- Volume 60 , Number 2
- Page: 201–7
Antibody responses to the 18-kDa Protein of Mycobacterium leprae in leprosy and tuberculosis patients
ABSTRACT
The 18-kDa protein of Mycobacterium leprae, as recognized by the monoclonal antibody L5, has a restricted species distribution, being confined to M. leprae and M. habana. We have developed a solid-phase ELISA using purified, recombinant M. leprae 18-kDa protein and compared the serological responses of Nepali leprosy and tuberculosis patients and endemic control subjects to the protein and the M . leprae phenolic glycolipid-I (PGL-I). Few control subjects had anti-18-kDa antibodies. A small proportion of paucibacillary (PB) leprosy and 42% of multibacillary (MB) leprosy patients had IgG anti-M leprae antibodies. A similar proportion (47%) of Nepali tuberculosis (TB) patients were seropositive, and IgG anti-18-kDa antibody levels were significantly higher in MB and TB patients than in control subjects. By comparison, IgM anti-PGL-I antibodies were detected in 88% of MB leprosy patients and only 7% of TB patients. The possible reasons for the 18-kDa protein seroreactivity in TB patients are discussed, and the anti-18-kDa assay is compared with other antibody assays for protein and nonprotein antigens of M. leprae. It is concluded that the sensitivity and specificity of the anti-M. leprae 18-kDa ELISA are insufficient for the assay to be of clinical utility in leprosy patients.RÉSUMÉ
La protéine de 18-kDa de Mycobacterium leprae, telle que reconnue par l'anticorps monoclonal L5, a une distribution restreinte pour l'espèce, ne se retrouvant que pour M. leprae et M. habana. Nous avons développé un ELISA en phase solide utilisant la protéine de 18-kDa de M. leprae, purifiée et recombinée, et nous avons comparé les réponses sérologiqucs de patients lépreux et tuberculeux du Népal et des témoins de pays endémiques vis-à-vis de la protéine et du glycolipide phénolique-I (PGL-I). Peu de témoins avaient des anticorps vis-à-vis de la protéine de 18-kDa. Une petite proportion des lépreux paucibacillaires (PB) et 42% des lépreux multibacillaires (MB) avaient des IgG anti-.M. leprae. Une proportion similaire (47%) de patients tuberculeux Népalais (TB) étaient séropositifs, et les taux d'anticorps IgG vis-à-vis de la protéine de 18-kDa étaient significativement plus élevés chez les patients MB et TB que chez les témoins. En comparaison, des anticorps IgM anti-PGL-I ont été détectés chez 88% des patients lépreux MB et seulement 7% des patients TB. Les raisons possibles de la séroréactivité de la protéine de 18-kDa chez, les patients TB sont discutées, et le test vis-à-vis de la protéine de 18kDa est comparé avec d'autres tests pour anticorps visà-vis d'antigènes protéiques et non protéiques de M. leprae. On en conclut que la sensibilité et la spécificité de l'ELISA pour les anticorps anti-18kDa de M. leprae sont insuffisants pour que le test ait une utilité clinique chez les malades de la lèpre.RESUMEN
La proteína de 18 kDa del Mycobacterium leprae reconocida por el anticuerpo monoclonal L5 tiene una distribución restringida y se encuentra confinada al M. leprae y al M. habana. En este estudio, utilizando una técnica inmunoenzimática (ELISA), hemos comparado la reactividad de los sueros de pacientes Nepalences con lepra of con tuberculosis, y de controles sanos de área endémica, con este antigeno recombinante de 18 kDa y con el glicolipido fenólico 1 (PGL-I) del M. leprae. Encontramos que muy pocos individuos control tuvieron anticuerpos anti-18 kDa. Una pequeña proporción de los pacientes con lepra paucibacilar (PB) y 42% de los pacientes multibacilares (MB) tuvieron anticuerpos IgG anti-,M. leprae. Una proporción similar (47%) de los pacientes con tuberculosis (TB) fueron scropositivos, y los niveles de anticuerpo IgG anti-18 kDa fueron significativamente mayores en los pacientes MB y TB que en los sujetos control. Comparativamente, el 88% de los pacientes con lepra MB y solo el 7% de los pacientes TB, tuvieron anticuerpos IgM anti-GLP-1. Se discuten las posibles razones de la seroreactividadade los pacientes TB con la proteína de 18 kDa y se compara el ensayo usando esta proteína, con ensayos para otros anticuerpos contra antígenos proteicos y no proteicos del M. leprae. Se concluye que por su baja sensibilidad y especificidad, el ELISA con la proteína de 18 kDa no resulta de ninguna utilidad clínica para los pacientes con lepra.In recent years there has been an expansion in the range of assays which can detect antibodies to Mycobacterium leprae. Interest has focused on the recognition of species-specific antibodies in leprosy patients (4, 7, 12, 25), changes in antibody levels with therapy (2, 8), and the possible early detection of leprosy in field studies (3). A variety of antigens have been used including the M. leprae -specific surface phenolic glycolipid-I (PGL-I) (6, 9), cell-wall-derived lipoarabinomannan (LAM) (17, 21, 23) and a number of defined proteins. All current methods are of limited sensitivity because only a minority of patients with active paucibacillary (PB) disease have antibodies even though most multibacillary (MB) patients are positive. For example, in a comparative study of newly diagnosed patients (21), only 20% of PB patients were seropositive to PGL-I antigen and 33% had antibodies to the M. leprae- specific epitope on the 35-kDa protein epitope defined by the murine monoclonal antibody (mAb) ML04 (25). Another significant shortcoming is the limited specificity of some assays which detect nonspecific humoral responses in healthy control subjects or in patients with other mycobacterial diseases, particularly tuberculosis.
