- Volume 60 , Number 2
- Page: 195–200
Sero-lmmunoreactivity of cloned protein antigens of Mycobacterium leprae
ABSTRACT
Sera f rom 173 leprosy patients with various types of disease (tuberculoid = TT, borderline tuberculoid = BT, borderline lepromatous = BL, and lepromatous = LL), 12 intrafamilial contacts, and 40 normal healthy individuals were assayed in an indirect enzyme-linked immunosorbent assay (ELISA) using Mycobacterium leprae antigens. Recombinant clones carrying M. leprae antigens, namely, Y3184 (12 kDa), Y3179 (18 kDa), Y3164 (28 kDa), Y3180 (36 kDa), and Y3178 (65 kDa) and a cell sonicate f rom armadillo-derived M. leprae were used for the study. A high degree of reactivity with the 65-kDa, 36-kDa, and 28 kDa protein lysates was observed in most of the sera f rom multibacillary patients, with a low degree of positivity with 18 kDa and 12 kDa. Only a few sera f rom paucibacillary patients showed positive reactions. The majority of the contacts' sera tested showed no reactivity with these antigens.RÉSUMÉ
Les serums de 173 patients lépreux présentant différents types de la maladie (tuberculoide = TT, borderline tuberculoide = BT, borderline lépromateux = BL et lépromateux = LL), 12 contacts intrafamiliaux, et 40 individus normaux en bonne santé ont été titrés par un test enzymatique indirect (ELISA) utilisant des antigènes de Mycobacterium leprae. Des clones recombinants porteurs des antigènes de M. leprae Y3184 ( 12 kDa), Y3179 (18 kDa), Y3164 (28 kDa), Y3180 (36 kDa), et Y3178 (65 kDa) et un sonicat cellulaire de M. leprae de tatou ont été utilisés pour cette étude. Un haut degré de réactivité a été observé avec les protéines de 65-kDa, 36-kDa et 28-kDa dans la majorité des serums de patients multibacillaires, et un degré faible de positivité pour les protéines de 18 kDa et 12 kDa. Seuls quelques sérums de patients paucibacillaires ont montré des réactions positives. La majorité des sérums de contacts n'a montré aucune réaction avec ces antigènes.RESUMEN
Usando un inmunoensayo enzimático (ELISA) con antígenos de Mycobacterium leprae se analizaron los sueros de 173 pacientes con diferentes tipos de lepra (tuberculoide, TT; tuberculoide subpolar, BT; lepromatosa subpolar, BL; y lepromatosa, LL), los sueros de 12 contactos familiares y los de 40 individuos sanos no relacionados. Para el estudio se usaron las siguientes clonas recombinantes portadoras de antígenos del M. leprae; Y3184 (12 kDa), Y3179 (18 kDa), Y3164 (28 kDa), Y3180 (36 kDa), y Y3178 (65 kDa), además de un sonicado de M. leprae cultivado en armadillo. En la mayoría de los pacientes con lepra multibacilar se observó un alto grado de reactividad con los lisados conteniendo las proteínas 65 kDa, 36 kDa, y 28 kDa, y un bajo grado de reactividad con las proteínas 18 kDa y 12 kDa. Sólo unos cuantos sueros de los pacientes paucibacilares mostraron reacciones positivas. La mayoría de los sueros de los contactos probados no mostraron reactividad con estos antígenos.An important aspect in the epidemiologic study of leprosy is the lack of reliable and appropriate technology for the detection of subclinical infection in patients infected with Mycobacterium leprae. Leprosy is one of the major disabling diseases and imposes a considerable burden in terms of morbidity. The disease presents a remarkably "broad spectrum of clinical stages ranging from tuberculoid leprosy (TT) to a stage designated as lepromatous leprosy (LL). Patients with TT exhibit strong cell-mediated immune responses to M. leprae, a low level of circulating antibody, and low levels of bacilli (paucibacillary) in their lesions(5,15). By contrast, most LL patients exhibit a high level of humoral response but fail to display specific cell-mediated responsiveness to M. leprae, and exhibit an abundant number of bacilli (multibacillary) in the lesions (5,15).
Our main objective was to screen several molecularly cloned protein antigens (12kDa, 18-kDa, 28-kDa, 36-kDa and 65-kDa) of M . leprae with sera from leprosy patients to determine if there is a specific distribution of antibodies to each of the protein antigens according to the clinical and bacteriological spectrum of the disease. This would permit the recognition of a possible sérodiagnostic and/or seroprognostic test which could be used in an epidemiological survey. All sera were screened by indirect enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Sera. Sera from leprosy patients in various stages of infection and their contacts were used for the study. The time course of the disease was similar in all patients screened. The patients had been clinically and histologically classified according to the Ridley-Jopling scheme (19) prior to the collection of the sera. Some of the scrum samples were collected from patients undergoing extensive chemotherapy. The sera were labeled accordingly, aliquoted, and maintained at - 20ºC. Sera from normal healthy individuals (NHS) and from contacts of leprosy patients (randomly chosen from an endemic area) were similarly maintained. These controls were not age and sex matched with the cases.
