- Volume 60 , Number 2
- Page: 208–24
Relationships between titers of antibodies immunoreacting against glycolipid antigens f rom Mycobacterium leprae and M. tuberculosis, the mitsuda and mantoux reactions, and bacteriological loads: implications in the pathogenesis, epidemiology and serodiagnosis of leprosy and tuberculosis
ABSTRACT
Analysis of cell-mediated immunity [(CMI) as judged f rom the Mantoux, Fernandez, and Mitsuda reactions and the presence of granulomas in biopsy material] against humoral immunity (measurements of anti-PGL-I, PGL-Tbl, and SL-IV IgG and IgM antibody titers by ELISA) were performed in selected human populations. The investigations yielded data indicating that humoral (B-cell) responses preceded protective CMI in both tuberculosis and leprosy. The B-cell responses were unrelated to (unfavorable) cell-mediated delayedtype hypersensitivity (DTH). Notwithstanding the difficulty in inferring sequential events f rom studies in humans, it was shown that in humoral responses there was an initial rise of specific IgM immunoglobulins that switched afterward to IgG production during subclinical tuberculosis and leprosy infections. In patent tuberculosis disease the IgM-to-IgG switch was observed in the majority of patients; in patent leprosy disease the switch was impaired in the majority of patients. The clinical, immunological, and laboratory data indicated that the B-cell responses were suppressed as protective CMI was re-established in the patients during the protracted subclinical infection. According to the data, the diagnosis of subclinical tuberculosis and leprosy may be accomplished using ELISA. The yearly risk of tuberculosis in apparently healthy persons but with significant antibody titers was estimated at 44%; the yearly risk for leprosy has not yet been established. The clinical, epidemiologic, and diagnostic implications of these findings are discussed.RÉSUMÉ
L'analyse de l'immunité à médiation cellulaire (CMI) évaluée à partir des réaction de Mantoux, Fernandez, et Mitsuda, et de la présence de granulomes dans le matériel de biopsie comparée à l'immunité humorale (mesure des titres d'anticorps IgG et IgM, vis-à-vis du PGL-I, PGL-Tbl, et SL-IV par ELISA) a été réalisée dans des populations humaines sélectionneées. Les recherches ont fourni des données indiquant que les responses humorales (cellules B) précédaient la protection de l'immunité cellulaire dans la tuberculose et la lèpre. Les réponses des cellules B n'étaient pas associées à une hypersensibilité retardée (défavorable) à médiation cellulaire. Malgré la difficulté de comprendre dès événements successifs à partir d'études dans les populations humaines, on a montré qu'il y avait une augmentation initiale, dans les réponses humorales, des immunoglobulines IgM spécifiques, qui évolue dans la suite vers une production d'IgG durant les infections subeliniques tuberculeuse et lépreuse. Durant la phase de tuberculose-maladie, le glissement d'IgM en IgG a été observé dans la majorité des patients; ce changement était altéré chez la majorité des patients présentant une lèpre symptômatique. Les données cliniques, immunologiques et de laboratoire ont montré que les réponses cellulaires B étaient supprimées tandis que l'immunité cellulaire protectrice était rétablie chez les patients au cours de l'infection subclinique prolongée. D'après les données, le diagnostic de tuberculose et lèpre subcliniques peut être établi par l'ELISA. Le risque annuel de tuberculose chez des personnes apparemment en bonne santé mais avec des titres significatifs d'anticorps a été estimé à 44%, le risque annuel pour la lèpre n'a pas encore ètè déterminé. Les implications cliniques, épidémiologiques et diagnostiques de ces observations sont discutées.RESUMEN
Se analizó la inmunidad mediada por células, CMI (reacciones de Mantoux, de Fernández y de Mitsuda, y presencia de granulomas en biopsias de piel), y la inmunidad humoral (anticuerpos IgG c IgM anti-PGL-I, anti-PGL-TBl, y anti-SL-IV, por ELISAs), en grupos de individuos seleccionados. Los resultados indicaron que las respuestas humorales (células B) precedieron a las respuestas celulares protectoras tanto en lepra como en tuberculosis. Las respuestas de las células B no estuvieron relacionadas con la (desfavorable) hipersensibilidad retardada mediada por células (DTH). No obstante la dificultad para inferir eventos sccuenciales a partir de estudios en humanos, se observó que inicialmente hubo una elevación en los niveles de inmunoglobulinas IgM específicas, que después cambió a la producción de IgG durante las infecciones subclínicas de la lepra y la tuberculosis. En la mayoría de los pacientes con tuberculosis se observó el cambio de IgM a IgG; en la mayoría de los pacientes con lepra este cambio no oceurrió. Los datos clinicos, inmunológicos y de laboratorio, indicaron que al reestablecerse la CMI durante la infección subclinica retardada, se suprimieron las respuestas de las células B. De acuerdo a los resultados, el diagnóstico de la tuberculosis y de la lepra subclínicas, podría hacerse usando la técnica de ELISA. Se estimó que el riesgo anual de tuberculosis en personas aparentemente sanas pero con títulos significativos de anticuerpos fue del 44%; el riesgo anual para los pacientes con lepra no se ha establecido. Se discuten las implicaciones clínicas, epidemiológicas y diagnósticas de estos hallazgos.The triglycosyl phenolphthiocerol dimycoserosates from Mycobacterium leprae (PGL-I) (32) and M . tuberculosis (PGL-Tb1) (17) are species-specific antigens (31,43) inducing in humans the production of IgG and IgM immunoglobulins (8, 12, 15, 54). The specificity of these antigens is supposedly determined by the nature of the terminal sugar in the trisaccharide which is a 3,6-di-omethyl- β -D-glucopyranose in PGL-I (32) and a 2,3,4-tri-methyl- α -L-fucopyranose in PGL-Tbl (17). Using ELISA, the high specificity of these antigens has been well established. However, for a specificity predetermined at 100%, the sensitivity of the assays in serodiagnosis has been variable between reports and generally was found to be low.
The low sensitivity of serodiagnosis in leprosy was soon understood as judged from three kinds of observations: a) anti-PGL-I antibody titers increased from the tuberculoid toward the lepromatous end of the Ridley-Jopling classification of leprosy (6, 31, 54) b) a reasonable direct correlation was found between the bacterial index (BI) and the antibody titers (38, 51); and c) a neat reciprocal relationship was found between the intensity of the Mitsuda reaction and the antibody titers (24). Consequently, the sensitivity of serodiagnosis should vary from one endemic region to another according to the incidence of lepromatous leprosy.
The low sensitivity of serodiagnosis in tuberculosis has been more difficult to understand. One hypothesis was that not all strains of tubercle bacilli synthesized the specific PGL-Tbl phenolic glycolipid (16); another was that low sensitivity could be related to an immune spectrum in tuberculosis (21, 24). Even though the existence of an immune spectrum in tuberculosis is still controversial (21, 36) it was suggested that the above considerations of the relationships between humoral, cell-mediated immunity, bacteriological loads and the clinical condition in leprosy might also apply to tuberculosis (21).
Following the above considerations, we reasoned that comparative studies relating antibody titers against species-specific antigens (PGL-I and PGL-Tbl) and antigens common to M. leprae and M. tuberculosis to bacteriological loads, the intensity of the Mitsuda and Mantoux reactions, and the clinical condition should give useful information about the immune responses to glycolipid antigens in mycobacterial diseases, and might help in developing new reagents for the diagnosis as well as the classification of cases within the immune spectrum. Among the glycolipid antigens common to both species that have been examined for serodiagnosis (the phosphomannosides 41; a lipoarabinomannan 10; an arabinogalactan 48; and an antigen designated SL-IV 15), we decided to use the SL-IV antigen from M . tuberculosis because it was known that antibodies immunoreacting with it were found in sera from leprosy cases (15). The SL-IV antigen was formerly characterized as a diacyl trehalose-2'-sulfate(18). However, its structure was recently revised as a 2-3-diacyl trehalose (37).