In an effort to find a serological assay which could detect anti- M. leprae antibodies with greater specificity and sensitivity, we have examined the antibody response to the M. leprae 18-kDa protein. Initial studies with murine mAb L5 demonstrated that the epitope on the 18-kDa protein recognized by this mAb was restricted to M . leprae. Subsequently, the gene encoding the 18-kDa protein was identified, and the recombinant protein expressed in Escherichia coli (4). Using this recombinant protein, we have examined the anti-18-kDa antibody response in Nepali leprosy and tuberculosis patients as well as healthy control subjects.
MATERIALS AND METHODS
Subjects
The leprosy patient groups consisted of 146 subjects diagnosed on clinical and bacteriological grounds according to the Ridley-Jopling classification (20) at Anandaban Leprosy Hospital, Kathmandu, Nepal. There were 101 male and 45 female patients with an age range of 9 to 80 years. The PB study group consisted of 8 primary neuritic (PN), 10 tuberculoid (TT), and 61 borderline tuberculoid (BT) leprosy patients. The 67 MB patients included 4 borderline (BB), 42 borderline lepromatous (BL), and 21 lepromatous (LL) leprosy patients. At the time of testing, 96 patients (48 PB and 48 MB) were untreated and 50 (31 PB and 19 MB) had received treatment with cither dapsone monotherapy (N = 9) for between 1 and 14 years, multidrug therapy (N = 39) for between 1 and 43 months, or monotherapy followed by multidrug therapy (N = 2). The bacterial index (BI) was determined as the mean of slit-skin smears taken from four sites, including two clinical lesions.
Sera from 44 patients with active pulmonary tuberculosis confirmed by sputum microscopy and chest X-ray were kindly supplied by Dr. D. Williams, Tansen Hospital, Palpa, and Dr. F. Garlick, Patan Hospital, Kathmandu, Nepal. Sera from 50 healthy nursing students were kindly provided by Mrs. B. Rai, UM N Nursing Campus, Kathmandu, Nepal.
Antigens
Recombinant M. leprae 18-kDa protein was expressed from E. coli transfected with the pML3 vector (4). The purified lyophilizEd protein was reconstituted in sterile phosphate buffered saline (PBS) and the pH adjusted to 7.5 with 1 N NaOH. Disaccharidc-bovinc serum albumin (dBSA) conjugate for the detection of anti-PGL-I antibodies was provided by the Immunology of Leprosy Program (IMMLEP) of the World Health Organization.