Preparation of M. leprae cell sonicate. Purified armadillo-derived M. leprae cells (obtained from Dr. P. J. Brennan and Dr. S. Hunter, Colorado State University, Fort Collins, Colorado, U.S.A.) were washed three times in 0.05% saline and disrupted by sonication at 4ºC for 12 min using a W-375 sonicator (Heat System-Ultrasonics, Plainview, New York, U.S.A.) with a 100 w energy output. Thereafter, the supernate, separated by centrifugation, was aliquoted and stored at - 20ºC after the addition of phenylmethylsulfonyl fluoride (PMSF; Sigma Chemical Co., St. Louis, Missouri, U.S.A.).
Preparation of crude lysate from lysogens. Lysogens of λgt1 1 with different gene (encoding M . leprae 12 kDa, 18 kDa, 28 kDa, 36 kDa, and 65 kDa) inserts maintained in Escherichia coli Y1089 were obtained from Dr. Richard Young, Massachusetts Institute of Technology, Cambridge, Massachusetts, U.S.A.) and λgt1 1 phage (without insert) maintained in E . coli Y1090 was obtained from Dr. J. Clark-Curtiss (Washington University, St. Louis, Missouri, U.S.A.). The lysogens obtained were as follows: Y3184-λgt1 1 recombinant encoding M . leprae 12-kDa determinant; Y3179-λgt1 1 recombinant encoding an M . leprae 18-kDa antigen determinant; Y3164-λgtl 1 recombinant encoding an M . leprae 28-kDa antigen determinant; Y3180-λgt1 1 recombinant encoding an M . leprae 36-kDa antigen determinant; and Y3178-λgt1 1 recombinant encoding an M . leprae 65-kDa antigen determinant.
A single colony of each lysogen was inoculated in 18 ml of Luria broth (LB; pH 7.5) containing 50 µ g/ml ampicillin (added only for Y3179 and Y3178, since Y3184, Y3164, and Y3180 do not grow in the presence of ampicillin), and incubated overnight at 32ºC in a shaker incubator. On the following day, 100 µl of the overnight culture was inoculated in 20 ml of LB containing 0.2% maltose. The cultures were grown with vigorous shaking in a waterbath maintained at 32ºC until an optical density (OD) of 0.5 was obtained at 595 nm. The lysogen was induced by shifting the temperature from 32ºC to 45ºC for a period of 20 min with vigorous shaking. Subsequently, isopropyl β-D-thiogalactopyranoside (IPTG; 10 raM) was added, and the culture was incubated for 1 hr at 38ºC. The intact cells were centrifuged and resuspended in phosphate buffered saline (PBS) or sodium dodecyl sulfate (SDS) buffer and stored at - 80ºC. Complete lysis of the cells was performed by freezethaw cycles as required. Each lysate was checked for the presence of M. leprae protein by immunoblotting (with the use of monoclonal antibody) and then used for further studies.
Monoclonal antibodies to M. leprae proteins. Monoclonal antibodies to each M. leprae protein (MC2404 for 65 kDa, MC5828 for 36 kDa, MC4742 for 28 kDa, MC8026 for 18 kDa, and MC8909 for 12 kDa) (7) were obtained from WHO IMMLEP, Geneva, Switzerland, through Dr. T. Shinnick (Centers for Disease Control, Atlanta, Georgia, U.S.A.).
Polyclonal antiserum to M. leprae cell sonicate. New Zealand white male rabbits were used for raising a hyperimmune antiserum to M . leprae cell sonicate. Three rabbits were used for this purpose. Prior to immunization, the animals were ear-bled to collect normal rabbit serum. Each rabbit was administered 1 ml of a 1:1 (v/v) mixture of the M . leprae cell sonicate and Freund's incomplete adjuvant (Difco Laboratories, Detroit, Michigan, U.S.A.) subcutaneously in the hind quarter. Six such injections were given spaced 1 week apart. This has been the established protocol in our laboratory for raising a strong hyperimmune serum. One week after the last injection, each animal was ear-bled, and the serum was analyzed by the immunodiffusion method (9, 18) to determine the antibody response. Since the response of each rabbit was similar, the animals were exsanguinated in the eighth week after the first injection and the scrum samples were pooled, aliquoted, and stored at -20ºC.
