- Volume 60 , Number 2
- Page: 173–84
Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the Leprosy Control Program of the all Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia
ABSTRACT
This paper reports on reactions in leprosy patients who were treated with multidrug therapy (MDT) in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Only those reactions which occurred in patients who had not been treated with dapsone before MDT and which required treatment with prednisolone were included. Until the end of the second year of MDT a reversal reaction had been diagnosed in 43.6% of 266 borderline lepromatous (BL) patients and in 19.2% of 109 lepromatous leprosy (LL) patients, and an erythema nodosum leprosum (ENL) reaction in 2.7% and 11.1% of the patients, respectively. The reversal reactions were observed in 4.9% of the BL patients and in 0% of the LL patients at the time of diagnosis of leprosy, in 26.3% and 12.8% of the patients during the first year of MDT, and in 12.4% and 6.4% during the second year of MDT. ENL reactions were seen in 0.8% of BL patients at diagnosis, 1.1% in the first year and 0.8% in the second year and 2.8% at diagnosis, 5.5% in the first year, and 2.8% in the second year for LL patients. During a 3½-year period, a total of 405 reactions were diagnosed among multibacillary (MB) patients on MDT; 365 of these reactions (90.1 %) were reversal reactions and only 40 (9.9%) were ENL reactions. The point in time of the reversal reactions showed that the risk of reversal reaction is highest during the first year of MDT. Thereafter there is a gradual decline, although reactions were still observed during the fifth year of MDT. A reversal reaction was diagnosed in 21.0% of 438 BT patients; in 3.4% of the patients the reaction was present at the time of diagnosis of leprosy; in 10.3% it occurred during MDT, and in 7.3% during the first year after release from MDT.During a period of 3½ years a total of 183 reversal reactions were diagnosed among BT patients. The point in time showed a declining trend in the risk of reversal reaction after starting MDT. The risk is highest during MDT, followed by the first 6 months after stopping MDT. However, reactions, although few, still occurred during the fourth year after stopping MDT .
The analysis of the results of prednisolone treatment in 161 patients who were treated for nerve function loss in the field showed that 142 patients (88.2%) regained complete or partial recovery of the nerve function(s), while no improvement was observed in 19 patients (11.8%). With the criteria defined for field treatment of reactions, about 85% of the patients could be treated with standard courses of prednisolone in the field. The main reasons for referral to the hospital were severe ENL reactions, associated med ical problems whether or not related to leprosy, examination for a possible relapse, recurrent reactions, and deterioration of nerve function. It was estimated that during the period in which all patients who needed prednisolone were required to be hospitalized, less than one third of the patients who developed a reaction were treated for it.
In addition to the finding that substantially more patients will be treated for their reactions if prednisolone is given in the field, other advantages of field treatment over hospital treatment are: a) it is much more convenient and economical for the patients, b) it has a positive effect on the credibility of the leprosy control services, c) it motivates the staff to do regular sensory and voluntary muscle testing, and d) it is substantially less expensive.
RÉSUMÉ
Cet article concerne les réactions chez les malades de la lèpre qui ont été traitées par polychimiothérapic (PCT) dans le programme de lutte contre la lèpre du "Ail Africa Leprosy and Rehabilitation Training Center (ALERT)" en Ethiopie. Seules les réactions survenues chez des patients qui N'avaient pas été traités par dapsone avant la PCT et qui nécessitaient un traitement par prednisolone ont été pris en compte. Jusqu'à la FIN de la deuxième année de PCT, une réaction d'inversion a été diagnostiquée chez 43.6% de 266 patients borderline lépromatcux (BL) et 19.2% de 109 patients lépromatcux (LL), et une réaction d'érythème noueux lépromatcux (ENL) chez respectivement 2.7% et 11.1% des patients. Des réactions d'inversion ont été observées chez 4.9% des patients BL et 0% des patients LL au moment du diagnostic de lèpre, chez 26.3% et 12.8% des patients durant la première année PCT, et chez 12.4% et 6.4% durant la deuxième année, contre 2.8% au diagnostic, 5.5% durant la première année et 2.8% durant la deuxième année pour les patients LL.Durant une période de 3½ ans, un total de 405 réactions ont été diagnostiquées parmi des patients multibacillaires (MB) sous PCT; 365 de ces réactions (90.1%) étaient des réactions d'inversion et seulement 40 (9.9%) étaient des réactions ENL. Le risque de réaction réverse est le plus élevé durant la première année de PCT. Par après, il y a une diminution progressive, bien que des réactions ont encore été observées durant la cinquième année de PCT.
Une réaction d'inversion a été diagnostiquée chez 21.0% de 438 patients BT; chez 3.4% des patients, la réaction était présente au moment du diagnostic de lèpre; chez 10.3% elle survint durant la PCT, et chez 7.3% durant la première année après l'arrêt de la PCT. Durant une période de 3½ ans, un total de 183 réactions d'inversion ont été diagnostiquées parmi les patients BT. Le risque de réaction réverse avait tendance à diminuer après le début de la PCT. Le risque est le plus élevé durant la PCT, puis durant les 6 premiers mois après l'arrêt de la PCT. Cependant des réactions, bien que peu nombreuses, sont encore survenues au cours de la quatrième année après l'arrêt de la PCT.