Serological assays
IgG anti-18-kDa antibodies. IgG anti-18kDa antibodies were detected by enzymelinked immunosorbent assay (ELISA). Round-bottom polystyrene plates (Nunc, Kamstrup, Denmark) were coated overnight at 37ºC with 50 µ l of recombinant M. leprae 18-kDa protein (pi8 protein) (10 µ g/ ml) in carbonate buffer, pH 9.6. The wells were washed once in PBS containing 0.05% Tween 20 (PBST) and incubated for 4 hr in 3% bovine serum albumin (BSA) in PBS. Then 50 µ l of patients' sera diluted 1:100 in 10% normal goat serum (NGS) was added to the wells in duplicate for 1 hr. The wells were washed three times with PBST atid 50 µ l of goat anti-human IgG-horseradish peroxidase conjugate (Cappell, West Chester, Pennsylvania, U.S.A.) diluted 1:2000 in 10% NGS was added for an additional hour. The wells were again washed three times, and the bound antibody was visualized by the addition of 75 µ l of 0.4 g/L O-phenylenediamine (Sigma, Dorset, U.K.) in 0.05 M citrate buffer, pH 5.0, containing 0.06% hydrogen peroxide. The reaction was stopped after 20 min by the addition of 50 µ l per well of 2.5 N sulfuric acid, and the plates were read at 492 nm in a Multiskan Reader (Flow Laboratories, Irvine, Scotland). The known positive and negative sera were included on each plate, and the variation in absorbance of the standards between plates was less than 10%.
IgM anti-PGL-I. Anti-PGL-I antibodies were measured by ELISA as previously described using dBSA at 250 ng/ml and serum diluted 1:300 (21). Samples with an absorbance at 492 nm of > 0.2 (the mean plus three standard deviations of 91 healthy Nepali control subjects) were considered positive.
Statistical methods
The differences in the proportions of study groups seropositive for the two antibodies were tested by the chi-squared statistic. Differences in the mean antibody levels in the various groups were measured by Student's t test.
RESULTS
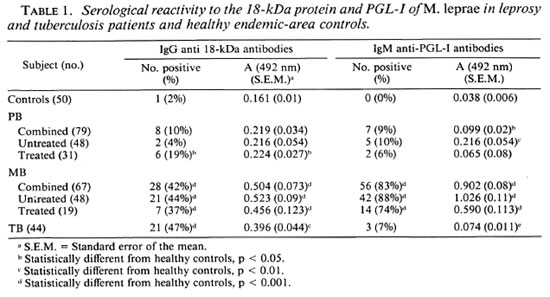
Low antibody responses to M . leprae 18kDa protein were found in healthy Nepali control subjects with a mean level of 0.161 (A492 nm). Using the criterion of the mean plus three standard deviations in control subjects as the negative cut-off (0.383), only 1 of the 50 control subjects (2%) was seropositive for anti-18-kDa antibodies. The level of IgG anti-18-kDa antibodies was low in PB leprosy patients (Figs. 1 and 2). There was no significant difference in the proportion seropositive or in the mean IgG anti-18-kDa levels between untreated PB patients and control subjects, although in treated PB patients the levels were slightly higher (Table 1). Anti-18-kDa antibodies were detected in about half of the untreated MB leprosy patients (Fig. 1), and the proportion seropositive and mean antibody levels (Fig. 2) were significantly higher than in control subjects (p < 0.001). Chemotherapy had only a slight effect on the IgG anti-18-kDa levels in treated MB patients (Table 1).
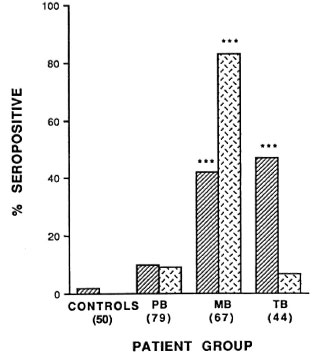
Fig. 1. Proportions of MB leprosy, PB leprosy, TB patients, and control subjects seropositive for antibodies to the 18-kDa protein  and the phenolic glycoli-pid-1 (PGL-I) antigen
and the phenolic glycoli-pid-1 (PGL-I) antigen  . Significant differences be-tween the patient and control groups arc shown as ***(p < 0.001).
. Significant differences be-tween the patient and control groups arc shown as ***(p < 0.001).
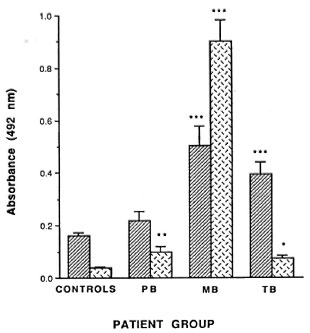
Fig. 2. Mean antibody level (A492 nm^S.E.M.) for IgG anti-M. leprae 18-kDa  and IgM anti-PGL-I
and IgM anti-PGL-I  antibodies in MB leprosy, P13 leprosy, TB patients,and control subjects. Significant differences betweenpatient and control groups are shown as * (p < 0.05); **(p < 0.01); *** (p < 0.001).
antibodies in MB leprosy, P13 leprosy, TB patients,and control subjects. Significant differences betweenpatient and control groups are shown as * (p < 0.05); **(p < 0.01); *** (p < 0.001).
