- Volume 60 , Number 2
- Page: 185–8
Intravascular hemolysis and acute renal failure following intermittent rifampin therapy
ABSTRACT
La insuficiencia renal es una rara complicación asociada con el uso de rifampina. También son extre + ND + madamente raros los casos de hemolisis intravascular que conducen a la insuficiencia renal en los pacientes tratado con este fármaco. En esta comunicación, reportamos el caso de 2 pacientes con lepra que desarrollaron hemolisis c insuficiencia renal aguda como consecuencia de su tratamiento con rifampina.RÉSUMÉ
L'insuffisance rénale est une complication rarement associée à l'utilisation de la rifampicinc. L'hémolyse intravasculaire menant à une insuffisance rénale aigüe suite à un traitement par rifampicinc est extrêmenent rare. On rapporte deux patients lépreux ayant développé une hémolyse et une insuffisance rénale aigüe à la suite d'un traitement par rifampicine.RESUMEN
La insuficiencia renal es una rara complicación asociada con el uso de rifampina. También son extre + ND + madamente raros los casos de hemolisis intravascular que conducen a la insuficiencia renal en los pacientes tratado con este fármaco. En esta comunicación, reportamos el caso de 2 pacientes con lepra que desarrollaron hemolisis c insuficiencia renal aguda como consecuencia de su tratamiento con rifampina.Rifampin is used widely in the treatment of tuberculosis and leprosy, and only minimal side effects arc reported with daily administration of the drug (18). Severe adverse reactions such as "flu"-likc syndrome, shock, dyspnea, hemolytic anemia, and renal failure have been reported when rifampin is given intermittently or restarted after an interruption (11, 18). We report here two patients who developed acute intravascular hemolysis and renal failure following intermittent rifampin therapy.
CASE 1
A 42-year-old male was diagnosed as having borderline tuberculoid leprosy in January 1988, and was started on oral dapsone 100 mg daily, clofazimine 200 mg daily, and rifampin 600 mg once a month. He continued this therapy until July 1988, after which he stopped taking all of the drugs on his own. One month later he restarted rifampin. Three hr after taking a single 600mg dose of this drug, he developed fever associated with chills and rigors, loin pain, nausea and vomiting, and became anuric. He was treated in a local hospital where his condition deteriorated. On the third day of this illness, his blood pressure was noted to be 80/50 mm of Hg. He was given intravenous fluids and referred to our institute.
On physical examination at the time of admission, he was febrile and had marked pallor, jaundice and acidotic breathing. His blood pressure was 130/80 mm of Hg; the chest, heart and abdomen were unremark able. He was drowsy and showed a flapping tremor, but there was no other neurological deficit. Laboratory investigations revealed evidence of severe intravascular hemolysis, hyperkalemia and renal failure (The Table).
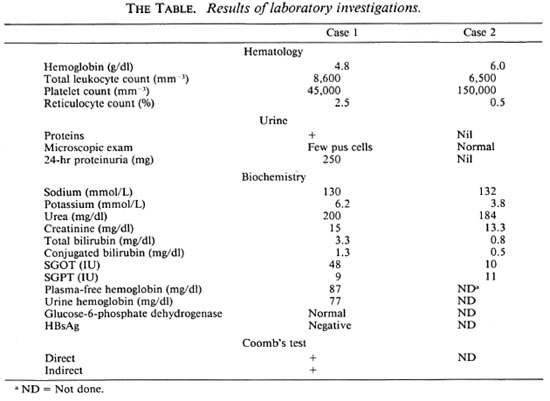
He was initiated on dialysis for renal failure and following 6 hemodialyses, he went into a diuretic phase on the 15th day of oliguria. His renal function returned to normal in the following 5 weeks. On follow up, his serum creatinine varied between 1.2 to 1.5 mg/dl. A kidney biopsy done during the oliguric phase was consistent with acute tubular necrosis; tubules contained pigment casts (The Figure).
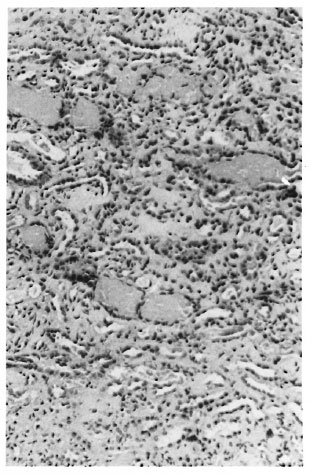
The Figure. Kidney biopsy of Case 1 showing pigment casts in many tubules and acute tubular necrosis.