L'analyse des résultats du traitement à la prednisolone chez 161 patients traités sur place pour perte de fonction nerveuse a montré que 142 patients (88.2%) ont récupéré totalement ou partiellement leur(s) fonction(s) nerveuse(s) tandis qu'aucune amélioration N'a été observée chez 19 patients ( 11.8%). EN se basant sur les critères établis pour le traitement sur place des réactions, environ 85% des patients pouvaient être traités sur place avec des schémas standards de prednisolone. Les raisons principales pour référer à l'hôpital étaient des réactions sévères d'ENL associées à des problèmes médicaux en rapport ou non avec la lèpre, un examen pour une rechute possible, des réactions récidivantes et une détérioration de la fonction nerveuse. Il a été estimé que durant la période au cours de laquelle tous les patients nécessitant de la prednisolone devaient être hospitalisés, moins d'un tiers des patients qui ont développé une réaction ont été traités pour celle-ci.
En plus du fait que significativement plus de patients sont traités pour leurs réactions si la prednisolone est administrée sur place, il y a d'autres avantages à ce traitement de terrain par rapport à l'hospitalisation: a) c'est beaucoup plus pratique et économique pour les patients, b) cela a un effet positif sur la crédibilité des services de lutte contre la lèpre; c) cela motive le personnel à tester régulièrement la sensibilité et les muscles volontaires, et d) c'est significativement moins cher.
RESUMEN
Este trabajo se refiere a las reacciones en los pacientes con lepra que fueron tratados con terapia multidroga (MDT) durante el programa de control de la lepra del All Africa Leprosy and Rehabilitation Training Center (ALERT) en Etiopia. Se incluyeron solo aquellos casos de reacciones ocurridas en los pacientes que no habian sido pretratados con dapsona y que requirieron del tratamiento con prednisolona. Hasta el final del segundo año de MDT se había diagnosticado una reacción reversa en el 43.6% de 266 pacientes lepromatosos subpolares (BL) y en el 19.2% de 109 pacientes con lepra lepromatosa (LL), y una reacción tipo eritema nodoso leproso (ENL) en el 2.7% y 11.1% de los pacientes BL y LL, respectivamente. Las reacciones reversas se observaron en el 4.9% de los pacientes BL y en el 0% de los pacientes LL al momento del diagnóstico de la enfermedad, en el 26.3% (BL) y 12.8% (LL) durante el primer año de MDT, y en el 12.4% (BL) y 6.4% (LL) durante el segundo año de MDT. Las reacciones ENL se observaron en el 0.8% de los pacientes BL al tiempo del diagnóstico, 1.1% en el primer año y 0.8% en el segundo año; en los pacientes LL, en el 2.8% en el tiempo del diagnóstico, 5.5% en el primer año, y 2.8% en el segundo año.Durante un periodo de 3.5 años, se diagnosticaron un total de 405 reacciones entre los pacientes multibacilares (MB) bajo MDT; 305 de estas (90.1%) fueron reacciones reversas y solo 40 (9.9%) fueron reacciones ENL. El análisis de los resultados indicó que el riesgo de las reacciones reversas es mayor durante el primer año de MDT. Después hay una disminución gradual, aunque aun se observaron reacciones durante el quinto año de MDT.
En el caso de la lepra tuberculoide subpolar (BT), se diagnosticaron reacciones reversas en el 21.0% de 438 pacientes BT; en 3.4% de los pacientes la reacción se presentó hacia el tiempo del diagnóstico, en el 10.3% de los casos la reacción apareció durante la MDT, y en el 7.3% de los mismos durante el primer año después de suspenderse el tratamiento. Durante un periodo de 3.5 años se diagnosticaron 183 reacciones reversas entre los pacientes BT. El análisis de los datos indicó que el riesgo de desarrollar una reacción reversa tendió a declinare después de empezar el tratamiento. El riesgo es mayor durante la MDT y menor durante los 6 meses siguientes a la suspensión del tratamiento, aunque algunas reacciones todavía ocurrieron durante el cuarto año después de haber suspendido la MDT.
El análisis de los resultados obtenidos en un grupo de 161 pacientes que fueron tratados con prednisolona por pérdida de función nerviosa, mostró que 142 pacientes (88.2%) recuperaron completa- o parcialmente la función nerviosa perdida mientras que no se observó ninguna mejoría en 19 de los pacientes (11.8%). Con los criterios definidos para el tratamiento ambulatorio de las reacciones, cerca del 85% de los pacientes pudieron ser tratados con esquemas estándar de prednisolona. Las razones principales para hospitalizar a los pacientes fueron reacciones ENL severas, problemas médicos asociados relacionados o no con la lepra, examen de una posible recaída, reacciones recurrentes, y deterioro de la función nerviosa. Se calculó que menos de ⅓ de los pacientes en reacción que necesitaban tratamiento con prednisolona, accedieron a hospitalizarse para recibir el tratamiento.
Además del hallazgo de que substancialmente más pacientes en reacción podrían ser tratados con prednisolona si esta se administra de manera ambulatoria, otras ventajas de este tratamiento sobre el tratamiento intrahospitalario son: (a) que es mâs conveniente para cl paciente, (b) que tienen un efecto positivo sobre la credibilidad de los scrvicios de control de la Lepra, (c) que motiva al Personal para realizarpruebasde funciôn nerviosa de manera regular, y (d) que es mucho mâs barato.