MATERIALS AND METHODS
Population studied. The sera used in this investigation were collected from 1532 individuals and included 248 bacteriologically confirmed tuberculosis cases, 65 professional contacts, 84 leprosy cases, and 1135 controls. All selected persons were adults residing either in a low-endemic region (France, N = 697) or in a high-endemic region (Manaus, Amazonia, Brazil, N = 835). Hereafter, the groups of individuals residing in the low-endemic and in the high-endemic regions are designated the LER and the HER populations, respectively. Subgroups within the LER and HER are indicated in the Results section as appropriate.
Clinical and laboratory examinations. Tuberculosis and leprosy were diagnosed using current clinical examinations. For the purpose of this study, all cases of tuberculosis were confirmed by the isolation and identification of M. tuberculosis using recommended methods (22), except in some cases of tuberculosis of the skin which were diagnosed on the basis of histopathology and the presence of acid-fast bacilli in smears; tuberculosis lesions were classified as recommended by Santa Cruz and Strayer (50). The leprosy patients were diagnosed and classified as recommended by Jopling (33).
Acid-fast stain. Smears were stained using the Kinyoun acid-fast staining procedure. The method used for tuberculosis was as recommended by the U.S. Centers for Disease Control (CDC) and the Pasteur Institut (22), and for leprosy as described by Fandinho, et al. (26). In tuberculosis, the smears were classified as proposed by David (20), and recommended by the American Thoracic Society (ATS) (3) and the Pasteur Institut (22, 23); in leprosy, the smears were classified as recommended by Jopling (33).
ELISAs. The PGL-Tbl and the SL-IV antigens were isolated from M. tuberculosis strain Canetti CIPT-14-001-0059, and the PGL-I antigen was isolated from the liver of an armadillo experimentally infected with M . leprae. The antigens were isolated using published methods (18, 32). For analytic purposes the authentic standards of PGL-Tbl and SL-IV were kindly supplied by M. DafTë (Centre de Recherche de Biochimie et Génétique Cellulaires, CNRS, Toulouse, France), and the authentic PGL-I was kindly supplied by R. J. W. Rees (WHO-IMMLEP). The ELISA method used was that of Cruaud, et al. (15). The purified antigens (PGL-I, 250 ng/well; PGL-Tbl, 250 ng/well; SL-IV, 100 ng/well) were dissolved in hexane and were added to the wells of fiat-bottom, 96-well plates and dried overnight at 37ºC. The subsequent steps and final calculation were as described in detail by Cruaud, et al. (15).
Mantoux, Mitsuda and Fernandez reactions. Tuberculin RT23 from WHO was supplied by the Ministry of Health (Brazil); the lepromin-H prepared from human lepromas was kindly supplied by M.-T. Orsi Souza of the Centra de Dermatologia Sanitaria Dr. Alfredo da Mata, Manaus, Amazonia, Brazil. Two tuberculin units of RT23 (5 bioequivalents of PPD-S) and lepromin-H containing 16 x 106 bacilli per 0.1 ml dose were used. Dual skin-test reactions were performed only in 534 control individuals (army recruits, 18-20 years old) in the HER. The Mitsuda reaction was performed in all leprosy cases. The Mantoux reaction was read 72 hr after injection as the diameter of induration in mm; the Fernandez reaction was read 72 hr after injection as the diameter of the erythema reaction in mm; the Mitsuda reaction was read 21 days after injection as the diameter of the nodule in mm. In the LER the Mantoux tests were performed using IP48 PPD containing 10 PPD-S bioequivalents.
Statistics. Statistics were performed using the Criket Graph and the StatView statistical programs for the Macintosh SE computer. Sensitivity and specificity values were calculated as described before (15).
RESULTS
Preliminary observations. To establish specificity levels of the assay is a prerequisite for using the antigens in serodiagnosis. The specificities of the assays using the cutoff levels of > 200 for the PGL-I and PGL-Tbl and > 300 for the SL-IV antigens are shown in Table 1. The specificity of the method was in the range of 99% to 100% in France (LER; Table 1) where leprosy is not present and where the incidence of tuberculosis is about 16 per 100,000 inhabitants. It did not differ significantly from our previous report (15). From these data it was concluded that serology values above 200 were positive test results for the PGL-I and PGL-Tbl antigens and that values above 300 were positive test results for the SL-IV antigen.
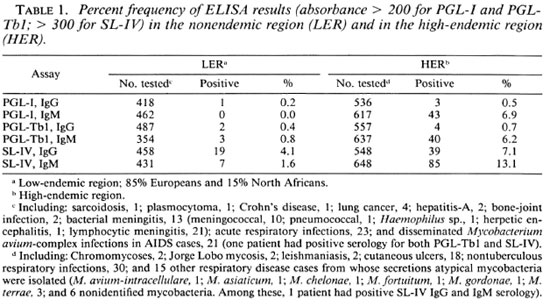
The frequency of the test results above the threshold values was significantly higher in the Brazilian population from Amazonia (HER; Table 1) where the incidences ofboth tuberculosis and leprosy are high (119 and 60 per 100,000 respectively; the prevalence of leprosy was estimated at 12 per 1000) (1). Because crossreactions were not detected with respect to a large variety of infectious and parasitic diseases and to colonization or infection by mycobacteria other than the tubercle bacilli (Table 1, legend), we concluded that the frequent occurrence of antibodies immunoreacting against the glycolipid antigens in sera of control subjects residing in the HER indicated previous contact with M. tuberculosis or M. leprae. Further data supporting the conclusion that the occurrence of specific antibodies in persons in apparent health is indicative of subclinal disease are presented below.
Antibody titers with respect to Mitsuda and Mantoux reactions in leprosy and tuberculosis patients. The distribution of ELISA results with respect to the Mitsuda test results in leprosy patients is shown in Figure 1. From the distribution ofthe paired data, a straightline fit could not satisfactorily describe the relationship between the two variables. Consequently, polynomial fits were traced, and the correlation coefficients were calculated (Fig. 1). As apparent, the increase in the values ofone ofthe variables resulted in decreasing values of the other. Analysis of the paired data showed that the highest titers of anti-PGL-I IgG-and IgM specific antibodies occurred in patients with negative (< 2 mm papules) Mitsuda reactions. At the other end of the Mitsuda scale, all patients with reactions > 6 mm had ELISA results < 200 (a negative test result), and within the 3 to 6 mm range of Mitsuda reactions ELISA results were variable. Using the SL-IV antigen from M. tuberculosis, the same relationships were found with respect to the IgG assays (Fig. 1 C), while no correlation was found between the Mitsuda reaction and the anti-SL-IV IgM assay results (Fig. 1 D). From a clinical viewpoint, it was concluded that PGL-I and SL-IV ELISA may be an interesting adjunct for the classification of recently discovered cases within the immune spectrum.