In October 1988, he resumed rifampin therapy again on the advice of another general practitioner and within 30 min of taking 600 mg of the drug, he developed fever of 105ºF associated with chills and rigors, lumbar pain, vomiting and oliguria. Within 48 hr, his scrum creatinine rose to 6 mg/dl and blood urea nitrogen to 100 mg/dl. The reticulocyte count was 2.5% and serum bilirubin 3.3 mg/dl with a conjugated fraction of 1.3 mg/dl. His plasma-free hemoglobin was 80 mg/dl, and Coomb's test (direct and indirect) was positive. On the fourth day, his urine output increased, and over the following week the blood biochemistries returned to normal.
CASE 2
A 40-year-old man had been taking homeopathic drugs for Hansen's disease since 1977. In 1982, he consulted a medical practitioner and was started on dapsone 100 mg/ day. Because of inadequate response to dapsone, rifampin 600 mg every 2 weeks was added in February 1988. Four months later, on the day of taking rifampin he developed dyspnea, vomiting and diarrhea. Over the next 2 days he developed generalized swelling over the body. There was no oliguria, hematuria, cola-colored urine, or jaundice. He was examined in a local hospital where his blood pressure was noted to be 160/100 mm of Hg. Laboratory investigations done on the seventh day showed a blood urea nitrogen of 210 mg/dl and serum creatinine of 13.4 mg/dl, and he was referred to our institute.
On examination at the time of admission, he looked markedly pale and had generalized anasarca. He had hypopigmented and anesthetic patches over the face. Peripheral nerves were thickened. Blood pressure was 130/80 mm of Hg. The chest, heart, and abdomen were unremarkable. Apart from the anesthetic patches, a central nervous system examination revealed no abnormality. Investigations (The Table) and the clinical course suggested a nonoliguric, acute renal failure.
Following two hemodialyses, his serum creatinine came down to 2.7 mg/dl on the seventh day and to 1.2 mg/dl on the 12th day. A percutaneous renal biopsy revealed histological changes of acute tubercular necrosis. Pigment casts were seen in several tubules.
DISCUSSION
Acute renal failure is a well known but rare complication of rifampin therapy. Among the reported cases, only 11 episodes of acute renal failure have occurred in patients receiving continuous therapy, while the remaining patients were on intermittent or interrupted therapy.
Most of the reported cases have shown histological lesions consistent with acute tubulo-interstitial nephritis (7, 17, 19) and acute tubular necrosis (1, 5, 10). Occasional cases have revealed acute cortical necrosis (4) and rapidly progressive glomerulonephritis (13, 16). Light-chain proteinuria (15) has also been reported following rifampin therapy. The exact pathogenesis of acute renal failure is not known. Rifampin-antibody complexes formed in the circulation have been suspected to play a role in a sensitized patient, thereby inducing a high renin secretion and preglomerular arteriolar constriction leading to renal cortical ischemia (14). Obstruction to blood flow resulting from swelling of the glomerular endothelial cells, leading to ischemic tubular injury, has also been postulated (2). Antirifampin antibodies and complement activation have also been suspected to cause acute renal failure (2).
The binding of rifampin antigen-antibody complexes to normal red blood cells may induce hemolysis (20) which is well known to predispose to acute renal failure(3, 9). The simultaneous occurrence of acute intravascular hemolysis and renal failure due to rifampin has been reported in only a few cases (6, 8, 12, 17).
In Case 1, who had developed a "flu"like syndrome, severe intravascular hemolysis and renal failure, the possibility of an associated glucose-6-phosphate dehydrogenase deficiency, which is not uncommon among our patients (3), was clearly excluded. Moreover, this patient had immune hemolytic anemia since the Coomb's test was positive. Rifampin antibody, however, could not be measured.
The temporal relationship of rifampin therapy with the onset of acute renal failure clearly supported rifampin to be the major pathogenetic factor in the causation of renal failure in Case 2. Since the tubules were loaded with pigment casts, it is possible that intravascular hemolysis had occurred in this patient also. The absence of hyperbilirubinemia had a high reticulocyte count are not surprising since this patient had presented 10 days after the onset of his illness. Both of these patients recovered completely. Recovery in rifampin-induced acute renal failure has been reported to be usually complete except for an occasional case (4).
REFERENCES
1. Campese, V. M., Marzullo, F., Schena, F. P. and Coratelli, P. Acute renal failure during intermittent rifampin therapy. Nephron 10(1973) 256-261.
