- Volume 60 , Number 2
- Page: 225–33
Human phagocyte respiratory burst by Mycobacterium bovis BCG and M. leprae: functional activation by BCG is mediated by complement and its receptors on monocytes
ABSTRACT
We have measured the role of serum components on two parameters of the phagocytosis reaction: a) the chemiluminescence (CL) response associated with the oxidative respiratory burst in response to Mycobacterium bovis BCG and M. leprae, and b) the uptake of these two mycobacteria by healthy human monocytes. Pre-incubations of fresh or heat-inactivated serum or serum containing EGTA or EDTA indicate that these two mycobacteria activate the alternative complement pathway. Monoclonal antibodies against CR1 and CR3 inhibit the responses of M. bovis BCG and M. leprae, demonstrating that complement receptors mediate the phagocytosis of these two mycobacteria. Thus, complement and its receptors on the surface of the monocytes (CR 1 and CR3) are important in the functional activation of phagocytosis of M . bovis BCG and M. leprae.RÉSUMÉ
Nous avons mesuré le rôle de deux composants sériques sur les paramètres de la phagocytose: a) la réponse chemoluminescente (CL) associée à l'oxydation respiratoire en réponse au BCG de Mycobacterium bovis et au M. leprae; et b) l'ingestion de ces deux mycobactéries par des monocytes humains normaux. La pré-incubation de sérum frais ou inactivé par la chaleur ou du sérum contenant de l'EGTA ou EDTA indique que ces deux mycobactéries activent les voies alternatives du complément. Des anticorps monoclonaux vis-à-vis de CR 1 et CR3 inhibent les responses du BCG de M. bovis et de M. leprae, démontrant que les récepteurs du complément interviennent dans la phagocytose de ces deux mycobactéries. En conséquence, le complément et ses récepteurs sur la surface des monocytes (CRI et CR3) sont importants pour l'activation de la fonction de phagocytose du BCG de M. bovis et de M. leprae.RESUMEN
Se estudió el efecto de algunos componentes del suero sobre dos parámetros de la función fagocítica de los monocitos de personas sanas: (a) la quimioluminiscencia (CL) asociada al estallido respiratorio en respuesta al estímulo con Mycobacterium bovis BCG, y M. leprae y (b) la ingestión de estas dos micobacterias. La preincubación de las micobacterias con suero fresco o suero inactivado por calor, or con suero conteniendo EDTA o EGTA, indicó que las dos micobacterias activan la vía alterna del complemento. Los anticuerpos monoclonales contra CRI y CR3 inhiben la respuesta al M. Bovis BCG y al M. leprae, demostrando que los receptores para complemento participan en la fagocitosis de estas dos micobacterias por los monocitos humanos.Cellular immunity plays a central role in the development of protective immunity against intracellular pathogens. In mycobacterial infections, specific cellular and humoral immunity are negatively correlated(2). Cellular immunity to mycobacteria is widely under investigation, and it is now clear that candidate vaccines against leprosy and tuberculosis must be able to induce cellular immunity. The role of the different components of serum in protective immunity against mycobacteria has drawn little attention. Although antibodies do not confer resistance to mycobacteria, components of complement and its receptors on the phagocytic cells might be important, particularly at the initial step of the immune response when contact between mycobacteria and the phagocytic cells occurs. Indeed, Mycobacterium leprae and its specific lipid, phenolic glycolipid-I (PGL-I), activate the alternative pathway of complement (14, 15), and CR1, CR3, and CR4 receptors for the C3 component of complement have been described as receptors for M. leprae and M . tuberculosis (16, 17) on human phagocytes and macrophages (18).
Thus, we investigated the role of serum components, particularly the components of complement, in the phagocytosis of M.bovis BCG and M. leprae by studying the chemiluminescence (CL) response associated with the oxidative respiratory burst and the uptake of mycobacteria by human monocytes from healthy subjects.