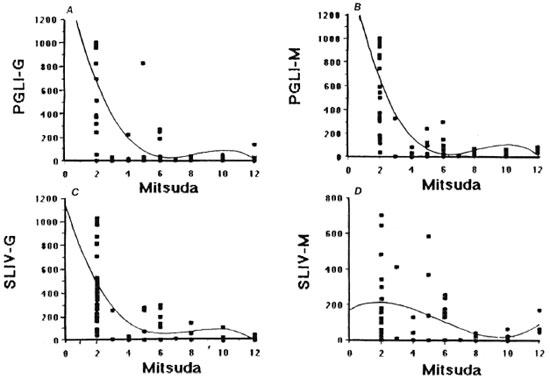
Fig. 1. Quantitative relationships between PGL-I and SL-IV antibody titersand the Mitsuda test reactions in 84 leprosy patients.
In pulmonary tuberculosis no correlation was found between the ELISA and the Mantoux test results (Table 2). In this group of 56 patients, 7 (12.5%) did not react to tuberculin, confirming previous reports (52) which indicated that when first discovered patients may be in an anergic state.
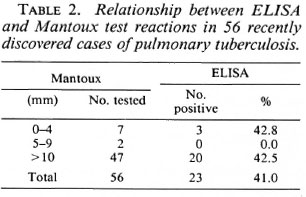
We conclude that antibody titers were inversely related to (protective) cell-mediated immunity (as judged from the Mitsuda test reaction) but not to (unfavorable) delayedtype hypersensitivity (as judged from the Mantoux test reaction). The absence of correlation between the PGL-I ELISA and the delayed-type hypersensitivity leprosin-A results in leprosy cases has been reported by others (13).
Antibody titers with respect to bacteriological loads. The finding that the quantitative relationships with respect to Mitsuda reactions were the same using a species-specific antigen (PGL-I) and a nonspecific antigen (SL-IV) made it necessary to find out if the same occurred with respect to bacterial loads. Previous workers (38, 51) found a good correlation between anti-PGL-I antibody titers and the BI. These observations were confirmed in this study. The correlation coefficients (data not shown) were r = 0.63; r = 0.76 and r = 0.72 for, respectively, IgG PGL-I, IgM PGL-I and SL-IV IgG. Virtually no correlation was observed between the BI and SL-IV IgM assays (r = 0.24).
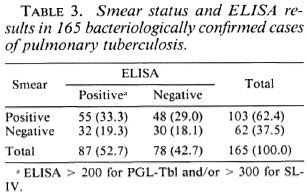
In the pulmonary tuberculosis cases there was no statistically significant difference (χ2 = 0.051, 90% < α < 50%) between the number of positive ELISA results and the number ofbacilli seen in smears (Table 3). However, from a clinical viewpoint the data showed that the sensitivity of sputum smear examination was 62.4%; the sensitivity of serodiagnosis was 52.7%; and the sensitivity of the combined use of both procedures was 81.8%. These differences were statistically significant ( ε = 10.3, p < 1%). Therefore, serological assays may be an interesting adjunct for the diagnosis oftuberculosis in suspected patients.
Antibody titers with respect to clinical condition. The mean, standard deviations, and the sensitivity of the ELISA results with respect to the Ridley-Jopling classification of leprosy patients are shown in Table 4. In this series of cases there was no significant difference between anti-PGL-I IgG and IgM assays; anti-PGL-I IgG and IgM and SL-IV IgG assays yielded values increasing from the tuberculoid toward the lepromatous pole of the spectrum; and the anti-SL-IV IgM assays were negative in most cases. In the 21 LL and 8 BL leprosy cases, the PGL-I IgM assay result was higher than the IgG assay result in 20 patients, the PGL-I IgG was higher than IgM in 8 patients, and the same ELISA result was found in 1 patient. With the SL-IV, the IgG assay result was higher than the IgM assay in 25 patients, the IgM assay was higher than the IgG in 2 patients, and the same ELISA result was found in 2 patients. From these observations it was concluded that the humoral immunity response to the glycolipid antigen isolated from M. tuberculosis followed the same general pattern as the humoral immunity response against the M . leprae PGLI-specific antigen, except that with the SL-IV antigen virtually all of the IgG responses were predominant while the PGL-I antigen showed both an IgG and an IgM response. It was therefore concluded that while the IgM to IgG switch was somehow blocked in the case of PGL-I, this was not the case with respect to SL-IV.
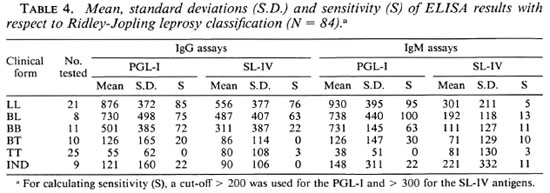
In tuberculosis there was no apparent relationship between antibody titers and the clinical condition (Table 5). The lack of correlation between antibody titers and the intensity of the Mantoux test (Table 2), bacterial loads (Table 3), and the clinical condition (Table 5) made us wonder if there was a relationship between antibody titers and the structure of the lesions (presence or absence of caseating granulomas). Because tuberculosis of the skin is relatively frequent in the HER and histopathological examinations are required for diagnosis, we performed ELISAs in sera from 31 individuals with suspected tuberculosis of the skin.
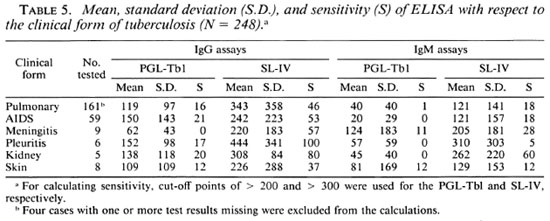
Tuberculosis of the skin. The diagnosis of cutaneous tuberculosis (Table 6) was established by isolation of M. tuberculosis inseven cases; in one case the disease wascaused by M. africanum . In six cases the presence of caseating tuberculoid granulomas and acid-fast bacilli in smears were the criteria for tentative diagnosis. As judged from the presence of tuberculoid granulomas and the results of tuberculin tests, all patients had developed cell-mediated immunity, except that in two cases we did not have the required information. Antibody titers above thres hold values were found infour cases (28.5%). Considering only cases bacteriologically confirmed, positive serology was obtained in 50%. Consequently, our observations indicated that all of our patients with cutaneous tuberculosis had recovered cell-mediated immunity when clinical disease was apparent, and that only about half of them produced the tested antibodies. Because we did not find tuberculosis of the skin in the absence of tuberculoid granulomas and four cases also had pulmonary tuberculosis, the relationship between cell-mediated immunity and humoral responses remained undecided. Considering that tuberculosis of the skin without tuberculoid granulomas has been described (30, 49) we continue to search for new cases. Nonetheless, the available data did not disagree with our hypothesis (see above) that development of protective cellmediated immunity results in suppression of specific B-cell responses.
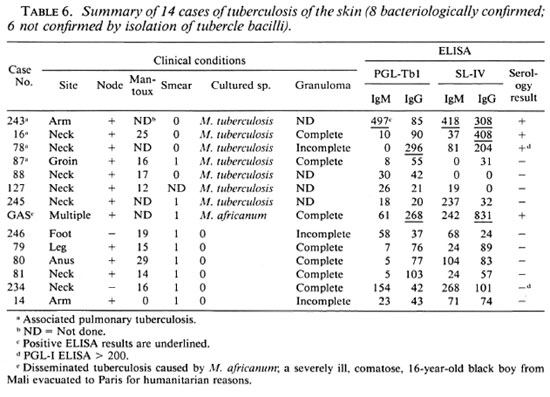
Professional contacts. An ELISA was performed in sera from 65 professionals with close contacts with leprosy and tuberculosis patients or who handle pathological specimens thereof. ELISA results in these sera are shown in Table 7. A positive serology result for tuberculosis was detected among three persons in the LER group (N = 22; 13.6%), and a positive serology result for tuberculosis was detected among six persons in the HER group (N = 43; 13.9%). In the LER group a positive test result for leprosy was detected in none, while in the HER group a positive test result for leprosy was detected in three individuals (8.1%). Clinical follow up of 6 months to 1 year for all professional contacts yielded the following information.