Indirect ELISA. Polystyrene, 96-well, flat-bottom microtiter plates (Falcon; Becton Dickinson Labware, Lincoln Park, New Jersey, U.S.A.) were coated with 100 /d of each lysate (containing M. leprae protein) per well for 3 hr at 37ºC. Each lysate was initially diluted 1:100 in carbonate bicarbonate buffer (pH 8.6) prior to use. M. leprae cell sonicate was also used as the coating antigen. The plates were then washed six times with PBS containing 0.05% Tween 20 (PBS-T) and blocked with 100 jd of 15% bovine serum albumin (BSA) in PBS at room temperature for 1 hr. The plates were washed once in PBS-T and stored overnight at 4ºC. On the following day, the wells were filled with 100 jul of test and control serum diluted 1:200 in Tris-buffered saline (TBS) and incubated at room temperature for 2 hr. Washing was followed by incubating with 100 µ l of 1:1000 diluted peroxidase-conjugated rabbit anti-human polyvalent immunoglobulin (Sigma) at room temperature for 1 hr. Subsequently, after washing six times with PBS-T, each well was incubated with 100 µ l of substrate solution (10 mg orthophenylene diamine dihydrochloride in 1 ml methanol mixed with 99 ml distilled water and 0.1 ml of 3% hydrogen peroxide) at room temperature for 30 min. The color development was stopped by the addition of 50 µ l of 4 N H2S04 per well, and the OD was measured after 15 min in a Titertek Multiskan reader using a 492 nm filter. All of the serum samples were tested in triplicate, and the mean OD was recorded for each sample. A positive reaction was defined as the OD which was greater than the mean control (NHS) value plus four standard deviations.
RESULTS
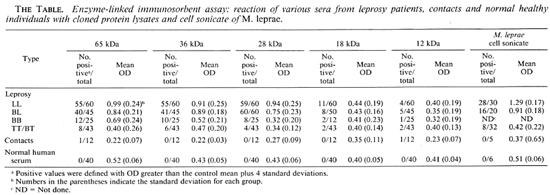
In this study, 225 serum samples were tested with cloned M. leprae antigens, while 93 serum samples were tested with M. leprae cell sonicate using an ELISA. Most serum samples from multibacillary patients (BL and LL), irrespective of the type and duration of treatment, reacted strongly with the 65-, 36- and 28-kDa lysates as well as with M. leprae cell sonicate (The Table). Reactivity of the samples gradually decreased toward the tuberculoid end of the spectrum. However, a small number of the sera also showed positive reactions with the 18- and 12-kDa lysates. Only one serum sample collected from a contact showed positive reaction with 65- and 12-kDa. Most of the control sera (NHS) showed minimal reaction with both the cloned antigens and with M. leprae cell sonicate. No reaction was observed when the sera were tested with the control lysate.
DISCUSSION
The present study gives the data on there activity of leprosy sera at all stages of infection with the M. leprae cell sonicate and the crude lysates of the cloned proteins by ELISA. As stated in the Methods section, the criterion for defining positive values in ELISA was chosen so as to ensure the exclusion of doubtful and false-positive results in the test. The results obtained indicate that the majority of the sera from multibacillary patients recognize the 65-, 36-and 28-kDa antigens to a high degree (93.1%). On the other hand, sera from the paucibacillary patients exhibited a considerably lower degree of reactivity (16%) to the same antigens. Whether the patients received treatment or were untreated did not influence the reactivity of the sera. Most of the serum samples showed no remarkable binding to either the 18- or 12-kDa lysates, indicating that these antigens are probably less immunoreactive than the other three. It is possible that if the antibodies to these antigens are present, they may be at a lower concentration and were undetectable by the method used. It should also be noted that the criteria for a positive test are vigorous, which may explain the relative lack of responses in paucibacillary patients.