Surprisingly, the proportion of seropositive tuberculosis patients (47%) was similar to that in MB patients (Fig. 1). While the mean levels of IgG anti-18-kDa antibodies were lower in TB patients, they were not significantly different from the levels in MB patients (Fig. 2).
By contrast, 9% of PB, 83% of MB, and only 7% of TB patients had detectable IgM anti-PGL-I antibodies (Fig. 1). The mean IgM anti-PGL-I antibody level in MB patients was significantly higher than in the control, PB and TB patient groups (Fig. 2). In the 96 untreated leprosy patients there were moderate correlations between the levels oflgG anti-M leprae 18-kDa antibodies and IgM anti-PGL-I antibodies (rs = 0.478, p < 0.001) and between the IgG anti-18kDa antibody levels and the BI (rs = 0.514, p < 0.001).
DISCUSSION
This study has demonstrated that 44% of untreated MB leprosy patients and a minority of PB patients mount an IgG antibody response to the 18-kDa protein (Table 1). A surprising finding was that a similar proportion (47%) of TB patients had IgG anti-18-kDa antibodies, although the mean antibody level was lower in these than in the MB leprosy patients. This appeared to be related to active mycobacterial infection since 98% of control subjects with similar environmental exposure to the patients were seronegative. What are the possible explanations for the 18-kDa response in TB patients?
First, there may be crossreactivity between antibodies to the M . leprae 18-kDa protein and other proteins. Initial studies had suggested that the 18-kDa protein was restricted to M. leprae, and it was distinct from the M. tuberculosis 19-kDa protein (1, 4). Further, probing genomic libraries of M. tuberculosis and M. bovis (BCG) with the M . leprae 18-kDa gene failed to demonstrate homologs in either species (unpublished observations). More recent evidence, however, indicates that the 18-kDa protein may share epitopes with other mycobacterial species. Polyclonal antisera raised against recombinant M. leprae 18-kDa protein showed crossreactivity with proteins in sonicates of M. smegmatis, M. tuberculosis and M . bovis (11). The amino acid sequence of the M. leprae 18-kDa protein shows a limited degree of homology with a family of low molecular weight plant heat-shock proteins (19). Moreover, an 18-kDa homolog has recently been demonstrated in the cultivable mycobacterium M . habana (14). The synthesis of the M . habana 18-kDa protein was significantly increased after heat shock, suggesting that the protein may be a member of a stress-inducible protein family. Therefore, the presence of homologous proteins or crossreactive epitopes on proteins of different molecular weights in other mycobacteria or unrelated bacterial species may account for the anti-18-kDa response observed in the TB patients. This crossreactivity also extends to T-cell epitopes on the 18-kDa protein because Dockerell and coworkers (10) have observed a T-cell response to the M . leprae 18-kDa protein in some TB patients.
A second possible explanation is that active infection with one mycobacterium, M. tuberculosis, may have stimulated a cellular response which provided T-cell help to recall an antibody response to specific epitopes on related M . leprae proteins. This could occur in subjects who had previously been exposed to leprosy. This phenomenon, termed "original antigenic sin," has been observed in leprosy patients who were shown to have titers of antibodies against M . tuberculosis -specific epitopes in the absence of active tuberculosis (5). This may be less prominent for the antibody response to the carbohydrate determinant on PGL-I because it stimulates a T-cell-independent IgM antibody response.