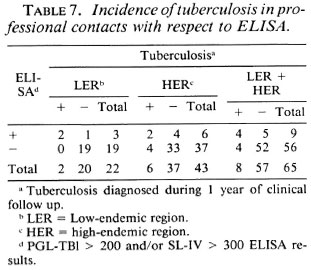
In the LER group there was one case of patent pulmonary tuberculosis 3 months after the serum was tested (SL-IV IgM ELISA value was 600; 4-mm Mantoux reaction) and one individual was a recent tuberculin converter (11-mm Mantoux reaction) with an anti-SL-IV IgM titer of 386, a chest X-ray considered suspect, and the individual was placed under specific chemotherapy. The remaining person with positive serology did not develop tuberculosis disease during the 12 months of clinical observation.
In the HER group six cases of pulmonary tuberculosis were detected, among which two had positive serology for tuberculosis (33.3%). The remaining persons with positive serology for tuberculosis did not develop apparent disease during the observation period (now 1 year after their sera were tested). With regard to the PGL-I (leprosy) test results, the three persons concerned did not develop leprosy for the 1-year period of clinical follow up.
Although the number of cases in each of the subgroups is small, the percentages arrived at allow the following preliminary conclusions. The risk of subclinical tuberculosis among the professional contacts was about the same in the LER and HER (13.6% and 13.9%, respectively). The risk of patent tuberculosis was significantly higher (χ2Yates = 6.92, 1% > α > 0.1%) in persons with positive serology (in case of positive serology the calculated risks were about 66%, 33%, and 45%, respectively, in the LER, HER and both groups). In case of negative serology, the calculated risks were 0.0%, 10.8%, and 7.1% in the LER, HER, and both groups. Judging from these results, it seemed that regular ELISA testing may be useful in the surveillance of tuberculosis among health personnel. In the case of leprosy, the observation period is still insufficient to draw conclusions about the value of the test in this context.
Antibody titers with respect to Mitsuda, Fernandez and Mantoux reactions in army recruits. From the foregoing data it appeared that high antibody titers in healthy individuals might signify the presence ofactual subclinical disease. To further examine this question 534 army recruits from the endemic region were tested (data shown in Fig. 2; Tables 8, 9, and 10).
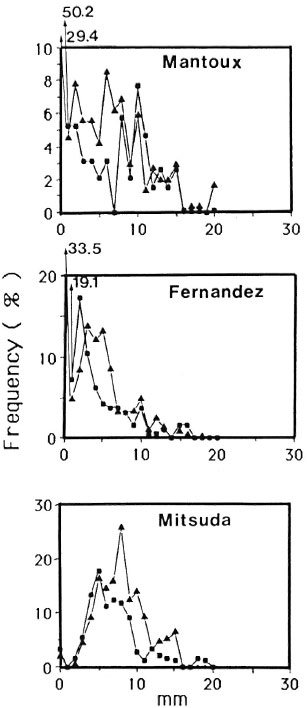
Fig. 2. Mantoux, Fernandez and Mitsuda reactions in 512 army recruits (199 nonvaccinated ■; 319 BCG vaccinated ▲). In upper figure, for the nonvaccinated group and values > 6 mm (N = 52), the mean and median values were, respectively, 11.4 and 11.0 mm. In bottom figure, the mean and median values were 6.0 and 8.0 for the nonvaccinated and BCG-vaccinated, respectively.
As shown in Figure 2, the relative frequency of different size reactions to tuberculin was distinctly bimodal in army recruits who did not receive BCG in childhood. BCG vaccination in childhood resulted in the disappearance of the bimodal shape of the curve.
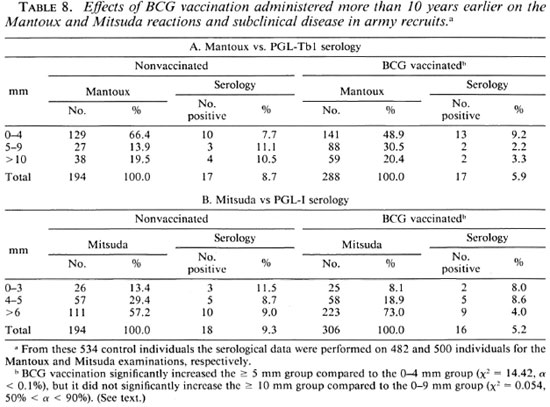
When the data were arranged according to the ATS classification of tuberculin reactions (Table 8A), it was apparent that previous BCG vaccination resulted in a decrease in the frequency of 0-4 mm reactions, in an increase of 5-9 mm reactions. The frequency of > 10 mm did not differ between the two groups of army recruits. From these data it was concluded that the > 10 mm criterion for tuberculosis infection (diagnosis of the infected not diseased) remained valid for the interpretation of tuberculin test reactions in adult individuals who had received BCG during their infancy (in this 18-20-year-old group, BCG vaccination > 10 years before).
The frequency of positive serological results (PGL-Tbl > 200) in both groups (Table 8A) was slightly less in the vaccinated group (8.7% and 5.9% in the nonvaccinated against the vaccinated groups). The difference between the two groups was not statistically significant (χ2 = 1-43, 20% > α > 30%). These data were tentatively interpreted to indicate that BCG vaccination did not give protection against subclinical tuberculosis. A most unexpected observation was the finding that about 8% of the individuals contained anti-PGL-Tbl-specific antibodies in their sera but were tuberculin negative (reactions of 0-4 mm). These observations raise the interesting question of whether there is in the human population a group of persons unable to develop delayedtype hypersensitivity during infection by tubercle bacilli since it is known that l%-5% of individuals do not develop delayed-type hypersensitivity even on repeated BCG vaccination (39).
The frequency distribution of different size reactions to lepromin was near-Gaussian in the nonvaccinated and the BCG-vaccinated (Fig. 2). The Gaussian distribution indicated that the Mitsuda reaction in nonleprous individuals is a characteristic biological parameter in humans, and that it was independent of previous contact with M. leprae, anotion that has been established before (47). The frequencies of positive PGL-I serological results were 9.3% and 5.2% in, respectively, the nonvaccinated and in the BCG-vaccinated individuals (Table 8B). The difference was not statistically significant (χ2= 3.07, 5% < α < 10%), indicating that BCG vaccination in infancy did not induce protective immunity against leprosy. The most interesting finding was that the ELISA results were independent of the Mitsuda reactions (Table 8B), indicating that the probability of subclinical disease was the same in all individuals. There was no difference between the 0-3-mm and > 4-mm groups (χ2Yates = 0.363, 50% < α < 90%); also there was no difference between the 0-5 mm and > 6 mm groups (χ2 = 1.96, 10% < α < 20%). Whether the outcome of infection was the same in Mitsuda-negative as compared to Mitsuda-positive reactors could not be ascertained at this time since clinical follow up of all individuals will not be completed before a minimum of 2-5 years. As to the question: Does BCG modify the Mitsuda reaction? The answer was: Yes, since it significantly increased the > 6-mm group as compared to the < 5-mm group (χ2= 13.14, α < 0.1%).