A major problem in leprosy is the development of reactions and, in particular, the loss of peripheral nerve function which, if not diagnosed and treated in time, will result in disabilities. Reactions are caused by immunologically mediated inflammation which is associated with Mycobacterium leprae antigens which persist in tissues after bacterial death (26). Although the development of nerve damage after the start of effective chemotherapy can hardly be prevented, it is certainly not inevitable that this should lead to irreversible loss of nerve function. If diagnosed soon after its occurrence and treated adequately, the loss of nerve function is often reversible. Increase in disability as a result of neglect of nerve function loss will be of influence in maintaining the fear and stigma of the disease, and patients may lose their confidence in the leprosy control services if they experience a worsening of their condition.
In most programs the treatment of leprosy reactions, requiring corticosteroids, was and still is exclusively the responsibility of hospitals. During recent years it has been more and more recognized that the leprosy control services should, besides early diagnosis of reactions, also take the major responsibility of the treatment of reactions (21, 24, 32, 33)
In this paper, findings on the occurrence of reactions in patients treated with multidrug therapy (MDT) in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia are presented. In addition, the experiences with diagnosing reactions, management of the reactions, and results of prednisolone treatment under routine field conditions are discussed. The problem of distinguishing between a relapse and a reversal reaction after stopping MDT is discussed in another paper (6), Reactions caused by a cellular immune response or delayedtype hypersensitivity, which occur in paucibacillary (PB) or multibacillary (MB) patients, are referred to as reversal reactions. Those caused by a humoral immune response, which occur in MB patients, are referred to as erythema nodosum leprosum (ENL) reactions.
MATERIALS AND METHODS
The ALERT leprosy control program is responsible for leprosy control in the Shoa Region, the central region of Ethiopia, which is divided into 11 rural districts and Addis Ababa. It covers about 85,000 sq. km. with an estimated population of 11 million (1989).
MDT, according to the 1982 recommendations of the World Health Organization (WHO) (29), was introduced in January 1983, and was implemented in the whole control area by January 1988. PB patients were clinically indeterminate, tuberculoid (TT) or borderline tuberculoid (BT) patients who had a bacterial index (BI) of not more than 1 at any site. MB patients were clinically borderline lepromatous [BL; including the few midborderline (BB) patients] or lepromatous (LL) patients who had a BI of more than 1 at any site. Since January 1990 patients who were clinically PB but had positive skin smears were included in the MB category, according to the recommendation of a WHO Study Group of 1988 (28), for the choice of the MDT regimen.
PB patients were treated with 6 fourweekly supervised doses of rifampin and 24 weeks of dapsone, unsupervised. The six doses of MDT should be taken within a maximum period of 9 months. MB patients were treated with 26 four-weekly supervised doses of rifampin and clofazimine and 2 years of daily dapsone and clofazimine, unsupervised. The 26 doses of MDT had to be completed within a maximum period of 3 years. Patients who, after the 26 doses of MDT, have positive skin smears should continue MDT until skin-smear negativity(3).
At the time of their release from MDT, the patients were educated about the importance of attending the service immediately after they observe (an increase of) loss of sensation and/or muscle strength. In order to detect reversal reactions at an.early stage, PB patients who were not treated with dapsone before MDT were instructed to attend at 3 and 6 months after stopping treatment. From registers and patient cards it was estimated that 60%-70% of the PB patients did attend during the third and/or sixth month after release from MDT.
Sensation testing (ST) of palms and soles and voluntary muscle strength testing (VMT) of the orbicularis oculi muscles, hands and feet were done at the time of diagnosis of leprosy, every month during MDT, prior to release from MDT, during follow-up examinations after release from MDT, and at any time a patient complained of loss of sensation or loss of muscle strength. The sensation on the palms and soles was routinely tested with a ballpoint pen on 10 standard points; on the ulnar side of the palm 4 standard points were tested, on the median side, 6 points.
Muscle strength was graded as strong, weak, or paralyzed. The muscle strength was considered weak if there was either full movement in the joint but reduced resistance against pressure, or both movement and resistance against pressure were reduced. The strength of the orbicularis muscle was tested on tight closure of the eyes. In case of lagophthalmos, the lid gap was recorded.
Until 1987 all patients who developed a reaction, which required treatment with prednisolone, had to be referred to the ALERT hospital. A serious drawback of this policy was that a substantial proportion of the patients (estimated at at least 50%) were not treated with prednisolone. The main reasons were that if the patients were either not able or not prepared to go to the hospital, they were not referred, e.g., because of transport problems, or they could not be admitted because of insufficient in-patient facilities. Based on these observations and the negative effect they had on the faith of the patients in the leprosy control services and on the motivation of the field staff for doing regular VM T or ST, the need for treatment of reactions in the field was more and more recognized.
During the last months of 1986, the senior staff of ALERT prepared a detailed manual for field treatment of leprosy reactions (2). After training of the field staff and some reorganization of the leprosy control services, the reaction treatment was introduced in three rural districts in April 1987. The implementation was closely supervised, and the operational aspects and results of the treatment were monitored. The treatment was gradually expanded, and its introduction into the whole control area was completed by the end of 1989.
An ENL reaction was considered severe if the nodules were ulcerating, if there was recent nerve function loss and/or signs of iridocyclitis, orchitis, or dactylitis.