Our data with regard to the scroreactivity of the different cloned proteins are in agreement with those of other investigators who have shown the presence of circulating antibodies in sera from leprosy patients in various stages of infection. Levis, et al. (14) have shown higher levels of antibodies to these antigens (especially the 65-kDa) in LL and BL patients as compared to TT or BT patients. Klatser, et al. (12) have reported that LL patients show high reactivity to all the antigens ranging from 12-kDa to 86-kDa. Similar observations with regard to the high degree of seroreactivity in multibacillary patients as opposed to paucibacillary patients have been made by Sathish, et al. (20) and Cherayil, et al. (3). Considering the scroreactivity of each individual protein, it is evident that the 65-kDa appears to be the most active component of the known antigenic mosaic of M. leprae in multibacillary leprosy, followed by the 36-kDa. The least reactive component appears to be the 12kDa, antibodies to which are present in the leprosy sera only to a minimum degree. The detection of circulating antibodies appears to be directly dependent upon the clinical status of the patients, with the LL and BL cases showing more humoral response than the TT and BT cases. Klatser, et al. (11) have shown that a large proportion of sera from patients in the TT or BT part of the spectrum react with the 36-kDa antigen. This appears to be in contrast to our observation and the observations of several others. Ehrenberg and Gebre (6) have reported that most of the sera from BT patients bind to M . leprae proteins greater than 70-kDa. Vega-Lopez, et al. (21) have reported the recognition of five proteins (33-, 25-, 18-, 15and 12-kDa) by a majority of LL sera using Western blotting; whereas Chakrabarty, et al. (2) have shown the recognition of only two proteins (33- and 12-kDa) by this method.
That sera from multibacillary leprosy patients possess a very high level of circulating antibodies as opposed to those from paucibacillary leprosy patients has been well documented. Navalkar, et al. (16, 17) first demonstrated this phenomenon when they studied the antibody levels in sera from patients in different stages of infection, using various mycobacterial antigens. Subsequently, Convit, et al. (4) and Kwapinski, et al. (13) confirmed these observations. Our observations with regard to the responsiveness of sera from intrafamilial contacts are not in total agreement with the observations made by other investigators. The lower positivity observed with contacts could be explained by the fact that the criteria chosen for a positive test in this study are vigorous. Amezcua, et al. (1) have reported a high percentage of seropositivity in household contacts of Mexican leprosy patients, while Gonzalez-Abreu, et al. (8) have observed an overall positivity of less than 10% with sera from contacts. Hartskeerl, et al. have reported the characterization of recombinant clones that produce M. leprae antigens which were recognized by sera from contacts of leprosy patients (10). However, none of the recombinant antigens recognized by the contacts' sera were identical or related to the well-characterized antigens of M. leprae used in this study.
Our results, in general, differ from the observations made by other investigators. It is likely that factors such as a) the clinical and bacteriological status of the patients evaluated, b) the source of sera, and c) the methodologies employed by individual investigators could possibly be attributed to the variability of results emanating from different laboratories. In this study, we have made an effort to identify antibodies not only against the M. leprae cell sonicate but also against the various cloned proteins of M. leprae. A common fact that emerges is that multibacillary leprosy patients, treated or untreated, exhibit a high degree of reactivity to the different antigenic preparations used as compared to the paucibacillary or bacillary-negative patients. It is, however, important to note that for the selection of an appropriate antigen for leprosy diagnosis, it is imperative to study a larger number of healthy controls, contacts and also other pathological sera from patients with mycobacterial infections that are prevalent in an endemic environment. Unavailability of such materials in greater numbers permitted us to screen only those samples that were made available to us.
Acknowledgment. This investigation was supported by the U.S. Leprosy Panel of the U.S. Japan Co-operative Medical Science Program, administered by the Geographic Medicine Branch, National Institute of Allergy and Infectious Diseases (Grant 127189).
We extend our sincere thanks to Dr. Thomas Gillis for his criticism and valuable suggestions. We would also like to extend our thanks to Dr. P. J. Brennan and Dr. S. Hunter for providing the purified M. leprae cells, to Dr. Richard Young for the lysogens, and to Dr. Thomas Shinnick for the monoclonal antibodies. Our sincere thanks are extended to Ms. S. Walton for the excellent job of typing this manuscript, Mr. J. Perry for the illustrations, and Sushant Navalkar for his timely help in the preparation of this manuscript.
REFERENCES
1. AMEZCUA, M. E., ESCOBAR-GUTIERREZ, A., BARBA-RUBIO, J., CAZARES, J. V., MAYEN, E., CHAVEZ-NUNEZ, M., PENA, R. C, RODRIGUEZ, R. and PASTEN, S. Prospective immunological follow-up in household contacts of Mexican leprosy patients. Int. J. Lepr. 58(1990)651-659.
2. CHAKRABARTY, A. K., MAIRE, M. H. and LAMBERT, P. H. SDS-PAGE analysis of M. leprae protein antigens reacting with antibodies from sera from lepromatous patients and infected armadillos. Clin. Exp. Immunol. 49(1982)523-531.
3. CHERAYIL, B. J. and YOUNG, R. A. A28-kDa protein from Mycobacterium leprae is a target of the human antibody response in lepromatous leprosy. J. Immun. 141(1988)4370-4375.