MATERIALS AND METHODS
Cell isolation. Peripheral blood was obtained by venipuncture using heparinized "Vacutainer" tubes (Becton, Dickinson & Co., Rutherford, New Jersey, U.S.A.) from healthy adults at the Pasteur Institut of Dakar, Senegal. Mononuclear cells were isolated on a Ficoll-Hypaque gradient (Sigma Chemical Co., St. Louis, Missouri, U.S.A.). The percentages of lymphocytes and monocytes were evaluated on May-Griinwald Giemsa smears. Viable cells were counted using Trypan blue dye.
Serum. Human sera were obtained from peripheral blood using nonheparinized tubes. Five sera from multibacillary (MB) and paucibacillary (PB) leprosy patients and five sera from normal individuals living in a nonendemic area (control sera) were pooled and passed through a 0.45-µm membrane filter (Multipore Corp., Bedford, Massachusetts, U.S.A.).
Reagents. Luminol (5-amino-2,3-dihydro-l-4-phthalazincdione) (Sigma) was dissolved in dimethylsulfoxide (DSMO; Sigma) and used at a final concentration of 10-4 M in phosphate-buffered saline (PBS). Phorbol myristate acetate (PMA; Prolabo, Paris, France) was used at 1.5 µ g/ml EDTA (cthylenediaminctetraacctic acid) and EGTA [ethyleneglycol-bis( β -aminoethyl ether)-N.A'-tetraacetic acid] were purchased from Sigma. Stock solutions were prepared in Hanks' solution as follows: a) 100 mM EDTA, pH 7.4, designated as EDTA; and b) 100 mM EGTA-100 mM MgCL, designated as EGTA.
Preparation of sera. Three preparations of serum were made as previously described (19). Briefly, 0.1 ml of EDTA or EGTA stock solution was added to 0.9 ml scrum, mixed well, and incubated for 1 hr at 37ºC, resulting in a final concentration of 10 mM. Sera were also inactivated by heating at 56ºC for 30 min in a waterbath.
Mycobacteria. Irradiated M. leprae (batch CD 128) extracted from armadillo tissues was provided by Dr. R. J. W. Rces through the WH O Immunology of Leprosy Program. M . bovis BCG IP Dakar was obtained in lyophilized samples from the Service du BCG, Institut Pasteur, Dakar. Viable M . bovis BCG IP Dakar was killed by heating for 120 min at 70ºC.
Antibodies. Monoclonal antibodies (mAb) anti-human CR1 (CD35) and antihuman CR3 (CD 1 lb) and an anti-HLA-DR were purchased from Becton, Dickinson & Co. Monoclonal antibodies containing sodium azide were dialyzed extensively against PBS before use.
Measurement of chemiluminescence. We used an automatic thermostated photometer and dispenser (1251 Luminometer and 1291 Dispenser; LKB Wallac, Turku, Finland) for measuring chemiluminescence (CL). Briefly, 100 µ l of a suspension of mononuclear cells adjusted to 105 monocytes per ml was transferred into a counting tube previously filled with 1 ml of a suspension of bacteria diluted in PBS to a ratio of 10 bacteria per mononuclear cell. The tube was placed in a dry bath at 37ºC under constant agitation, and 200 µ l of luminol solution was subsequently added. The tube was then immediately placed into a photometer counting chamber (λ = 425 nm). A measurement mode was available for continuous recording over 150 min. Maximal high intensity (MLI) in mV at the peak of the course for 104 monocytes was recorded for each combination performed. The results are expressed as a chemiluminescence index (CLI) as follows:

The maximal intensity for MLI-stimulated cells was found in the presence of stimulating agents and the light intensity for MLI-unstimulated cells was determined in the presence of Hanks' solution instead of stimulating agents.
The influence of serum concentration in the mycobacterial CL response was tested by incubating monocytes with mycobacteria in the presence of varying concentrations of serum from 0.1% to 10% (v/v). In studies using mAb on the M . bovis BCG CL response, the mononuclear cells were preincubated for 30 min at 37ºC in 1% sera and mAb before the addition of M . bovis BCG. In some experiments the results are expressed in percentages of inhibition that represent decreases from the control without monoclonal antibodies.