A reversal reaction was considered severe if one or more of the following signs were present: a) pain or tenderness in one or more nerves with or without nerve function loss; b) change in VMT of < 6 months' duration; c) change in ST of < 6 months' duration. A change was considered significant when one found: in a hand-an unquestionable increase of loss of sensation in two or more points occurring in the area which is supplied by the same peripheral nerve trunk; in the feet-an unquestionable increase of loss of sensation in two or more points; d) skin reaction in a patch overlying a major nerve trunk or around an eye.
Patients with severe ENL should be referred to the hospital, those with severe reversal reaction should be treated in the field. The following exceptions were made: a) Patients with ulcerating nodules as the only sign of a severe ENL reaction could be treated in the field if they were not able to or unwilling to go to the hospital, b) Patients with a severe reversal reaction and having a deep ulcer(s), nerve abscess, severe eye problems, urine positive for glucose or protein, were suspected of having tuberculosis or any other systemic disease had to be referred to the hospital, c) Patients with a severe reversal reaction who were pregnant or between 6 and 12 years of age and patients who developed the reversal reaction more than 1 year after release from MDT had to be referred to the hospital, but could be treated in the field if they were not able to or unwilling to go to the hospital.
The decision about whether or not the patient should be treated in the field had to be made by a leprosy control supervisor. During field visits and training courses the performance of the field staff in nerve function testing was assessed by the leprosy control senior staff, including a physiotherapist.
Initially, a 12-week standard course of prednisolone was prescribed for all patients with severe reversal reaction. Based on the observation that in about one third of BL patients the reversal reaction recurred during the tailing-off or immediately after stopping prednisolone, the course of prednisolone for MB patients was extended to 20 weeks. Thereafter, very few patients developed recurrent reversal reaction. The initial dose of prednisolone was 40 mg daily given for 2 weeks. To patients with ulcerating nodules as the only sign of severe ENL, pregnant patients, and children between the ages of 6 and 12 years, who were treated in the field, modified courses of prednisolone were prescribed.
Patients whose nerve function deteriorated while on prednisolone were referred to the hospital. The supervisors prepared monthly reports about the patients who had been diagnosed with leprosy reaction. For each patient who was treated with prednisolone in the field they prepared a report with details on the clinical findings at the time of diagnosis of the reaction and the findings at the end of the prednisolone course.
Data about reactions are presented for 7 of the 11 rural districts of the Shoa Region and Addis Ababa, where field treatment of reaction was introduced during 1987 and 1988. During the period of study (July 1987 to December 1990), approximately 70% of all new patients diagnosed with leprosy in the ALERT leprosy control services resided in the study area. In the analysis, only reactions which required treatment with prednisolone and which were diagnosed in patients who had not been treated with dapsone before MDT are included. Patients who developed a recurrent reaction were included only once.
RESULTS
The data on the incidence of reversal reactions and ENL reactions at the time of diagnosis of leprosy and during the first 2 years of MDT in 266 BL and 109 LL patients, who started MDT during the period July 1987 to December 1988, are presented in Table 1. The 13 BL patients who presented with a reversal reaction at the time they were diagnosed with leprosy had one or more tender nerves, 11 of them with corresponding nerve damage. The incidence of reversal reactions is significantly higher in BL than in LL patients (χ2 = 18.7, p < 0.005). The incidence of ENL reactions is significantly higher for LL patients (χ2 = 9.6, p < 0.005).
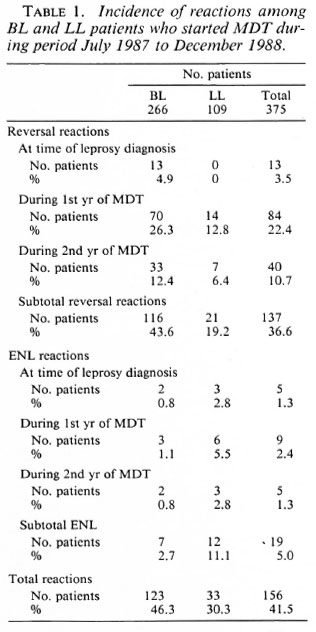
Table 2 presents the point in time of all reversal and ENL reactions which were diagnosed among BL and LL patients during a 3 ½ -year period, at the time the patients were diagnosed with leprosy and during MDT. During the same period, a reversal reaction after release from MDT was diagnosed in 10 BL and 4 LL self-reporting patients.
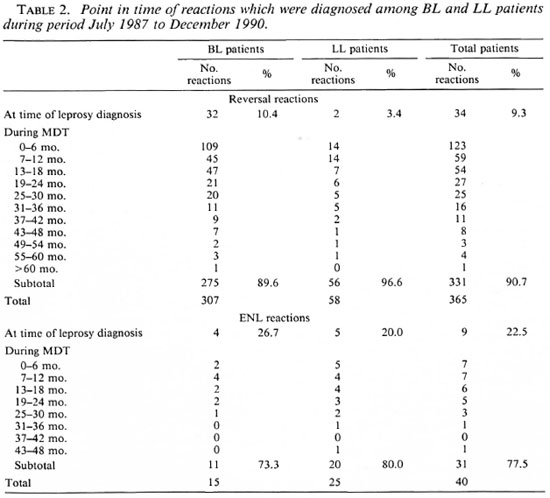
The incidence of reversal reactions in BT patients who started MDT during two consecutive years is given in Table 3. The reactions which were diagnosed during the first year after release from MDT were observed among self-reporting patients. In addition to the 438 BT patients, 67 TT patients were treated with MDT. In none of these TT patients was a reversal reaction diagnosed.