4. CONVIT, J. and ULRICH, M. Recent advances in the immunology of leprosy. Int. J. Dermatol. 15(1976)157-170.
5. DHARMENDRA. Classifications of leprosy. In: Leprosy. Hastings, R. C, ed. London: Churchill Livingstone, 1985, pp. 88-99.
6. EHRENBERG, J. P. and GEBRE, N. Analysis of the antigenic profile of Mycobacterium leprae: crossreactive and unique specificities of human and rabbit antibodies. Scand. J. Immunol. 26(1987)673-681.
7. ENGERS, H. D., ABE, M., BLOOM, B. R., MEHRA, V., BRITTON, W., BUCHANAN, T. M., KHANOLKAR, S. K., YOUNG, D. B., CLOSS, O., GILLIS, T., HARBOE, M., IVANYI, J., KOLK, A. H. J. and SHEPARD, C. C. Results of a World Health Organization-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. (Letter). Infect. Immun. 48(1985)603-605.
8. GONZALEZ-ABREU, E., MORA, N., PEREZ, M., PEREIRA, M., PEREZ, J. and GONZALEZ, A. B. Sere-diagnosis of leprosy in patients' contacts by enzyme-linked immunosorbent assay. Lepr. Rev. 61(1990)145-150.
9. HANSON, L. A. Immunological analysis of streptococcal antigens and human sera by means of diffusion-in-gel methods. Int. Arch. Allergy 14(1959)279-291.
10. HARTSKEERL, R. A., VANRENS, R. M., STABEL, L. F. E. M., DEWIT, M. Y. L. and KLATSER, P. R. Selection and characterization of recombinant clones that produce Mycobacterium leprae antigens recognized by antibodies in sera from household contacts of leprosy patients. Infect. Immun. 58(1990)2821-2827.
11. KLATSER, P. R., DEWIT, M. Y. L. and KOLK, A. H. J. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
12. KLATSER. P. R.. VANRENS, M. M. and EGGELTE, T. A. Immunochemical characterization of Mycobacterium leprae antigens by the SDS-polyacrylamide gel electrophoresis immunoperoxidasc technique (SGIP) using patients' sera. Clin. Exp. Immunol. 56(1989)537-544.
13. KWAPINSKI, J. B. G., ALCASID. A., KWAPINSKI, E. H. and NAIRN, V. The immunology of cytoplasmic antigens of mycobacteria. Can. J. Microbiol. 18(1972)1201-1211.
14. LEVIS, W. R., MEEKER, H. C, SCHULLER-LEVIS, G. B., GILLIS. T. P.. MARINO, L. J.. JR. and ZABRISKIE, J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid 1 and protein antigens. J. Clin. Microbiol. 24(1986)917-921.
15. NAVALKAR, R. G. Immunology of leprosy. CRC Grit. Rev. Microbiol. (1980)25-47(145 ref).
16. NAVALKAR. R. G., NORLIN, M. and OUCHTERLONY, O. Characterization of leprosy sera with various mycobacterial antigens using double diffusion-ingel analysis. II. Int. Arch. Allergy 28(1965) 250-260.
17. NORLIN, M., NAVALKAR, R. G., OUCHTERLONY, O. and LINO, A. Characterization of leprosy sera with various mycobacterial antigens using double diffusion-in-gel analysis. 3. Acta Pathol. Microbiol. Scand. 67(1966)555-562.
18. OUCHTERLONY, O. Diffusion-in-gel methods for immunologcal analysis. II. Prog. Allergy 6(1962)30-154.
19. RIDLEY, D. S. and JOPLING, W . H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
20. SATHISH, M., ESSER, R. E., THOLE, J. E. R. and CLARK-CURTISS, J. E. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect. Immun. 58(1990)1327-1336.
21. VEGA-LOPEZ, F.. STOKER, N. G., LOCNISKAR, M. F., DOCKRELL. H. M., GRANT, K. A. and Me-ADAM. K. P. W. J. Recognition of mycobacterial antigens by sera from patients with leprosy. J. Clin. Microbiol. 26(1988)2474-2479.
1. M.S., Research Assistant; Department of Microbiology and Immunology, Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
2. Ph.D., Research Associate; Department of Microbiology and Immunology, Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
3. Ph.D., Assistant Professor; Department of Microbiology and Immunology, Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
4. Ph.D., Professor and Chairman, Department of Microbiology and Immunology, Morehouse School of Medicine, 720 Westview Drive SW, Atlanta, Georgia 30310, U.S.A.
Reprint requests to Professor Navalkar.
Received for publication on 25 November 1991.
Accepted for publication in revised form on 12 March 1992.