- Volume 60 , Number 1
- Page: 87–9
Does previous BCG vaccination interfere with the serodiagnosis of tuberculosis using Mycobacterium tuberculosis-specific glycolipid antigens?
To the Editor:
There is evidence to the effect that the development of protective T-cell-mediated protective immunity results in suppression of humoral B-cell immunity in leprosy and tuberculosis (4). BCG vaccination is believed to induce protective immunity against tuberculosis. It may be reasonably assumed that BCG-vaccinated individuals who subsequently are diagnosed with tuberculosis should build up protective immunity more efficiently than nonBCG-vaccinated individuals. Therefore the sensitivity of serodiagnosis should be lower in BCG-vaccinated tuberculosis patients. To examine this question, we decided to conduct a study in patients residing where BCG coverage was wide and where the incidence of tuberculosis was high as a means of detecting sufficient numbers of tuberculous patients who had received BCG vaccination.
The city of Manaus (Amazonas, Brazil) was selected for the study because, according to the health authorities, about 50% of the population had been vaccinated 1 month after birth and the incidence of smear-positive cases of tuberculosis was estimated at 69.2/100,000 inhabitants for the last decade (ranging from 70.5 in 1981 to 58.9 in 1989). During the same period, the population in the state of Amazonas increased by about 30% (1.5 million in 1981 and 2.0 million in 1989).
As part of the clinical history of all patients who are personally observed at the National Research Institute of the Amazonia (INPA), previous BCG vaccination is registered. Evidence of BCG vaccination is established by searching for the characteristic vaccinal scar in the deltoid region. This scar is easily differentiated from the smallpox vaccination scar which can still be found in the older age groups. The frequency distribution of 273 confirmed tuberculosis cases in respect to previous BCG vaccination and decade of life is shown in The Figure. The proportion of patients who received BCG vaccination was 48.3% and, as shown in The Figure, the proportion of vaccinated patients was over 50% during the first four decades of life, dropping sharply thereafter. Judging from these unexpected findings, we decided that a sample of about 50 successive cases of tuberculosis should be enough to answer the question of whether previous BCG vaccination might interfere with the sensitivity of serodiagnosis. As shown in The Figure, the sampled population was representative of the whole population of tuberculosis patients.
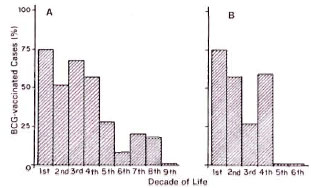
The figure. Tuberculosis disease in BCG-vaccinated patients. A = Frequency distribution of BCG-vaccinated tuberculosis patients with respect to decade of life (number vaccinated, 132; number examined,273). B = Frequency distribution of BCG-vaccinatedcases among 53 successive tuberculosis cases selected for ELISA with respect to decade of life (number vac-cinated 26).
Tuberculosis disease was confirmed in all cases indicated above by isolation and identification of Mycobacterium tuberculosis using current methods (3). To start the study, blood was collected from successive patients before treatment, and the sera were kept frozen until ready to use. When the total number of bacteriologically confirmed tuberculosis cases reached 53 their sera were used for ELISA. The serology procedure used was as described previously (1). The antigens used were the PGL-Tb1 and the SL-IV glycolipids isolated from the strain Cannetti of M. tuberculosis (2,7,8). ELISA values were assayed at a 1/250 serum dilution, and were scored as positive if >200 for the PGL-Tb1 and/or > 300 for the SL-IV antigens (1).
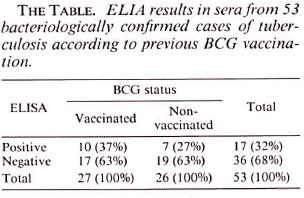
The ELISA results in the sera of the 53 cases selected for the study are shown in The Table. The sensitivities of serodiagnosis were 26.9% and 37.0% in, respectively, the nonvaccinated and the BCG-vaccinated patients. The difference between the two groups was not statistically significant (χ2 = 3.36, α = > 0.05). We have therefore concluded that previous BCG vaccination did not adversely affect serodiagnosis. However, the conclusion must be tempered because the unexpected high proportion of tuberculosis patients who had been BCG vaccinated suggests that the vaccination was ineffective.