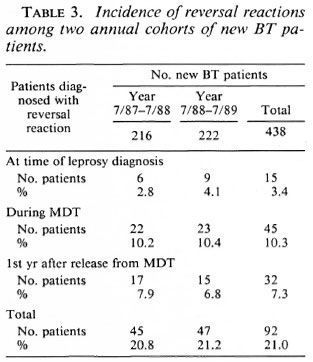
The point in time of all reversal reactions which had been diagnosed in BT patients during a 3 ½ -year period is presented in Table 4. The 77 reactions after stopping MDT were diagnosed in self-reporting patients. In particular, for the patients who developed a reaction more than 1 year after release from MDT, the possibility of a relapse of leprosy was considered. Of the 26 patients who developed a reaction more than 1 year after slopping MDT, 21 (80.8%) responded within a few weeks to prednisolone treatment. This finding, and the observation that these patients did not report with recurrent signs of activity after stopping prednisolone, is in favor of the diagnosis of reversal reaction. Of three patients (11.5%) data were incomplete and in two patients (7.7%), whose nerve function did not improve, the records indicated that the nerve function loss most probably existed for more than 6 months.
During the 3 ½ -year period, 602 reactions were diagnosed in PB and MB patients. These were 405 reactions in MB patients (Table 2), 183 reactions in PB patients (Table 4), and 14 reactions in MB patients after release from MDT. In addition, during the same period 132 reactions were diagnosed in patients who had been treated with dapsonc before they were put on MDT, were still on dapsone, or had been released from dapsone in the study area. These were 734 reactions in total; on average, 210 reactions per year. Because about 70% of the patients resided in the study area, in the whole control area approximately 300 patients could be expected to develop a reaction. During the year before the introduction of field treatment of reactions, a total of only 80 patients had been treated for a reaction at the hospital (1). Thus, less than one third of those patients who developed a reaction were treated with prednisolone before field treatment of reactions was introduced.
Of the 734 patients who were diagnosed with a reaction, 112 patients (15.3%) were referred to the hospital and 622 (84.7%) were treated in the field. The main reasons for hospital referral were: a severe ENL reaction; associated medical problems, whether or not related to leprosy; examination for possible relapse; recurrent reversal reaction; and deterioration of nerve function on prednisolone. Two patients who were started on prednisolone in the field were referred because of complications; in one patient glucose was found in the urine and one patient developed signs suspect of pulmonary tuberculosis.
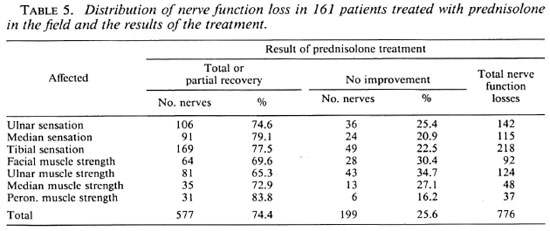
Of 178 patients who had been treated with prednisolone in the field the information at the time of diagnosis of the reaction and the results of the prednisolone treatment were analyzed: 161 (90.4%) of these patients had recent nerve function loss. In 93 (57.8%) of them this was associated with pain or tenderness in one or more nerves. Twelve patients (6.7%) had nerve tenderness without recent nerve function loss, and five patients (2.8%) had a skin reaction in a patch around an eye without facial nerve function loss. Of the 161 patients with recent nerve function loss, in 51 patients (31.7%) only one nerve was affected. Sensation loss on the soles, which was observed in 24 of these 51 patients (47.1%), was the commonest single problem. In the remaining 110 patients (68.3%) more than one nerve was affected.
In these 161 patients, a total of 766 nerve functions were impaired. In Table 5 the distribution of the nerve function loss and the results of prednisolone treatment are presented. Weakness or paralysis of the orbicularis muscle coincided in 37 of 80 eyes (46.3%) showing weakness, and in 9 of 12 eyes (75.0%) showing paralysis with an active skin patch around one or both eyes. In 142 of the 161 patients (88.2%) there was complete or partial recovery of nerve function loss, and there was no improvement in 19 patients (11.8%). In all 17 patients with nerve tenderness or a skin reaction in a patch around an eye, the tenderness or skin reaction subsided on treatment with prednisolone.
DISCUSSION
Surprisingly few data have been published on the frequency of occurrence of reactions. Pfaltzgraff, et al. reported that reversal reactions are most common in patients with BT leprosy, even without chemotherapy (23). In their experience, well over half of BT patients develop a reversal reaction, usually between 2 and 6 months after the start of dapsone. They also reported that about 50% of BL patients will develop a reversal reaction, usually between 2 and 12 months after starting dapsone, and over one half of LL patients and one quarter of BL patients experience an ENL reaction. Rose, et al. reported that during the dapsone era about one third of BL patients developed a reversal reaction in the first year of treatment, and perhaps one half over 5 years(24)
Boerrigter, et al. observed 2.2% marked reactions, leading to steroid treatment, in 503 patients at the time of the diagnosis of leprosy, and 1.7% marked reactions during MDT (8). During the first year of active follow up after stopping MDT, 4.8% of 227 self-reporting patients and 0 of 153 patients found by active case-finding developed a marked reaction. Their data indicate that the chance of late reversal reaction is higher in self-reporting patients than in actively detected cases. Katoch, et al. reported 9% reversal reactions in PB patients during the first 12 months after stopping MDT (15).