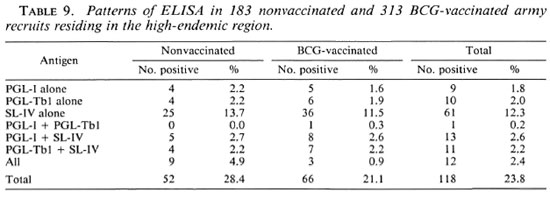
As indicated above, the SL-IV antigen is common to tuberculosis and leprosy and, consequently, the analysis of data with respect to the Mantoux and Mitsuda reactions was not pertinent. The comparison of SL-IV serological results with serological results using the highly specific PGL-I and PGL-Tbl antigens is shown in Table 9. The SL-IV antigen yielded positive test results more often than the species-specific antigens. In most instances a positive test result against the species-specific antigens was also associated with a positive test result for SL-IV (25/34, 73.5% in case of PGL-I, 23/33 = 69.7% in case of PGL-Tbl) and 12 individuals (9 in the nonvaccinated group and 3 in the BCG-vaccinated group) had positive ELISA results with respect to the three antigens. From these data we concluded that the SL-IV was more sensitive than the species-specific antigens for case finding, but the full benefit of the ELISA requires the use of all three antigens.
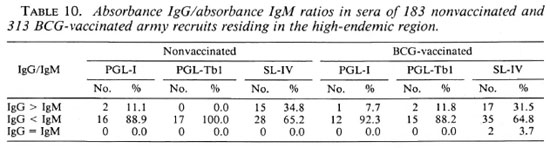
When IgM and IgG absorbance values were compared (Table 10) it was found that in the majority of individuals with positive serology the IgM values were higher than the IgG values with respect to all of the antigens used. This was an interesting finding because, as shown above, IgM levels are often predominant in patent leprosy (Fig. 1, Table 4), while IgG levels are predominant in patent tuberculosis (Table 5). From these observations we concluded that the IgM-to-IgG shift with respect to PGL-I (but not to SL-IV) is somehow blocked in leprosy. In tuberculosis the IgM-to-IgG shift occurred for both the PGL-Tbl and SL-IV antigens.
The data with respect to the Fernandez reaction (Fig. 2) were more difficult to interpret because the differences between the groups did not seem to be significant and no relationships were found between the reaction and any of the other parameters ex-amined. Furthermore, most reactions were in the 2-3-mm range, and were possibly of a nonspecific nature. Consequently, no conclusions could be drawn from the data, and further studies are necessary to clarify the nature of the Fernandez reaction.
DISCUSSION
To our knowledge, this was the first study in which an attempt was made to infer comparative immunopathogenesis of tuberculosis and leprosy by relating humoral B-cell responses to cellular T-cell responses. The study demonstrated that the approach was rewarding and deserving of further investigations. To conduct the study we examined the sera from individuals who were tested using tuberculin (Mantoux test) and/or lepromin (Fernandez and Mitsuda tests), bacteriological examinations (Kinyoun acid-fast microscopy; culture studies), and histopathological examinations as appropriate for the various subgroups examined. The ELISA data were then analyzed against the other clinical and laboratory data. The tested populations included 697 persons residing in a low tuberculosis endemy region (LER) where leprosy has been eliminated (France) and 835 persons residing in a region of high endemy (HER) for tuberculosis and leprosy (Amazonia, Brazil). In the discussion that follows, the evidence supporting the thesis that humoral immunity is related to cell-mediated immunity and proceeds along a definite time curve will be discussed first. Then we will analyze our data with respect to the value of the ELISA in clinical microbiology.
Tuberculosis and leprosy are diseases in which infection is not inexorably followed by disease. In tuberculosis it was well established that infection by tubercle bacilli does not cause disease in the great majority of individuals, which introduced the concept of the "infected not diseased" detected using the Mantoux test (3). As shown in this report, the diagnosis of subclinical disease may be accomplished by assaying for IgM and IgG immunoglobulins reacting against the PGL-Tb 1 and SL-IV glycolipid antigens from M. tuberculosis. While the risk of tuberculosis disease was estimated at 3% to 5% in the newly infected during the first year (reviewed in (20 and 53) and and at 1-10 per 10,000 in the "infected not diseased" in community studies (reviewed in 20 and 53), the risk was calculated to be 44% (C.I. 95% from 14.5% to 77%) during the first year in individuals with subclinical tuberculosis (present study). In the case of leprosy there is no test useful in clinical medicine to identify the "infected not diseased." Indeed, the product available (lepromin) induces skintest reactions (Mitsuda reactions) in the majority of individuals in the absence of exposure to the agent (47, and present study).
As in tuberculosis (see above), subclinical leprosy was diagnosed using a PGI-I ELISA but, in this case, the risk of leprosy disease was not yet established. Because antibodies are absent in sera from the "infected not diseased," IgM immunoglobulins are predominant in subclinical disease, and IgG immunoglobulins may be predominant in patent disease, we concluded that as infection progresses into subclinical disease IgM immunoglobulins are produced first, later switching to IgG production. The IgM-to-IgG switch was apparent in most cases of patent tuberculosis but was somehow impaired in patent leprosy. In this respect, it is of interest that serial anti-PGL-I IgM and IgG assays during experimental leprosy of mangabey monkeys clearly showed that the rise of IgM antibodies was transitory and preceded IgG production in most of the tested animals (29), but in armadillos this was less apparent (56).
According to our data, the B-cell response was suppressed as protective cell-mediated immunity was progressively established during the protracted subclinical disease latent period. Leprosy patients who develop specific cell-mediated immunity (Mitsuda reactors) respond poorly to the antigens that produce specific circulating immunoglobulins, as judged from clinical studies showing that antibody titers and the sensitivity of serodiagnosis increase from the tuberculoid toward the lepromatous end of the leprosy spectrum. (8, 12, 54) A more-detailed quantitative analysis (24, and present study) revealed that there was a strong reciprocal relationship between the degree of cell-mediated immunity (expressed as the intensity of the Mitsuda reaction) and antibody titers against the PGL-I and the SL-IV antigens (this study). These quantitative relationships support our contention that the development of protective cell-mediated immunity results in the suppression of B-cell responses against specific antigens of M. leprae and vice versa.
However, the above quantitative relationships that apply to leprosy disease were not found in healthy individuals because significant antibody levels were detected in apparently healthy lepromin-positive individuals. In 1950 Rotberg, et al. (47) described the results of a comparative study of the Fernandez and Mitsuda reactions in healthy persons residing in a nonendemic region (New York, USA) and in an endemic region (Sao Paulo, Brazil). They showed that the frequency of positive reactions to lepromin was about the same in both populations and was, therefore, independent of exposure to M. leprae. These earlier observations were confirmed in the present study showing that the percent distribution of Mitsuda reactions in army recruits was Gaussian (mean = 6.8; median = 6.0 in the nonvaccinated; mean = 7.9; median = 8.0 in the BCG-vaccinated), indicating that the response to lepromin was a normal and characteristic parameter in humans. An ELISA performed with sera of these persons added new information since it was shown that healthy Mitsuda reactors produced IgM antibodies upon exposure to M . leprae as often as the Mitsuda-negative healthy reactors. Furthermore our data demonstrated that the presence of circulating specific immunoglobulins required exposure to leprosy bacilli, because these antibodies were not detected in sera from controls residing in the LER region (N = 462, positive test results < 0.2%) while they were frequently detected in sera from persons residing in the HER region (N = 617; positive test results = 6.9%).