Although the purpose of this study 'was not to evaluate the efficacy of BCG vaccination, our observations led to the following considerations. It was suggested that BCG vaccination may enhance or depress the protective immunity against tuberculosis that is supposedly induced by environmental mycobacteria (11). The occurrence of environmental mycobacteria was also advanced as one possible explanation for the failure of BCG vaccination in the Chingleput, India, evaluation of its efficacy (6). Previous studies in Manaus showed that environmental mycobacteria, including potentially pathogenic species, were isolated from 25.4% of all clinical specimens examined (10), from 31.3% of hand and forearm wa shings of healthy individuals (9), and from 17.9% of hand washings of leprosy patients (5). In view of the above results and considerations, we concluded that an indepth investigation of this matter is urgently needed in regions like the Amazonia where environmental mycobacteria are highly prevalent. Because of these observations, we began to collect information on previous BCG vaccination in leprosy patients for use in verifying if it might somehow interfere with anti-PGL-I titers in this disease.
- Julia Ignez Salem, M.D., Ph.D.
Chief Laboratory of Mycobacteriology
- Maria Francisca de A. Costa
Nurse, Laboratory of Mycobacteriology
National Research Institute of the Amazonia (INPA)
P.O. Box 478
69011 Manaus, Amazonia, Brazil
- Philippe Cruaud, M.Sc. (Pharm.)
Unité de la Tuberculose et des Mycobactéries
- Hugo L. David, M.D., Ph.D.
Chef, Unité de la Tuberculose et des Mycobactéries
Institut Pasteur
25 Rue du Dr. Roux
75724 Paris 15, France
Acknowledgment. This project was partially supported by the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq, Brazil).
REFERENCES
1. CRUAUD, P., YAMASHITA, J. T., MARTIN CASA-BONA, N., PAPA, F. and DAVID, H. L. Evaluation of a novel 2, 3-diacyl-trehalose-2'-sulfate (SL-IV) antigen for case finding and diagnosis of leprosy and tuberculosis. Res. Microbiol. 141(1990)679-694.
2. DAFFÉ, M., LACAVE, C, LANEELLE, M.-A. and LANEELLE, G. Structure of the major triglycosyl phenol phtioccrol of Mycobacterium tuberculosis (strain Canctti). Eur. J. Biochem. 167(1987)155-160.
3. DAVID, H. L., LEVY-FREBAULT. V. and THOREL, M. F. Méthodes de Laboratoirepour Microbacteriology Clinique. Comission des Laboratories de Reference at d'Expertise de l'lnslitut Pasteur, eds. Paris: Institut Pasteur, 1989.
4. DAVID, H. L., MARÓJA, M. F. and CRUAUD, P. Quantitative relationship between anti-PGL-Ispecilic antibody levels and the lepromin reaction. Int. J. Lepr. 59(1991)332-334.
5. FANDINHO, F. C. O., SALEM, J. I., GONTIJO-FILHO, P. P., MARÓJA, M. F. and DAVID, H. L. Mycobacterial flora of the skin in Leprosy. Int. J. Lepr. 59(1991)569-575.
6. GRANGE, J. M. Environmental mycobacteria and BCG vaccination. Tubercle 67(1986)1-4.
7. LEMASSU, A., LANÉELLE, M.-A. and DAFFÉ, M. Revised structure of a trehalose-containing glycolipid of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 78(1991)171-176.
8. PAPA, F., CRUAUD, P. and DAVID, H. L. Antigenicity and specificity of selected glycolipid fractions from Mycobacterium tuberculosis. Res. Microbiol. 140(1989)569-578.
9. SALEM, J. I., GONTIJO-FILHO, P., LÉVY-FREBAULT, V. and DAVID, H. L. Isolation and characterization of mycobacteria colonizing the healthy skin. Acta Leprol. 7suppl.1(1989)18-20.
10. SALEM, J. I., MARÓJA, M. F., CARVALHO, F. F., LIMA, M. O. and FEUILLET, A. Mycobacteria other than tubercle bacilli in sputum specimens from patients in Manaus (Amazonia, Brazil). Acta Amazonica 19(1989)349-354.
11. STANFORD, J. L., SHIELD, M. J. and ROOK. G. A. W. How environmental mycobacteria may predetermine the protective efficiency of BCG. Tubercle 62(1981)55-62.
Reprint requests to Dr. David.