Groenen, et al., in a prospective study on the relationship between intensive bactericidal therapy and leprosy reactions, observed in 335 PB patients 6% reactions, the majority of which were mild. In MB patients treated with the MDT regimen recommended by the WHO, 17% reversal reactions and no ENL reactions were observed, compared with 45% and 55% reversal reactions and 20% and 14% ENL reactions in patients treated with regimens which contained ethionamide. They concluded that more aggressive bactericidal regimens cause more reactions (9).
The report frequencies of occurrence of reactions are, to a large extent, not comparable because of: a) some studies were done in a control area and others in a hospital. From the very nature of patients who attend a hospital clinic, the frequency of reactions may be substantially higher there than in the control area, b) different criteria were used, particularly for reversal reactions. In some studies mild and severe reactions were included; in others, only severe reactions. Some investigators reported only those reactions which were spontaneously presented by patients, c) in some studies findings were reported for all PB patients; in others, only for BT patients, d) some studies were done in areas where most patients were self-reporting; in other studies, many patients were actively detected cases, e) in some studies follow up of patients after stopping MDT was active; in others, the reactions were observed in self-reporting patients. Nevertheless, it certainly can be concluded that a substantial proportion of leprosy patients undergo a reaction.
In this study, the vast majority of the reactions in MB patients were reversal reactions. Only 19 (12.2%) of the 156 reactions which were either present at the time of the diagnosis of leprosy or occurred during the first 2 years of MDT (Table 1) and 40 (9.9%) of the total of 405 reactions (Table 2) were ENL reactions. A decline in ENL reactions after the introduction of the WHO-recommended MDT regimen has also been observed by others (22, 27, 31, 33). The antiinflammatory activity of the daily dose of 50 mg clofazimine appears adequate to prevent ENL reactions in many patients. This is a very important advantage of the MDT regimen for MB patients recommended by the WH O over regimens which include a thioamide instead of clofazimine.
Although diagnosis and proper management of severe ENL reactions remain important, a major challenge in patients treated with the WHO-recommended MDT regimen is the early diagnosis and adequate treatment of severe reversal reactions. The development of reversal reactions in MB patients is a particular problem in BL patients. Because BL patients have some degree of cell-mediated immunity to M . leprae (this is absent in polar LL patients), this is what one expects. The LL patients diagnosed with a reversal reaction most probably were either established subpolar LL patients or patients who downgraded from borderline leprosy. Other possibilities are that some BL patients were incorrectly classified as LL or that part of the ENL reactions, notably those with nerve function loss as the predominant sign, were diagnosed as reversal reactions.
The risk of reversal reactions among MB patients appears highest during the first year of MDT; thereafter, there is a steady decline (Table 2). Because reactions were still observed during the third and subsequent years of MDT, still more reactions can be expected among the patients who are presented in Table 1. Taking into consideration that 43.6% of the BL patients and 19.2% of the LL patients were diagnosed with a reversal reaction, either at the time of diagnosis of leprosy or within the first 2 years of MDT (Table 1), probably on the order of 50% of BL patients and 25% of the LL patients will develop a reversal reaction before they are released from MDT.
Because reactions in BT patients after stopping MDT were diagnosed among selfreporting patients, and not all patients who developed a reaction may have presented themselves, the finding that 21.0% of BT patients developed a reversal reaction may be an underestimate. On the other hand, there might have been some overdiagnosis of reactions because of the anxiety of field staff to prevent (deterioration of) nerve function loss. Through training, close supervision, and regular assessment of the findings by senior staff members this, to a large extent, was prevented.
The observation that a substantial proportion of the reversal reactions in BT patients occurred more than 6 months after starting MDT is quite different from the finding by Naafs, et al. in patients treated with dapsone. They reported that 98% of the reversal reactions in BT patients are found either at the time of diagnosis of leprosy (60%) or during the first 6 months of dapsone (38%) (19). They are of the opinion that the suppression of cell-mediated immunity by dapsone prevents the occurrence of reversal reactions, particularly after the total antigen load released from dead bacilli has decreased (17, 19). Suppression of cell-mediated immunity by dapsone has been shown in vitro (5) and in vivo (4). Katoch, et al. reported that continuation of treatment with 1 year of dapsone after MDT will prevent the occurrence of reactions (14, 15). However, this has not been proven in a double-blind clinical trial. In our opinion there is no justification yet for the continuation of dapsone in patients who do not require antileprosy treatment and of whom the majority will not develop a reversal reaction after 6 months of MDT.
The finding that 7.3% of the patients developed reversal reactions during the first year after stopping MDT is comparable with the observation by others that 4.8% (8) and 9.0% (15) of the patients developed a reversal reaction during the first year of follow up. Because reversal reactions occurred more than 1 year after release from MDT, in the cohorts of patients presented in Table 3 more reactions can be expected. Rose, et al. reported that the majority of the reversal reactions in BT patients develop during the first 6 months of treatment, but they are not rare during the second 6 months and, with increasing rarity, undoubtedly may occur during the second, third and fourth years (24). A similar declining trend can be observed in the present material. The risk of reaction after stopping MDT appears to be highest during the first year, particularly during the first 6 months. Therefore, the main emphasis should be given to examination of the patients during the first year after stopping MDT. The usual recommendation that PB patients should be examined annually after stopping MDT (28) is insufficient for the early detection of reactions. In our view, at least during the first year after release from MDT, patients should be scheduled for examination at 3-month intervals.