Mycobacterial phagocytosis assay. Peripheral blood mononuclear cells were allowed to adhere to eight wells of Lab Teck slides (Miles Laboratories, Inc., Naperville, Illinois, U.S.A.) at a concentration of 1 x 106 per well in RPMI medium containing 10% fetal calf serum at 37ºC in 5% humidified CO2. After 2 hr of incubation, the nonadherent cells were removed by vigorous pipetting of the slides with warm Hanks' solution, and the wells were then replenished with fresh medium.
Each mycobacterial suspension was briefly sonicated to disrupt clumps and adjusted to a ratio of 10 bacteria per monocyte and added to the adherent cell cultures. The cultures were incubated at 37ºC in 5% humidified C0 2. At 15, 30, 45, 60, 120, and 180 min after the addition of the mycobacteria, the supernatants were discarded and the plates were rinsed in warm Hanks' solution and fixed in methanol for 1 min. Then the cultures were stained with an acid-fast bacillus stain (Ziehl-Neelsen). The number of wells with at least one bacterium was determined by examination with a Leitz microscope with a 100 x water immersion lens. Three-hundred to 400 cells were counted, and the results are expressed as the percentage of cells with associated mycobacteria (representing bacteria both attached to and ingested by the monocytes). The number of bacteria per monocyte was also evaluated. The influence of serum concentration was tested under the same conditions as described in the CL assay. In studies using mAb, the mononuclear cells were preincubated with the mAb and 1% sera for 30 min before the addition of the mycobacteria.
Statistical analysis. The Student's / test was used to compare the different experimental groups.
RESULTS
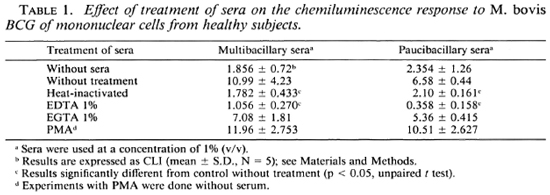
Effect of serum concentration on the mycobacteria-triggered CL response of mononuclear cells. The influence of serum components on the mycobacteria-triggered CL response of mononuclear cells was evaluated using a pool of sera from MB and PB leprosy patients and normal controls. Figures 1 and 2 show the effect of sera on the CL response of mononuclear cells to M . bovis BCG and M . leprae. On one hand, the CL response to M . bovis BCG was significantly increased in the presence of fresh (noninactivated) sera. Indeed, the CL response was serum dependent to a point and was maximal in the presence of 0.5% or 1% (v/v) fresh sera. Fresh sera from MB and PB leprosy patients as well as normal controls showed this phenomenon. On the other hand, the addition of varying concentrations of fresh or heat-inactivated sera neither increased nor decreased the CL response of human mononuclear cells to M. leprae. Figure 2 demonstrates a small but insignificant difference in response between leprosy patient serum (A and B) and normal serum (C).
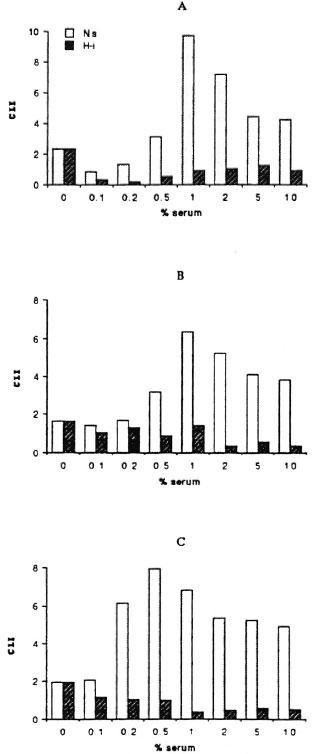
Fig. 1. Effects of varying concentrations of fresh (Ns), heat-inactivated (Hi) sera from multibacillary (A), paucibacillary (B) leprosy patients, and normal controls (C) on the CL response to BCG (ratio 10/1) of mononuclear cells from healthy subjects.
Fig. 2. Effects of varying concentrations of fresh (Ns) or heat-inactivated (Hi) sera from multibacillary (A) and paucibacillary (B) leprosy patients, and normal controls (C) on the CL response to M. leprae (ratio 10/ 1) of mononuclear cells from healthy subjects. (Note different scale compared to Fig. 1.)