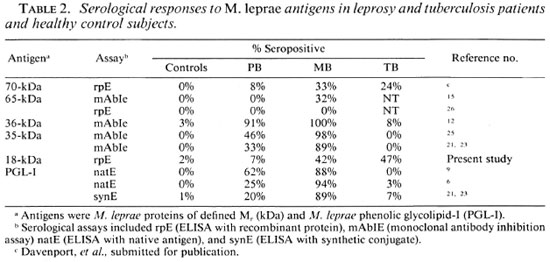
In addition to problems of specificity, the 18-kDa antibody assay had a lower sensitivity than the PGL-I assay (Table 1). This was not dissimilar to the levels of seroreactivity observed with the M . leprae 70-and 65-kDa proteins in ELISAs (Table 2) (15 and Davenport, et al. submitted for publication). By contrast, mAb inhibition assays have detected antibodies to M . leprae-spe cific epitopes on the 35-kDa and 36-kDa proteins in the majority of leprosy patients (12 , 25). However, false-positives may occur with inhibition assays due to steric hindrance of mAb binding caused by serum antibodies binding to adjacent rather than the mAb-defined epitopes. This was the case with the 65-kDa protein because sera with apparent reactivity with mAb-defined epitopes on the protein in an inhibition assay (15) failed to bind to the recombinant 65kDa antigen in a direct ELISA (26). Studies with synthetic peptides of the 36-kDa protein have shown that the mAb F47-9 and leprosy sera, which inhibited F47-9 binding to the protein, in fact bound to different portions of the molecule (13). By comparison the high degree of specificity observed with the 35-kDa mAb inhibition assay (Table 2)(21, 25) was maintained when the recently purified M . leprae 35-kDa protein was shown to bind mAb ML04 and leprosy sera but not TB sera (18). The sensitivity of the anti-35-kDa assay is still limited in tuberculoid leprosy since 33% of PB patient sera (21) had detectable anti-35-kDa antibodies compared to 20% with IgM anti-PGL-I antibodies and 42% with either or both antibodies.
Although anti- 18-kDa responses are of limited value, IgM anti-PGL-I and anti-35kDa responses may be of assistance in the management of clinical leprosy despite their limitations in sensitivity. Both in PB (21) and primary neuritic (22) leprosy, increasing nerve involvement is associated with a rise in antibody levels to both antigens. In fact, PGL-I serology has an equivalent sensitivity to nerve biopsy in the diagnosis of primary neuritic leprosy (22). Further, during chemotherapy the fall in IgM anti-PGL-I antibody levels is a useful monitor of the response to therapy(2, 8, 16). Finally, the presence of IgM anti-PGL-I antibodies at the time of diagnosis in borderline leprosy patients is a major risk factor for the subsequent development of type 1 reactions during therapy (24).
Acknowledgment. The Mycobacterial Research Laboratory is fully supported by The Leprosy Mission (International). We are grateful to the doctors and staff of Anandaban Hospital for their excellent cooperation and to Mrs. S. Failbus for her technical assistance.
REFERENCES
1. ASHBRIDGE, K. R., BOOTH, R. J., WATSON, J. D. and LATHIGRA, R. B. Nucleotide sequence of the 19 kDAantigen gene from Mycobacterium tuberculosis. Nucleic Acids Res. 17(1989)1249.
2. BACH, M.-A., WALLACH, D., FLAGUEL, B., HOFFENBACH, A. and COTTENTOT, A. Antibodies to phenolic glycolipid-I in leprosy patients-evolution during chemotherapy. Int. J. Lepr. 54(1986)256-267.
3. BAGSHAWE, A. F., GARSIA, R. J., BAUMGART, K. and ASTBURY, L. IgMserum antibodies to phenolic glycolipid-I and clinical leprosy: two years' observation in Acommunity with hyperendemic leprosy. Int. J. Lepr. 58(1990)25-30.
4. BOOTH, R. J., HARRIS, D. P., LOVE, J. M. and WATSON, J. D. Antigenic proteins of Mycobacterium leprae: complete sequence of the gene for the 18kDAprotein. J. Immunol. 140(1988)597-603.
5. BOTHAMLEY, G., BACH, J. S., BRITTON, W. J., and IVANYI, J. Antibodies to M. tuberculosis -specific epitopes in lepromatous leprosy. Clin. Exp. Immunol. 1992 (in press).
6. BRETT, S. J., P AYNE, S. N., GIGG, J., BURGESS, P. and GIGG, R. Use of synthetic glycoconjugates containing the M. leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
7. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
8. CHANTEAU, S., CARTEL, J-L., CELERIER, P., PLICHART , R., DESFORGES, S. and Roux, J. PGL-I antigen and antibody detection in leprosy patients: evolution under chemotherapy. Int. J. Lepr. 57(1989)735-743.
9. CHO, S.-N., YANAGIHARA, D. L., HUNTER. S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in the scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
10. DOCKERELL, H. M., STOCKER, N. G., LEE, S. P., JACKSON, M., GRANT, K. A., JOUY, N. E., LUCAS, S. B., HASAN, R., HUSSAIN, R. and MCADAM, K. P. W. J. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57(1989)1979-1983.