Consequently, the Mitsuda test reaction may not be interpreted the same way in health and in disease. In the healthy, the Mitsuda reaction must represent native resistance to leprosy; in the diseased, it represents specific protective immunity induced by the leprosy bacilli. According to the data the antigens were equally processed in the healthy irrespective of their Mitsuda status. The question remains whether, upon infection by leprosy bacilli as judged from ELISA, the healthy Mitsuda reactor develops specific immunity more efficiently than the nonreactor. Also, the question remains whether the clinical forms of leprosy are related to the presence of native immunity, that is, whether tuberculoid leprosy follows infection of the Mitsuda-positive healthy individual and lepromatous leprosy follows infection of the Mitsuda-negative healthy individual. The clinical follow up of army recruits now in progress should help to clarify this matter. However, 2 to 5 years of clinical supervision may be necessary before conclusive data will be available.
In tuberculosis, although the antigens were highly specific the sensitivity of the ELISA as judged from assays in bacteriologically confirmed tuberculosis was low (the overall sensitivity was in the 50%-60º/o range). The low sensitivity of the ELISA in patent tuberculosis may also be attributed to a reciprocal relationship between B-cell proliferation and T-cell protective immunity. Indeed, it has been well established that in the absence of immunosuppression all patients suffering from tuberculosis are capable of building up specific cell-mediated immunity (9, 40). Supportive evidence from our study was the finding that tuberculoid granulomas were found in all biopsy samples examined and, although the tuberculin reaction is not a measurement of (protective) cell-mediated immunity, the finding that only 12.5% of recently discovered cases had tuberculin reactions of 0-4 mm also indicated that most patients had competent T-cell responses. Therefore, it was apparent that in tuberculosis as in leprosy the development of the specific protective cell-mediated immunity results in suppression of the B-cell response against specific antigens. In this respect it is pertinent to note that in mice experimentally infected with M. avium, a quantitative relationship between B-cell activation and immunosuppression was recently described (27).
One unexpected observation in our findings was that some individuals (8.4%) in whose sera antibodies were detected did not react to tuberculin, which brought to light the possibility that in human populations there are individuals unable to build up delayed-type hypersensitivity upon infection by tubercle bacilli (that some individuals do not develop delayed-type hypersensitivity even upon repeated vaccination with BCG has been well documented 36, 39). In light of the above considerations, it is also possible that these persons had actual primary infections detected before delayed-type hypersensitivity was established. A minimum of 1 to 2 years of clinical follow up is necessary to clarify these possibilities, to verify if the probability of tuberculosis disease is higher in these individuals, and to evaluate the severity of the disease in the various subgroups.
Although it is difficult to infer fundamental mechanisms from studies in human populations, the cumulative data allow us to advance the following preliminary explanations. The phenol glycolipid (PGL-I) from M. leprae accumulates in large quantities in the tissues of patients with lepromatous leprosy (11), and in tissues of experimentally infected armadillos (31, 32); cultured macrophages phagocytize the bacteria which are slowly digested with a relative lack of antigen transfer to the surface of macrophages (44); and PGL-I was shown to suppress T-cell responses to a variety of effector molecules (28, 32, 35). The fact that the phenolic glycolipid antigen from M. tuberculosis (PGL-Tb 1) might also accumulate in tissues of tuberculosis patients or in animals experimentally infected has not been examined yet. However, it was found that it persists in cultured macrophages (unpublished observations). Although a monomycolate of trehalose was reported to occur in M . leprae (19), we did not detect the SL-IV antigen in the tissues of armadillos infected with M. leprae. Their possible accumulation in the tissues of animals infected with M. tuberculosis has not yet been examined, but it was established that these molecules are rapidly processed in cultured macrophages(44). Furthermore, the powerful and highly efficient drugs used in tuberculosis rapidly stop the progression of the infection and, consequently, the accumulation of the antigens in tissues may not be expected. The differential processing of the antigens, the distinct host-parasite relationships in leprosy and tuberculosis, and the efficacy of antituberculosis treatment were considered in the following comparative analysis of the immunological data.
Using PGL-I as the antigen, IgM is the predominant immunoglobulin class in sera from leprosy patients, which led some investigators to the conclusion that a failure to activate T cells could be responsible for the lack of the IgM-to-IgG switch (12) or to the complete lack of the IgG response (55). The present study docs not seem to support these conclusions. Indeed, the IgM-to-IgG switch is impaired but is not absent in the case of PGL-I, and it proceeded as expected when another glycolipid (SL-IV) was used as the antigen. These findings did not seem to support the notion of defective antigen presentation or the absence of specific reactive T cells in the circulation (33, 34, 36).
In our opinion the comparative immunopathological data on leprosy and tuberculosis herein reported are more consistent with views attributing the diversity of the clinicopathological features to the complexity of the regulatory mechanism controlling cell-mediated immunity We have already proposed (24) that the simplest comprehensive explanation of these complexities was to assume that for every specific T cell there was a twin specific B-cell clone, their ratio being strictly regulated, and that during the subclinical phase of the disease whichever was primed to proliferate, the other would be suppressed. Regulation of the T-cell/B-cell twin lines could operate by direct cell-to-cell signals and, consequently, we began to culture B cells and T cells, specifically responding to the antigen as a way to test the hypothesis. In this respect, it was shown by others (42) that T cells from lepromatous patients recovered reactivity when cultured in vitro. In our laboratory we found that B cells from a tuberculoid patient produced anti-PGL-I IgG antibodies even though antibodies immunoreacting against PGL-I were not significantly detected in his serum (unpublished observation).
The quantitative relationships between anti-PGL-I antibody levels and bacteriological loads (bacterial index, BI) reported by others were confirmed in our study. The antibody levels/BI relationship was not observed in tuberculosis, perhaps because the numbers of bacilli in smears are not a true representation of bacterial loads in the tissues and, in tuberculosis, the number of bacilli in the patient's body is many orders of magnitude less than in multibacillary leprosy (the number of tubercle bacilli in a lung cavity was estimated at 108; in a lepromatous nodule the number of leprosy bacilli was estimated to be 7 x 109 per gram of tissue 9, 33). These quantitative differences in bacteriological loads may be attributed to the fact that M. tuberculosis, not M. leprae, is a deadly pathogen.
Irrespective of further investigations to elucidate the fundamental nature of the foregoing, our study justifies some epidemiological and clinical considerations. Contrary to the Mantoux test (2, and present study) criteria to define a positive Mitsuda reaction have not been firmly established but our data clearly demonstrated that Mitsuda reactions > 6 mm is the best criterion to classify a positive test reaction. Judging from our data, Mitsuda reactions of > 6 mm in the absence of detectable amounts of antibodies characterize the polar forms of tuberculoid leprosy; Mitsuda reactions of 0- to 2-mm in presence of high titers of antibodies (> 500 using the PGL-I antigen) characterize the polar forms of lepromatous leprosy. Any other combination of Mitsuda reactions versus serology should apply to all other forms of leprosy. From these observations we conclude that an ELISA using the glycolipid antigens is a laboratory examination that could be used as an aid for the classification of recently discovered patients within the immune spectrum. From our data and the data in the literature (4, 5, 14, 25, 45, 49) it was apparent that anti-PGL-I and SL-IV IgM antibodies may be found in the sera of leprosy contacts, that some of the contacts were Mitsuda reactors (it is unfortunate that previous workers did not report the actual intensity of the Mitsuda reaction), and that clinical leprosy was diagnosed in some of these individuals. However, views on the value of the ELISA PGL-I for predicting leprosy in these populations differed widely.
