions suggestive of active disease. The MLPA test was performed using the Serodia-Leprae kit (Fujirebio Inc., Tokyo, Japan). Serum samples were diluted to 1:16 and 1:32 in 96well, U-bottom microtiter plates. Twenty-five µ l of unsensitized gelatin particles and 25 µ l gelatin particles sensitized with synthetic trisaccharide of PGL-I (NT-P-BSA) were mixed with 25 µ l of 1:16- and 25 µ l l:32-diluted serum samples, respectively. After being incubated for 2 hr at room temperature, the plates were read for agglutination. Serum samples showing agglutination at the 1:32 dilution of sera were considered positive. ELISA with ND-O-BSA antigen, the synthetic disaccharide of PGL-I (kindly supplied by IMMLEP, WHO) was carried out as described in our previous paper (4). Serum samples were tested at the 1:300 dilution, and those showing OD values > 0.200 were considered positive. The SACT was done following the method of Sinha, et al. (10) but using peroxidase- instead of isotope-labeled monoclonals. Serum samples were applied at the 1:10 dilution and those causing 50% inhibition of ML0 4 binding to M. leprae sonicate (ID50) were considered positive.
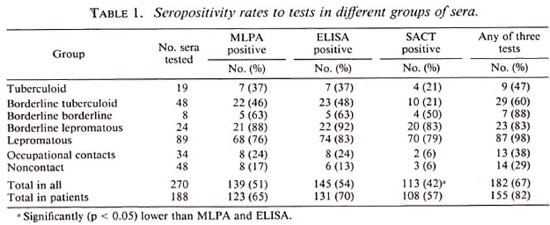
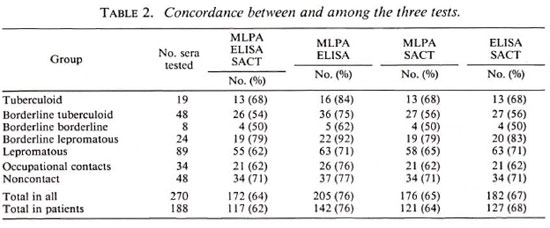
Table 1 summarizes the percent positivity for each test in different groups of subjects. Overall seropositivity rates were found to be 51%, 54%, and 42% for the MLPA test, ELISA, and SACT, respectively. These rates increased to 65%, 70%, and 57% when sera of patients were considered separately. Also, when we analyzed the patients showing positivity to any one of the tests, the seropositivity increased to 82%, suggesting that simultaneous application of all three tests could detect most of the M. leprae infections. In general, the seropositivity rates of the three tests did not show much difference in multibacillary patients. However, in paucibacillary patients even though the seropositivity rates were more or less similar for the MLPA test (43%) and the ELISA (45%), it was much lower (21%) for the SACT, indicating that the prevalence of antibodies to the 35-kDa antigen of M. leprae in paucibacillary patients is limited. It was also found that all three tests could give concordant results only in 64% ofthe serum samples (Table 2). Slightly higher concordances were noticed when two of the tests were compared. While the MLPA test and ELISA showed 76% concordance, the MLPA test and SACT and the ELISA and SACT showed 65% and 67%, respectively. The results in 20% of sera, however, showed no concordance between the tests. While the MLPA test (65.4%) and ELISA (69.7%) showed more or less similar sensitivity, the sensitivity of the SACT (57%) was observed to be lower than the other two although it was not statistically significant.
Similar to this study, others have also observed lower ELISA (1,3,12) and SACT (10) positivity in paucibacillary leprosy patients as compared to multibacillary patients. However, in contrast to our results, Roche et al. (9) have reported higher positivity to the SACT (33%) and lower positivity to ELISA (20%) in a group of paucibacillary leprosy patients from Nepal. This is surprising because, unlike ours, the patients included in their study were untreated and, as such, are supposed to show more positivity. It appears that this discrepancy is not due to the cut-off value employed to determine ELISA positivity since both studies have used 0.200 OD as the cut-off mark. A study involving both of these tests in untreated leprosy patients from a different area may throw some light on this point.
Our results suggest that the MLPA test is equally efficient in detecting M. leprae infection. Although the concordance between the MLPA test and the ELISA in this study (76%) is relatively less than that reported (89%-100%) by I/.umi, el al. (5), it may partly be due to the different antigens used in the ELISA. Whereas synthetic disaccharide (ND-O-BSA) has been employed in our ELISA, Izumi, et al. (5) have used synthetic trisaccharide (NT-P-BSA) similar to the one used for the MLPA test. These disaccharide and trisaccharide antigens show some difference in binding with anti-PGL-I antibodies, as already reported (2). Recently, Wu, et al. (11) have also reported the development of an agglutination test by sensitizing latex particles with native PGL-I and ND-O-BSA. Although attempts were made to sensitize latex particles with ND-O-BSA in our laboratory, the results were not encouraging because the sensitization did not last for long.