Concerning the treatment of severe reversal reactions, there is wide variation in the recommended duration of treatment with corticosteroids as well as in the dosages of prednisolone, the most commonly used corticosteroid (6, 8, 11-13, 18, 20, 22, 24, 25, 30). Recommendations about dosages range from individually tailored regimens to semiand fully standardized regimens. Some advise an initial daily dose of 60-80 mg prednisolone; others found that an initial dosage of 30 mg or 40 mg of prednisolone is adequate in most patients. The advised period of treatment is 3 to 9 months for BT patients and 6 to 24 months for BL patients. When the guidelines for field treatment of leprosy reactions in the ALERT leprosy control program were prepared, major considerations were that the initial doses of prednisolone should be high enough, and prednisolone should be given long enough, to control the reaction in the vast majority of patients, while the risk of complications should be low. Further, the period of treatment should be operationally feasible. At the ALERT hospital reversal reactions had already been treated for several years with standard courses of prednisolone, with an initial dose of 40 mg daily. For treatment in the field it was decided to give a standard regimen, while the total period of treatment was shortened from 24 to 12 weeks and, subsequently, lengthened to 20 weeks for MB patients. The observation that BL patients need a longer period of treatment with prednisolone has also been reported by others (18).
Even though in most programs patients who require treatment with corticosteroids should be admitted to the hospital, where assessment of patients can be done frequently, very little has been published about the results of corticosteroid treatment. Touw-Lagendijk, et al. studied the improvement of median and ulnar nerve function in borderline leprosy patients who had been treated with a 6-month course of prednisolone (25). In 80.6% of 36 patients, who were followed for 1 year after completion of prednisolone, the nerve function improved. The nerve function remained unchanged in 11.1% of the patients and deteriorated in 8.3%. In addition to ST and VMT, motor nerve conduction velocity (MCV) was examined, while nerve indexes were used for assessment of the improvement of nerve function. Because of different assessment techniques and a follow-up period of 1 year, their findings are not comparable with those presented in this paper.
Kiran, et al. reported on the results of treatment of recent facial nerve damage with lagophthalmos, using a semistandardized steroid regimen (16). After completion of the steroid course, 75% of 36 eyes had complete closure or only a slight lid gap of 2 mm or less on gentle closure. Hogeweg, et al. observed that 85% of the patients with recent facial nerve damage showed significant patches over the malar region or around the eye, and that 45% of the patients with a significant red and raised patch in reaction developed lagophthalmos (10). In the present material also, significant percentages of patients diagnosed with facial nerve function loss had an active patch around one or both eyes. Because patients with a facial patch in reaction have a high risk of developing facial nerve function loss, it appears appropriate to treat such patients with a course of prednisolone rather than waiting until they have developed lagophthalmos. Although there is very little material to compare with, an overall result of complete or partial recovery of nerve function loss in 88.2% of the patients should be considered satisfactory. Others observed that nerve function may improve further after stopping prednisolone (25). This was not assessed in the present study.
From the experience in the ALERT leprosy control program a number of points emerge:
a) The vast majority of patients, in this analysis about 85%, can be treated with standard courses of prednisolone in the field. However, hospital admission of some patients remains essential, particularly for patients with severe ENL reaction, those with additional medical problems (whether or not related to leprosy), and patients whose nerve functions deteriorate while on a standard prednisolone course. Further, until operational criteria for differentiation between a relapse and a late reversal reaction have been defined, patients who develop nerve function loss after release from MDT should be referred to the hospital for further examination.
b) The standard courses of prednisolone appear adequate to reverse nerve function loss in the majority of the patients.
c) If treatment of reactions remains exclusively the responsibility of hospitals, a substantial proportion of the patients may not be treated at all. In this study it was observed that before the introduction of field treatment of reactions, less than one third of the patients who developed a reaction were treated with prednisolone.
d) Detailed instructions on the diagnosis of reactions, selection of patients for field treatment, prednisolone courses, follow up and monitoring of patients, and the results of the treatment should be developed.
e) The field staff should be trained and closely supervised.
In addition, the following advantages of field treatment of reactions over hospital treatment were observed:
a) It is much more convenient and economical for the patients.
b) It has a positive effect on the credibility of the leprosy control services.
c) It motivates the staff for doing regular ST and VMT examinations.
d) It is substantially less expensive than hospital treatment.
Acknowledgment. We should like to express out thanks to the ALERT leprosy control supervisors who recorded the data and to the ALERT/AHRI Research Committee for approving the study on operational aspects of MDT. Our thanks also go to Dr. D. Frommel for his encouragement and comments, and to Prof. Dr. A. S. Muller and Dr. B. Naafs for their comments and suggestions.
REFERENCES
1. ALERT. Annual report. Addis Ababa: Armauer Hansen Research Institute, 1986, p. 51.
2. ALERT. Manual for field treatment of leprosy reactions. Addis Ababa: Armauer Hansen Research Institute, 1986.
3. ALERT. Manual for implementation of MDT. 3rd. rev. ed. Addis Ababa: Armauer Hansen Research Institute, 1990.
4. Barnetson, R. St.C, Pearson, J. M. H. and Rees, R. J. W. Evidence for prevention of borderline leprosy reaction by dapsone. Lancet 2( 1976)1171-1172.