Considering the importance of and the conflicting opinions about this matter we began to collect sera and to perform Mitsuda and Mantoux tests in dermatology cases with lesions of undetermined nature attending dermatology outpatient clinics in the highly endemic region. This was justified considering the report by Binford (7) of a 6-year-old boy who returned to the USA after living in a leprosy-endemic region and who had a skin lesion histologically diagnosed as nonspecific dermatitis. He developed lepromatous leprosy at the age of 14, and a review of the skin sections performed 8 years before showed the presence of acidfast bacilli in deep terminal nerves. Also, clinical experience in Manaus, Brazil has shown that a definitive diagnosis of leprosy is obtained in a significant number of cases for whom a diagnosis of nonspecific dermatitis was made months before.
In the case of tuberculosis, assays of serial sera (6) showed that the peak of antibody titers was reached several months before clinical tuberculosis was apparent. The titers declined afterward and, in some of the tested cases, it was already below the positivity threshold when the disease was first diagnosed. Consequently, as in leprosy, the sensitivity of the ELISA for the serodiagnosis of clinical tuberculosis must be necessarily low (in our studies, about 50% of all cases examined).
In our opinion, the foregoing observations explain why the highly specific antigens may be inadequately sensitive for serodiagnosis. Nonetheless, the overall evaluation of available information indicates that the ELISA remains a useful laboratory procedure for case-finding among populations at high risk (in leprosy, all patients attending outpatient dermatology clinics in endemic regions; in tuberculosis, all HIV-infected individuals; in life threatening infections such as suspected tuberculosis meningitis; in regular check-ups of exposed personnel; and, perhaps, in nursing homes for the aged). Furthermore, an ELISA as described in this report may be used as a rapid, reliable and nonexpensive method for the classification of patients within the immune spectrum.
The question remains whether positive serology may justify preventive treatment of these cases. For recommending preventive treatment it is still necessary to establish a significant level of antibody titers perhaps well above the positivity threshold levels as estimated from control subjects. However, clinical follow up of the studied populations is still required to decide on the appropriate titer for making treatment decisions.
Acknowledgment. We thank M. Gonçalves for her technical help in performing the ELISAs during thèse investigations. We also thank V. Vincent and A. Feuillet for their help in the identification of mycobacterial isolates. Special acknowledgments are addressed to the military authorities of the Amazonia for their cooperation and help in this investigation. The Brazilian group received financial support from the Conselho Nacional de Desenvolvimento Cientifico e Technologico (CNPq).
REFERENCES
1. ANONYMOUS. Situação da hanseniase no Brasil. Bull. Natl. Epidem. (Brazil) 1987.
2. ANONYMOUS. The tuberculin skin test. (American Lung Association). Am. Rev. Respir. Dis. 124(1981)356-363.
3. ANONYMOUS. Diagnostic standards and classification of tuberculosis and other mycobacterial diseases. (American Thoracic Society) Am. Rev. Respir. Dis. 123(1981)343-348.
4. AGIS, F., S CHLICH, P., CARTEL, J.-L., GUIDI, C. and BACH, M.-A. Use of anti- M. leprae phenolic glycolipid-l antibody detection for early diagnosis and prognosis of leprosy. Int. J. Lepr. 56(1988)527-536.
5. BAGSHAWE, A. F., GARSIA, R. J., BAUMGART, K. and ASTBURY, L. IgM scrum antibodies to phenolic glycolipid-I and clinical leprosy: two years' observation in a community with hyperendemic leprosy. Int. J. Lepr. 58(1991)25-30.
6. BERLIE, M.-C, PETIT, J.-C. and DAVID, H. L. Use of the SL-IV and the PGL-Tbl glycolipid antigens in ELISA for the diagnosis of tuberculosis in AI DS patients. Zentralbl. Bakteriol. 275(1991)351-357.
7. BINFORD, C. H. The histologic recognition of the early lesions of leprosy. Int. J. Lepr. 39(1971)225-230.
8. BRETT, S. J., DRAPER, P., PAYNE, S. N. and REES, R. J. W. Serological reactivity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-279.
9. CANETTI, G. Le Bacille de Koch dans la Lesion Tuberculcuse du Poumon. Paris: Editions Medicales Flammarion, 1946.
10. CHANDRAMUKJ, A., BOTHAMLEY, G. H., BRENNAN, P. J. and IVANYI, J. Levels of antibody to defined antigens of Mycobacterium tuberculosis in tuberculous meningitis. J. Clin. Microbiol. 27(1989)821-825.
11. CHO, S.-N., HUNTER, S. W., GELBER, R. H., REA, T. H. and BRENNAN, P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigenemia in leprosy. J. Infect. Dis. 153(1986)560-569.
12. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and its use in the scrodiagnosis of leprosy. Infect. Immun. 41(1982)1077-1083.
13. CREE, I. A., CAIRNS, W., SMITH, S. and BECK, J. S. A quantitative study of the relationship between systematic and histological parameters of immunity in individual leprosy patients. Int. J. Lepr. 58(1990)347-352.
14. CRÉE, I. A., SMITH, W. C. and BECK, J. S. Serum antibody responses to mycobacteria in leprosy patients and their contacts. Lepr. Rev. 59(1988)317-327.
15. CRUAUD, P., YAMASHITA, J. T., MARTIN-CASABONA, N., PAPA, F. and DAVID, H. L. Evaluation of a novel 2, 3-diacyl-trehalose-2'-sulfate (SL-IV) antigen for case finding and diagnosis of leprosy and tuberculosis. Res. Microbiol. 141(1990)679-694.
16. DAFFE, M., CHO, S.-N., CHATTERJEE, D. and BRENNAN, P. Chemical synthesis and seroreactivity of a neoantigen containing the oligosaccharide hapten of the Mycobacterium tuberculosis-speciûc phenolic glycolipid. J. Infect. Dis. 163(1991)161-168.
17. DAFFE, M., LACAVE, C, LANEELLE, M.-A. and LANEELLE, G. Structure of the major triglycosyl phenol phthioccrol of Mycobacterium tuberculosis (strain Canetti). Eur. J. Biochem. 167(1987)155-160.
18. DAFFE, M., PAPA, F., LASZLO, A. and DAVID, H. L. Glycolipids of recent clinical isolates of Mycobacterium tuberculosis: clinical characterization and immunoreactivity. J. Gen. Microbiol. 135(1989)2759-2766.
19. DHARIVAL, K. R., YANG, Y.-M., FALES, H. M. and GOREN, M. B. Detection of trehalose monomycolate in M. leprae grown in armadillo tissues. J. Gen. Microbiol. 133(1987)201-209.
20. DAVID, H. L. Bacteriology of the Mycobacterioses. Atlanta: Centers for Disease Control, 1976. DHEW Pub. No. CDC 76-8316.
21. DAVID, H. L. The spectrum of tuberculosis and leprosy: what can be the significance of specific humoral responses? Res. Microbiol. 141(1989)197-206.
22. DAVID, H. L. Diagnostic bactériologique des infections à mycobactéries atypiques. Méd. Mal. Infect. 21(1991)82-87.
23. DAVID, H. L., LÉVY-FRÉBAULT, V. and THOREL, M.-F. Méthodes de Laboratoire pour Mycobactériologie Clinique. Commission des Laboratoires de Référence et d'Expertise de l'Institut Pasteur, eds. Paris: Institut Pasteur, 1989.
24. DAVID, H. L., MAROJA, M. F. and CRUAUD, P. Quantitative relationship between anti-PGL-Ispecific antibody levels and the lepromin reactions. Int. J. Lepr. 59(1991)332-334.
25. DESFORGES, S., BOBIN, P., BRETHES, B., HUERRE, M., MOREAU, J.-P. and BACH, M.-A. Specific anti- M. leprae PGL-I antibodies and Mitsuda reactions in the management of household contacts in New Caledonia. Int. J. Lepr. 57(1989)794-800.