2. Chan, W. C, O'Mahoney, M. G., Yu, C. Y . and Yu, R. Y. H. Renal failure during intermittent rifampicin therapy. Tubercle 56(1975)191-198.
3. Chugh, K. S., Singhal, P. C, Sharma, B. K., Mahakur, A. C, Pal, Y., Datta, B. N. and Das, K. C. Acute renal failure due to intravascular hemolysis in north Indian patients. Am. J. Med. Sei. 174(1977)139-146.
4. Cochran, M., Morrhead, P. J. and Platts, M. Permanent renal damage with rifampicin. (Letter) Lancet 1(1975)1428.
5. Cohn, J. R., Fye, D. L., Sills, J. M. and Francos, G. C. Rifampicin-induced renal failure. Tubercle 66(1985)289-293.
6. Conen, D., Blumberg, A., Webers, F. and Schubothe, H. Hämolytische krise and akutes Nierenversagen unter Rifampicin. Schweiz. Med. Wochenschr. 15(1979)558-561.
7. Cordonnier, D. and Muller, J. M. Acute renal failure after rifampicin. Lancet 2(1972)1364-1365.
8. Criel, A. and Verwilghen, R. L. Intravascular hemolysis and renal failure caused by intermittent rifampicin treatment. Blut 40(1980)147-150.
9. Falmenbaum, W., Gehr , M., Gross, M., Kaufman, J. and Hamburger, R. Acute renal failure associated with myoglobinuria and hemoglobinuria. In: Acute Renal Failure. Brenner, B. M. and Lazarus, J. M., eds. Philadelphia: W. B. Saunders, 1983, pp. 269-282.
10. Flynn, C. T., Rainford, D. J. and Hope, E. Acute renal failure and rifampicin: dangcrof unsuspected intermittent dosage. Br. Med. J. 2(1974)482.
11. Girling, D. J. and Hitze, K. L. Adverse reactions to rifampicin. Bull. WHO 57(1979)45-49.
12. Haase, W., Rohle, H. D., Warnecke, F. and Wiek, K. Hämolytische kris durch Rifampicin. Praxis Pncumol. 25(1971)466-468.
13. Hirsch, D. J., Bia, F. J., Kashgarian, M. and Bia, M. J. Rapidly progressive glomerulonephritis during antituberculous therapy. Am. J. Nephrol. 3(1983)7-10.
14. Kleinknecht, D., Humiierg, J. C. and Decroix, G. Acute renal failure after rifampicin. Lancet 2(1972)1238-1239.
15. Kumar, S., Mehta, J. and Trivedi, H. Light chain proteinuria and reversible renal failure in rifampicin treated patients with tuberculosis. Chest 70(1975)564.
16. Murray, A. N., Cassidy, M. J. and Templecamp, C. Rapidly progressive glomerulonephritis associated with rifampicin for pulmonary tuberculosis. Nephron 46(1987)373-376.
17. Nessi, R., Bonoldi. G. L.. Redaelli. B. and diFilipo, G. Acute renal failure after rifampicin; a case report and survey of literature. Nephron 16(1976)148-159.
18. Poole, G., Stradling, P. and Worlledge, S. Potential serious side effects of high-dose twice weekly rifampicin. Br. Med. J. 3(1971)343-347.
19. Power, D. A., Russell, G., Smith, F. W., Simpson, J. G., MacLeod, A. M., Friend, J. A. R. and Catto, G. R. Acute renal failure due to con D. tinuous rifampicin. Clin. Nephrol. 20(1983)155-159.
20. Sors, C, Sarrazin, A. and Homberg, J. C. Accidents hemolytiqucs rccivivants d'origine immuno-allergique au cours d'un traitemcnt intermittent par la rifampicine. Rev. Tuberc. (Paris) 36(1975)405-416.
1. M.D., D.M., Senior Resident; Department of Nephrology, Postgraduate Institute of Medical Education & Research, Chandigarh 160012, India.
2. M.D., D.M.; Department of Nephrology, Postgraduate Institute of Medical Education & Research, Chandigarh 160012, India.
3. M.D., D.M., D.N.B.; Department of Nephrology, Postgraduate Institute of Medical Education & Research, Chandigarh 160012, India.
4. M.D., F.A.C.P., Department of Nephrology, Postgraduate Institute of Medical Education & Research, Chandigarh 160012, India.
Reprint requests to Dr. Chugh.
Received for publication on 9 December 1991.
Accepted for publication in revised form on 29 April 1992.