5. Beiguelman, B. and Pisani, R. C. B. Effect of DDS on phytohemagglutinin-induced lymphocyte transformation. Int. J. Lepr. 42(1974)412-415.
6. Becx-Bleumink, M. Relapses among leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992) 161-172.
7. Becx-Bleumink, M., Berhe, D. and Mannetje, W. The management of nerve damage in the leprosy control services. Lepr. Rev. 61(1990)1-11.
8. Boerrigter, G., Ponnighaus, J. M. and Fine, P. E. M. Preliminary appraisal of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
9. Groenen, G., Janssens, L., KAYEMBE, T., Nollet, E., Coussens, L. and Pattyn, S. R. Prospective study on the relationship between intensive bactericidal therapy and leprosy reactions. Int. J. Lepr. 54(1986)236-244.
10. Hogeweg, M., Kiran, K. U. and Suneetha. S. The significance of facial patches and type 1 reactions for the development of facial nerve damage in leprosy; a retrospective study among 1226 paucibacillary leprosy patients. Lepr. Rev. 62(1991)143-149.
11. Imkamp, F. M. J. H. Standardized schemes for steroid treatment in ENL and reversal reactions. Int. J. Lepr. 53(1985)313-317.
12. Jacobson, R. R. Treatment. In: Leprosy. Hastings, R. C, ed. London: Churchill Livingstone, 1985, pp. 211-217.
13. Job, C. K. Nerve damage in leprosy. Int. J. Lepr. 57(1989) 532-539.
14. Katoch, K. Recent trends in chemotherapy of paucibacillary leprosy. ICMR Bull. 20(1990)53-57.
15. Katoch, K., Ramanathan, U., Natrajan, M., Bagoa, A. K., Bhatia, A. S., Saxena, R. K. and Ramu, G. Relapses in paucibacillary patients after treatment with three short-term regimens containing rifampin. Int. J. Lepr. 57(1989)458-464.
16. Kiran, K. U., Hogeweg, M. and Suneetha, S. Treatment of recent facial nerve damage with lagophthalmos and using a semistandard steroid regimen. Lepr. Rev. 62(1991)150-154.
17. Naafs, B., Lyons, N. F., M atemera, B..O. and Ellis, B. P. B. Short term WHO advised multiple drug treatment of paucibacillary' patients. (Letter) Indian J. Lepr. 58(1986)348-353.
18. Naafs, B., Pearson. J. M. H. and Wheate, H. W. The prevention of permanent nerve damage; comparison of short and long-term steroid treatment. Int. J. Lepr. 47(1979)7-12.
19. Naafs, B. and Wheate, H. W. The time interval between the start of anti-leprosy treatment and the development of reactions in borderline patients. Lepr. Rev. 49(1978)153-157.
20. Pearson, J. M. H. The use of corticosteroids in leprosy. Lepr. Rev. 52(1981)293-298.
21. Pearson, J. M. H. The evaluation of nerve damage in leprosy. Lepr. Rev. 53(1982)119-130.
22. Pfaltzgraff, R. E. The management of reaction in leprosy. Int. J. Lepr. 57(1989)103-109.
23. Pfaltzgraff, R. E. and Bryceson, A. Clinical leprosy. In: Leprosy. Hastings, R. C ed. London: Churchill Livingstone. 1985, pp. 165-171.
24. Rose, P. and Waters, M. F. R. Reversal reactions in leprosy and their management. Lepr. Rev. 62(1991)113-121.
25. Touw-Langendijk, E. M. J., Brandsma, J. W. and Andersen, J. G. Treatment of ulnar and median nerve function loss in borderline leprosy. Lepr. Rev. 55(1984)41-46.
26. Waters, M. F. R. The chemotherapy of leprosy. In: The Biology of the Mycobacteria, VolumeClinicalAspects of Mycobacterial. London: Academic Press, 1989, pp. 405-474.
27. UNDP/World Bank/WHO Special Programme for Research an d Training in Tropical Diseases. Report of the fifth meeting of the Scientific Working Group in the Chemotherapy of Leprosy. Geneva: World Health Organization, 1986. TDR/ THELEP-SWG(5)86.3.
28. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
29. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
30. World Health Organization. AGuide to leprosy control. 2nd ed. Geneva: World Health Organization, 1988.
31. World Health Organization. Report of a consultation on implementation of multidrug therapy for leprosy control. Geneva: World Health Organization, 1985. WHO/LEP/85.1.
32. World Health Organization. Report of a consultation on disability prevention and rehabilitation in leprosy, Geneva, 9-11 March 1987. Geneva: World Health Organization, 1987. WHO/ CDS/LEP/87.3.
33. World Health Organization. Report of the third coordinating meeting on implementation of multidrug therapy (MDT) in leprosy control programmes, The Hague, 1988. Geneva: World Health Organization, 1988. WHO/CDS/LEP/88.4.
1. M.D., D.T.P.H.,All Africa Leprosy and Rehabilitation Training Center (ALERT) and Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia.
2. B.Sc, M.P.H., M.D., All Africa Leprosy and Rehabilitation Training Center (ALERT) and Armauer Hansen Research Institute (AHRI), Addis Ababa, Ethiopia.
Reprint requests to Dr. Becx-Bleumink, Plasweg 15, 3768 AK Soest, The Netherlands.
Received for publication on 15 October 1991.
Accepted for publication in revised form on 11 March 1992.