11. DOHERTY, T. M. Epitopes recognized by the murine immune response to 18 kilodalton protein of Mycobacterium leprae, Ph.D. thesis, University of Auckland, 1991.
12. KLATSER, P. R., DE WIT, M. Y. L. and KOLK, A. H. J. An ELISA inhibition test using monoclonal antibody for the serology of leprosy. Clin Exp. Immunol. 62(1985)468-473.
13. KLATSER, P. R., DE WIT, M. Y., KOLK, A. H. J. and HARTSKEERL, R. A. Characterization of murine B cell epitopes on the Mycobacterium leprae proline rich antigen by the use of synthetic peptides. Infect. Immun. 59(1991)433-436.
14. LAMB, F. I., SINGH, N. B. and COLSTON, M. J. The specific 18 kilodalton antigen of Mycobacterium leprae is present in Mycobacterium habana and functions as Aheat-shock protein. J. Immunol. 144(1990)1922-1925.
15. LEVIS, W. R., MEEKER, H. C, SCHULLER-LEVIS, G. B., GILLIS, T. P., MARINO, L. J. and ZABRISKIE, J. Serodiagnosis of leprosy: relationships between antibodies to Mycobacterium leprae phenolic glycolipid I and protein antigens. J. Clin. Microbiol. 24(1986)917-921.
16. MEEKER, H. C, LEVIS, W. R., SERSEN, E., SCHULLER-LEVIS, G., BRENNAN, P. J. and BUCHANAN, T. M. ELISAdetection of IgM antibodics against phenolic glycolipid I in the management of leprosy: Acomparison between laboratories. Int. J. Lepr. 54(1986)530-539.
17. MILLER, R. A., HARNISCH, J. B. and BUCHANAN, T. M. Antibodies t o mycobacterial arabinomannan in leprosy: correlation with reactional states and variation during treatment. Int. J. Lepr. 52(1984)133-139.
18. MOHAGHEGHPOUR, N., MUNN, M. W., GELBER, R. H. and ENGLEMAN, E. G. Identification of an immunostimulating protein from Mycobacterium leprae. Infect, Immun. 58(1990)703-710.
19. NERLAND, A. H., MUSTAFA, A. S., SWEETSER, D., GODAL, T. and YOUNG, R. A. Aprotein antigen of Mycobacterium leprae is related to Afamily of small heat shock proteins. J. Bacteriol. 170(1988)5919-5920.
20. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity: Afive group system. Int. J. Lepr. 34(1966)255-273.
21. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., LUDWIG, FL, THEUVENET, W. J. and ADIGA, R. B. Heterogeneity of serological responses in paucibacillary leprosy: differential responses to protein and carbohydrate antigens and correlations with clinical parameters. Int. J. Lepr. 58(1990)319-327.
22. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., THEUVENET, W. J., LAVENDER, M. and ADIGA, R. B. Serology in primary' neuritic leprosy. Trans. R. Soc. Trop. Med. Hyg. 85(1991)299-302.
23. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., WILLIAMS, D., PRADHAN, H. M. and THEUVENET, W. J. Operational value of serological measurements in multibacillary leprosy: clinical and bacteriological correlates of the antibody response. Int. J. Lepr. 58(1990)480-490.
24. ROCHE, P. W., THEUVENET, W. J. and BRITTON, W. J. Risk factors for type I reactions in borderline leprosy patients. Lancet 338(1991)654-656.
25. SINHA, S., SENGUPTA, U., RAMU, G. and IVANYI, J. Serological survey of leprosy and control subjects by Amonoclonal antibody based immunoassay. Int. J. Lepr. 53(1985)33-38.
26. VADIEE, A. R., GILLIS, T. P. and SHANNON, E. J. Confirmation of Afalse-positive result associated with Acompetition inhibition assay used for detecting antibodies to Aprotein epitope of Mycobacterium leprae. Clin. Exp. Immunol. 79(1990)397-402.
1. B. App.Sc, Mycobacterial Research Laboratory, Anandaban Leprosy Hospital, P.O. Box 151, Kathmandu, Nepal.
2. Ph.D.; Department of Molecular Medicine. School of Medicine. University of Auckland. Auckland, New Zealand.
3. Ph.D., Centenary Institute of Cancer Medicine and Cell Biology, University of Sydney, Sydney NSW 2006, Australia.
Reprint requests to Dr. Britton.
Received for publication on 5 December 1991.
Accepted for publication in revised form on 19 March 1991.