26. FANDINHO, F. C. O., ORSI SOUZA, A.-T. and SALEM, J. A. Comparison of the Ziehl-Neclsen and Kinyoun methods in staining smears from leprosy patients. Int. J. Lepr. 58(1990)389-391.
27. FERREIRA, P., SOARES, R. and ARALA-CHAVES, M. Susceptibility to infection with Mycobacterium avium is paradoxically correlated with increased synthesis of specific antibacterial antibodies. Int. Immunol. 3(1991)445-452.
28. FOURNIE, J.-J., ADAMS, E., MULLINS, R. J. and BASTEN, A. Inhibition of lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect. Immun. 57(1989)3653-3659.
29. GORMUS, B. J., OHASHI, D. K., OHKAWA, S., WALSH, G. P., MEYERS, W. M., BRENNAN, P. J. and TRYGG, C. Serologic responses to Mycobacterium leprae specific phenolic glycolipid-I in sooty mangabcy monkeys with experimental leprosy. Int. J. Lepr. 56(1988)537-545.
30. GRADY, E. Cutaneous inoculation tuberculosis: a report of eight cases. Am. Rev. Tuberc. 63(1951)526-537.
31. HUNTER, S. W. and BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity. J. Bactcriol. 147(1981)728-735.
32. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)15072-15078.
33. JOPLING, W. H. Handbook of Leprosy. 2nd edn. London: William Heinemann Medical Books Ltd., 1978.
34. KAPLAN, G. and COHN, Z. A. Leprosy and cellmediated immunity. Curr. Opin. Immunol. 3(1991)91-96.
35. KOSTER, F. T., SCOLLARD, D. M., UMLAND, E. T., FISHBEIN, D. B., HANLY, W. C, BRENNAN, P. J. and NELSON, K. E. Cellular and humoral immune response to a phenolic glycolipid antigen (PhenGL-I) in patients with leprosy. J. Clin. Microbiol. 25(1987)552-556.
36. LAGRANGE, P. H. and HURTEL, B. Ancrgy and other immunologic perturbances in mycobacterial infections: overview. In: Mycobacterium tuberculosis: Interactions with the Immune System. Bendinelli, M. and Friedman, H., eds. New York: Plenum Press, 1988.
37. LEMASSU, A., LANEELLE, M.-A. and DAFFE, M. Revised structure of a trehalosc-containing glycolipid of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 78(1991)171-176.
38. LYONS, N. F., SHANNON, E. J., ELLIS, B. P. and NAAFS, B. Association of Ig Gand IgM antibodies to phenolic glycolipid-I antigen of Mycobacterium leprae with disease parameters in multibacillary leprosy patients. Lepr. Rev. 59(1988)45-52.
39. MANDE, B. BCG: Manual Pratique de Vaccination. Centre International de l'Enfrancc, ed. Paris: Editions Medicales Flammarion, 1966.
40. MEDLAR, E. M. Behavior of pulmonary tuberculosis lesions; a pathological study. Am. Rev. Tuberc. 71 (Part II) (1955) i-xii; 1-244.
41. MEHTA, P. K. and KHULLER, G. Comparative evaluation of the diagnostic significance of circulaiing immune complexes and antibodies to phosphatidylinositomannoside in pulmonary tuberculosis by enzyme-linked immunosorbent assay. Med. Microbiol. Immunol. 178(1989)229-233.
42. MOHAGHEGHPOUR, N.. GELBER, R. II. and ENGLE-MAN. E. T. Cell defect in lepromatous leprosy is reversible in vitro in absence of exogenous growth factors. J. Immunol. 138(1987)570-578.
43. PAPA. F.. CRUAUD, P. and DAVID, H. L. Antigenicity and specificity of selected glycolipid fractions from Mycobacterium tuberculosis. Res. Microbiol. 140(1989)569-574.
44. RASTOGI. N.. CADOU, S. and HELLIO. R. Differential handling of bacterial antigens in macrophages infected with Mycobacterium leprae as studied by immunogold labeling of ultrathin sections. Int. J. Lepr. 59(1991)278-291.
45. ROCHE, P. W., BRITTON. W. J., FAILIIUS. S. S., WILLIAMS, D., PRADIIAN, H. M. and THEUVENET, W. J. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58(1990)480-490.
46. ROOK, G. A. W. The immunology of leprosy. Tubercle 64(1983)297-312.
47. ROTHERG. A.. BECHELLI, L. M. and KEIL, H. The Mitsuda reaction in a nonleprousarea. Int. J. Lepr. 28(1960)209-221.
48. ROY, A. Serological study of leprosy employing ELISA with arabinogalactan of Mycobacterium smegmatis as antigen. Lepr. Rev. 57(1986)137-145.
49. SAAD, M. H. F., MEDIEROS, M. A., GALLO, M. E. N., GONTUO, P. P. and FONSLCA, L. S. IgM immunoglobulins reacting with the phenolic glycolipid-1 antigen from Mycobacterium leprae in sera of leprosy patients and their contacts. Mem. Inst. Oswaldo Cruz 85(1990)191-194.
50. SANTA-CRUZ, D. J. and STRAYER, D. S. The histologic spectrum of the cutaneous mycobacterioses. Hum. Pathol. 13(1982)485-495.
51. SCHWERER, B., MEEKER, H. C, SERSEN. G. and LEVIS. W. R. IgM antibodies against phenolic glycolipid-I from Mycobacterium leprae in leprosysera: relationships to bacterial index and erythema nodosum leprosum. Acta Leprol. (Geneve) 95(1984)395-402.
52. STEAD, W. W. Tuberculin tests. (Letter) N. Engl. J. Med. 286(1972)844.
53. TOMAN. K. Tuberculosis case-finding and chemotherapy. Geneva: World Health Organization, 1979.
54. YOUNG, D. B. and BUCHANAN, T. M. A serologic test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
55. YOUNG. D. B.. DISSANAYAKE, S., MILLER, R. A., KHANOLKAR, S. R. and BUCHANAN, T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149(1984)870-873.
56. VADIEE, A. R., SHANNON, E. J., GILLIS. T. P., MSHANA, R. N. and HASTINGS, R. C. Armadillo Ig Gand IgM antibody responses to phenolic glycolipid-] during experimental infection with M. leprae. Int. J. Lepr. 56(1988)422-427.
1. M.D., Ph.D.; in Pharmacy, Unite de la Tuberculose et des Mycobactcrics, Institut Pasteur, 75724 Paris 15, France.
2. M.Sc. in Pharmacy, Unite de la Tuberculose et des Mycobactcrics, Institut Pasteur, 75724 Paris 15, France.
3. M.Sc. in Pharmacy, Clinical Pathologist, Hôpital Jean Verdier, 93140 Bondy, France.
4. M.D., Hôpital Saint Antoine, 75724 Paris 12, France.
5. M.D., Instituto de Dermatologia Tropical e Venereologia "Alfredo Da Matta," 69011 Manaus, Amazonia, Brazil.
6. M.D., Instituto de Dermatologia Tropical e Venereologia "Alfredo Da Matta," 69011 Manaus, Amazonia, Brazil.
7. M.D., Ph.D.; Instituto Nacional de Pesquizas da Amazonia, 69011 Manaus, Amazonia, Brazil.
8. Nurse, Instituto Nacional de Pesquizas da Amazonia, 69011 Manaus, Amazonia, Brazil.
Received for publication on 7 October 1991.
Accepted for publication in revised form on 13 February 1992.