- Volume 60 , Number 1
- Page: 92–4
Reactive oxygen intermediates in the phagocytes f rom leprosy patients: correlation with reactional states and variation during treatment
To the Editor:
It is well known that lepromatous leprosy patients' lymphocytes are unresponsive to Mycobacterium leprae in vitro. Theoretically, this defect in cell-mediated immunity toward M. leprae could be due to a macrophage defect in these individuals. Monocytes from lepromatous leprosy patients secrete subnormal quantities of H2O2 after stimulation with the potent secretagogue, phorbol myristrate acetate (PMA) (4). M. leprae fails to stimulate significant release from human monocytes, human neutrophils, or mouse peritoneal macrophages (1,3). On the other hand, monocyte activation does occur during reactional episodes in leprosy patients, implying that some other pathway must be operating during reaction which does not operate in the absence of reactions in the patients (5). To further study monocyte function in rcactional and nonreactional leprosy patients we measured monocyte activation by luminol-dcpendcnt chemiluminescence and lymphocyte proliferation in response to M. leprae.
Subjects. The leprosy patients were attending the outpatient leprosy unit of the Oswaldo Cruz Foundation, Rio dc Janeiro, Brazil. The clinical diagnosis was confirmed by skin biopsy in each case. We studied 8 patients with polar lepromatous (LL), 8 with borderline lepromatous (BL), 8 with borderline tuberculoid (BT) disease, and 8 healthy laboratory controls. Five of the 8 LL patients were experiencing erythema nodosum leprosum (ENL). Three of the 8 BL patients had ENL and 1 had reversal reaction. Two of the 8 BT patients had neuritis. Two of the 5 LL patients were not being treated with anti-reactional drugs. All of the other patients with reactions were receiving treatment with clofazimine and/or thalidomide.
Peripheral blood mononuclear cells were prepared with Ficol-Hypaque gradient centrifugation (2). After 24-hr incubation on glass coverslips, the adherent cells were transferred to liquid scintillation vials containing RPMI 1640 medium supplemented with 10% v/v fetal calf serum and luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) at a final concentration of 5 x 10-5 M. Upon oxidation, luminol exhibits striking chemiluminescence. This was quantitated using a liquid scintillation counter, measuring chemiluminscence for 1 min after stimulation of the monocytes with PMA at a concentration of 0.5 µ g/ml and subtracting the background chemiluminescence of the monocyte preparation before the addition of PMA.
Resting monocytes from nonreactional BL and LL patients showed lower background chemiluminesence than monocytes from healthy laboratory controls (an average of 22% of control values). Monocytes from BL and LL patients with reactions, on the other hand, showed higher resting chemiluminescence than cells from healthy controls (22% of control values). Upon stimulation with PMA there was an increase in chemiluminescence with monocytes from the healthy controls and from BT patients (Fig. 1). In nonrcactional LL patients there was no chcmiluminesccncc with PMA stimulation. In contrast, monocytes from LL patients with ENL showed activation with PMA. In patients with ENL reactions being treated with thalidomide, chemiluminescence induced by PMA was lower than that seen in patients with untreated ENL.
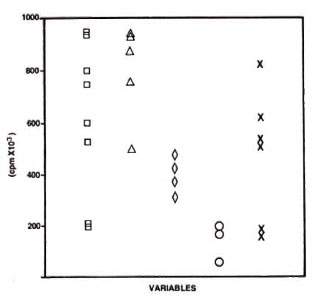
Fig. 1. Enhancement of chemiluminescence response by monocytes from laboratory controls (□), BT patients (∆), BL patients ( ◊), LL patients (○), andlepromatous patients with ENL (x). Amount of chemiluminescence is expressed as ∆cpm = PMA stimulated count - PMA free count. Monocytes were cultured for 1 day, washed, and transferred tochemiluminescence reaction medium, and then stimulated with PMA (0.5 µ g/ml).
As expected, lymphocyte proliferation in vitro in response to M. leprae was positive in some of the healthy controls and BT patients, but was essentially negative in BL and LL patients (Fig. 2).
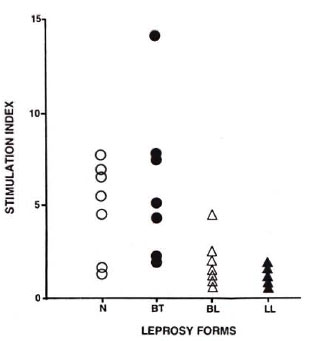
Fig 2. Lymphocyte transformation test (LTT) measured by [3H] TdR incorporation for laboratory controls (N) and leprosy forms (BT, BL, LL). Amount of LTT response is expressed as Stimulation Index = antigen-stimulated culture count/antigen-free culture count. Mononuclear cells were cultured for 5 days in presence or absence of M. leprae . [3H] TdR was added and after 18 hr of labeling, thymidine uptake was as-sayed with a liquid scintillation counter.
Although the number of patients studied in each group is small, the results suggest that during ENL the monocytes of LL patients can respond to PMA although their lymphocytes remain unresponsive to M. leprae.
- Dilvani O. Santos, B.Sc, M.Sc.
Department of Cellular and Molecular Biology
Federal Fluminense University
Av. Barros-Terra s/n
Valonguinho
Niteroi, R. J., Brazil 20400
- Jorge L. Salgado, B.Sc.
Andre L. Moreira, M.D.
Leprosy Unit
Oswaldo Cruz Foundation
- Euzenir N. Sarno, M.D., Ph.D.
Chairman
Leprosy Unit
Oswaldo Cruz Foundation
Av. Brasil 4365
Manguinhos
Rio de Janeiro. R.J., Brazil 21040
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases (TDR) and from CNPq (Brazil).
REFERENCES
1. HOLZER, T. J., NELSON, K. E., SCHAUF, V., CRISPEN, R. G. and ANDERSEN, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51(1986)514-520.
2. HORWITZ, M. H., LEVIS, W. K. and COHN, Z. Defective production of monocyte-activating cytokines in Lepromatous leprosy. J. Exp. Med. 159(1984)666-672.
3. LAUNOIS, P., MAILLERE, B., DIEYE, A., SARTHOU, J. L. and BACH, M.-A. Human phagocyte oxidative burst activation by BCG, M. leprae, and atypical mycobacteria: defective activation by M. leprae is not reversed by interfcron-γ. Cell. Immunol. 124(1989)168-174.
4. NATHAN, C. F., KAPLAN, G. and LEVIS, W. R. Local and systemic effects of intradermal recombinant interferon^ in patients with lepromatous leprosy. N. Engl. J. Med. 3(1986)6-15.
5. RIDLEY, M. J. and RIDLEY, D. S. The immunopathology of erythema nodosum leprosum: the role of extravascular complexes. Lepr. Rev. 54(1983)95-107.