Since the performance of the MLPA test is easier than the ELISA, it is highly suitable for screening populations for M. leprae infection, permitting more samples to be handled per day. A further advantage is that the results of this test can be assessed with the naked eye and, therefore, it can be performed in the field or in peripheral laboratories. We believe that the introduction of this test in place of the ELISA in ongoing seroepidemiological studies would enable the investigator to screen more of the population with a sensitivity corresponding to the ELISA. However, the question of whether the serological studies would really be useful in detecting leprosy in the population, because the infection often heals naturally, can be answered only if the results of the longitudinal epidemiological studies currently underway in several parts of the world are reported. Nonetheless, since it has been shown that the MLPA test gives titer values similar to the ELISA it may have greater application in monitoring the effect of multidrug therapy in leprosy patients.
- Subramanian Dhandayuthapani, Ph.D.
Senior Research Officer
- Duraisamy Anandan, B.Sc.
Technical Assistant
- Vivekanand N. Bhatia, M.D.
Director (Microbiology)
Central Leprosy Teaching and Research Institute
Chengalpattu 603001, India
Acknowledgment. The authors thank Dr. J. Ivanyi, Director, MRC Tuberculosis and Related Infections Unit, Hammersmith Hosptial, London, for the gift of ML04 monoclonal antibody used in this study. The authors also thank Dr. S. Izumi for critically going through the script. The technical assistance of Ms. B. Vasanthi and G. Yusuff is gratefully acknowledged.
REFERENCES
1. BRETT, S. J.. DRAPER, P.. PAYNE, S. N. and REES, R. J. W. Serological activity of characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
2. CHANTEAU, S., CARTEL, J. L., Roux, R., PLICHART, R. and BACH, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid-I in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-776.
3. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
4. DHANDAYUTHAPANI. S., ANANDAN, D., VASANTHI, B. and BHATIA, V.N. Use of eluates of filter paper blood spots in ELISA for the serodiagnosis of leprosy. Indian. J. Med. Res. 89(1989)150-157.
5. IZUMI, S., FUJIWARA, T., IKEDA, M.. NISHIMURA, Y., SUGIYAMA, K. and KAWATSU, K. Novel gelatin particle agglutination test for serodiagnosis of leprosy in the field. J. Clin. Microbiol. 28(1990)525-529.
6. KLATSER, P. R.. DE WIT, M. Y. L. and KOLK, A. H. J. An ELISA inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
7. KUMAR, S., MOUDGIL, K. D., BAND, A. H., NARAYANAN, P. R., GUPTA, S. K., SHARMA, A. K. and TALWAR, G. P. A dot enzyme immunoassay for detection of IgM antibodies against phenolic glycolipid-I in sera from leprosy patients. Indian J. Lepr. 58(1986)185-190.
8. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
9. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., LUDWIG, H., THEUVENET, W. J. and ADIGA, R. B. Heterogeneity of serological responses in paucibacillary leprosy; differential responses to protein and carbohydrate antigens and correlation with clinical parameters. Int. J. Lepr. 58(1990)319-327.
10. SINHA, S., SENOUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by a monoclonal antibody based immunoassay. Int. J. Lepr. 53(1985)33-38. of antibodies to phenolic glycolipid-I. Lepr. Rev. YOUNG, D. B. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
11. Wu, Q., YE. G.. YIN, Y., LI, H., LIU, Q. and WEI, W. Rapid serodiagnosis for leprosy; a preliminary study on latex agglutination test. Int. J. Lepr. 58(1990)328-333.
12. YOUNG, D. B. and BUCHANAN, T. M. A serologicaltest for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
13. YOUNG, D. B., FOHN, M. J., KHANOLKAR, S. R. and BUCHANAN, T. M. A spot test for detection of antibodies to phenolic glycolipid-I. Lepr. Rev. 56(1985)193-198.
Reprint requests to Dr. Dhandayuthapani at his present address: National Institute for Leprosy Research, 4-2-1 Aobacho, Higashimurayamashi, Tokyo 189, Japan.
Present address for Dr. Bhatia: Department of Serologist and Chemical Examiner, 3 Kyd Street, Calcutta, West Bengal, India.