- Volume 60 , Number 1
- Page: 96–8
Comparison of PGL-I level with AFB numbers in foot pad suspension
To the Editor:
Since the time that phenolic glycolipid-I (PGL-I) was first isolated and characterized as a Mycobacterium leprae- specific product (4,5), it has been widely used in serological tests for leprosy. Besides its use for purposes of detecting antibodies to the antigen, PGL-I has been found in various clinical specimens, such as serum (1-3,10), urine (6), and tissues (8,9), etc. However, PGL-I has never been assayed in tissues of mouse foot pads inoculated with M. leprae where it could prove to be a useful surrogate of acid-fast bacilli (AFB) numbers. Therefore, we attempted to measure the levels of PGL-I in a mouse foot pad suspension using the dot enzyme-linked immunosorbent assay (ELISA) described previously (2,3). Briefly, foot pad suspensions (1.0-1.7 ml) were lyophilized, and the lipids were extracted with chloroform : methanol (2:1) solution. After application to a florisil column, the chloroform : methanol (19:1) elute was examined for the presence of PGL-I by dot-ELISA using rabbit anti-; M. leprae antiserum containing anti-PGL-I antibodies. A series of normal mouse foot pad suspensions containing different amounts of the standard PGL-I were processed using the same procedures to determine the test parameters for the PGL-I assay. The numbers of AFB in these foot pad suspensions were obtained microscopically by standard procedures (7), 60 oil immersion fields being counted. If there were no AFB in 60 fields, no M. leprae were considered to have been present in the sample.
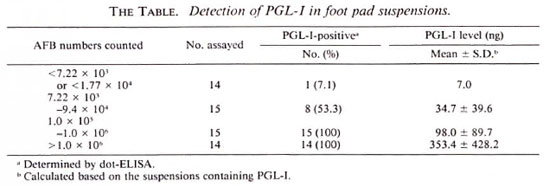
A total of 58 foot pad suspensions were examined. PGL-I was detectable in all suspensions, 29 in number, containing more than 1.0 x 105 AFB (The Table). Of 15 suspensions containing 7.22 x 103 to 9.4 x 104 AFB, 8 specimens had detectable PGL-I. Also, PGL-I was detectable by dot-ELISA in one of 14 suspensions containing less than 7.22 x 103 or less than 1.77 x 104, which were considered AFB negative. When the PGL-I level was compared with the total AFB numbers in each suspension, there was a strong correlation (r = 0.834) (The Figure). Interestingly, PGL-I concentration in foot pad suspension was much higher (about 20 times) than the calculated PGL-I amount based on the report that about 2.3% of M. leprae dry weight was PGL-I (4,5). This observation supports the contention that the live bacilli actively secrete PGL-I into the surrounding tissues, and that the antigen may be trapped in tissues for a long time. The results also showed that the PGL-I level in tissues corresponded approximately to the AFB numbers, especially at the critical level (105 AFB) when the growth of the usual 5 x 103 M. leprae inoculum would have demonstrated unequivocal multiplication (7). Therefore, it should be possible to use the PGL-I detection techniques instead of counting the AFB to determine bacillary load in foot pad suspensions.
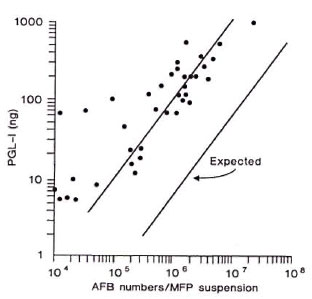
The figure. Comparison of PGL-I level with AFB numbers in the foot pad suspensions. Each dot indicates a suspension. The "expected line" was drawn based on PGL-I amount calculated from the numbers of M. leprae.
- Sang-Nae Cho, D.V.M., Ph.D.
Joo-Deuk Kim, M.D., Ph.D.
Department of Microbiology
Yonsei University College of Medicine
C.P.O. Box 8044
Seoul 120-752, Republic of Korea
- Robert H. Gelber, M.D.
Kuzell Institute for Arthritis and Infectious Diseases
Medical Research Institute of San Francisco at California Pacific Medical Center
2200 Webster Street
San Francisco, CA 94115-1896, U.S.A.
- Patrick J. Brennan, Ph.D.
Department of Microbiology
Colorado State University
Fort Collins, CO 80523, U.S.A.
Acknowledgment. This work was supported by a research grant (1989) from the Heiser Program for Research in Leprosy and a contract from the G. W. Long Hansen's Disease Center. Carville, Louisiana. U.S.A. We are grateful to Ms. E. O. Shin, Lydia P. Murray, Pat Siu. and Mabel Tsang for technical assistance.
REFERENCES
1. CHANTEAU, S., CARTEL, J.-L., PERANI. E., N'DELI, L., Roux, J. and GROSSET, J.-H. Relationships between PGL-I antigen in serum, tissue and viability of Mycobacterium leprae as determined by mouse footpad assay in multibacillary patients during short-term clinical trial. Lepr. Rev. 61(1990)330-340.
2. CHO, S.-N., CELLONA. R. V., FAJARDO, T. T., JR., ABALOS. R. M., DELA CRUZ. E. C, WALSH, G. P., KIM, J. D. and BRENNAN, P. J. Detection of phenolic glycolipid-I antigen and antibody in sera from new and relapsed lepromatous patients treated with various drug regimens. Int. J. Lepr. 59(1991)25-31.
3. CHO, S.-N., HUNTER. S. W., GELBER, R. H., REA, T. H. and BRENNAN, P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigencmia in leprosy. J. Infect. Dis. 153(1986)560-569.
4. HUNTER. S. W. and BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacterid. 147(1981)728-735.
5. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. 257(1982)15072-15078.
6. KALDANY, R. R. J. and NURLIGN, A. Developmment of a dot-ELISA for detection of leprosy antigenuria under field conditions. Lepr. Rev. 57 Suppl. 2(1986)95-100.
7. SHEPARD, C. C. and MCRAE, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36(1986)78-82.
8. VEMURI, N., KHANDKE, L., MAHADEVAN, P. R., HUNTER, S. W. and BRENNAN. P. J. Isolation of phenolic glycolipid I from human lepromatous nodules. Int. J. Lepr. 53(1985)487-489.
9. VENKATESAN, K., MINNIKIN, D. E., SINGH, H., RAMU, G. and BHARADWAJ, V. P. Detection of mycobacterial lipids in skin biopsies from leprosy -patients. FEMS Microbiol. Lett. 44(1987)167-172.
10. YOUNG, D. B., HARNISCH, J. P., KNIGHT, J. and BUCHANAN, T. M. Detection of phenolic glycolipid I in sera from patients with lepromatous leprosy. J. Infect. Dis. 152(1985)1078-1081.Chem.