Using an enzyme-linked immunosorbent assay (ELISA) as previously described ('), we observed that the sera from lepromatous leprosy patients contained high titers (1/ 2000-1/1500) of anti- M. leprae, anti- M. bovis BCG, and anti-PGL-I antibodies (data not shown). Little or no antibody activity to these antigens was found in PB leprosy patients and normal controls. Figure 1 shows that serum with or without specific antibodies gave the same pattern of response. Thus, the role of serum components other than antibodies was implicated as being responsible.
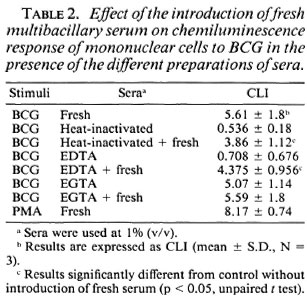
Since inactivation by heating the sera for 30 min at 56ºC reduced the CL phenomenon in response to M . bovis BCG (Fig. 1, Table 1), we studied the different pathways for the activation of the complement by using as chelators EDTA or EGTA. Heating the serum or treating it with EDTA reduced the effect of serum (p < 0.05 compared with fresh sera, Table 1). EGTA-treated sera has the same effect as fresh serum on the CL response. As shown in Table 1, the sera from MB and PB leprosy patients gave the same pattern of reactivity. Moreover, when fresh sera were added back to heat-inactivated sera, an increase of the CL response of the mononuclear cells to M . bovis BCG was observed (Table 2).
Effects of anti-human CR1 and anti-human CR3 mAbs on mycobacteria-triggered CL response of mononuclear cells. Since the M . bovis BCG CL response is dependent upon serum components, and particularly on complement components as described above, we explored the role of the receptors of complement components (C3b and C3bi for CR1, and C3bi, C3dg for CR3) on mononuclear cells.
We used monoclonal antibodies to human CR1 and human CR3 to inhibit the CL response of mononuclear cells to M . bovis BCG. As shown in Figure 3, mAb against human CR1 and CR3 significantly inhibited the CL response of mononuclear cells to M. bovis BCG. The mAb against CR3 had stronger activity than the mAb against CR 1. Inhibition (decrease from controls without monoclonal antibodies) for anti-CRl was from 21 % to 46%; for anti-CR3, from 81 % to 84%. When a combination of the two mAbs against CR1 and CR3 was added, 95% to 96% inhibition occurred. The capacity of the mononuclear cells to respond to stimuli, as measured by the response of the mononuclear cells to PMA, was not impaired by any of the mAb tested. The control monoclonal antibody against HLA-DR did not inhibit the CL response to M . bovis BCG. Since M . leprae did not induce oxygen-free radical production by mononuclear cells, we could not evaluate the effect of complement component receptors on the CL phenomenon.
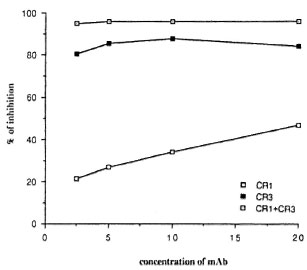
Fig. 3. Effects of concentration ( µ g/ml) of anti-human CR1 and CR3 monoclonal antibodies on M. bovis BCG-triggercd CL response of human mononuclear cells. Results expressed as mean of four experiments in percentages of inhibition that represent a decrease from the control without monoclonal antibody (S.D. did not exceed 15%).
Effect of serum on M. bovis BCG and M. leprae phagocytosis. First, the effect of the serum concentration on phagocytosis of the mycobacteria by mononuclear cells was evaluated. A concentration of 1% (v/v) fresh serum gave the maximal response (data not shown). To evaluate the effect of serum on the phagocytosis of mycobacteria, we incubated the monocytes with fresh serum, heat-inactivated serum, and EDTA- and EGTA-treated serum. As previously described (11) in the absence of sera, phagocytosis of M . leprae was lower than that of M. bovis BCG. Figure 4 shows the results of sera from MB leprosy patients, but sera from PB leprosy patients and normal controls gave the same pattern of response (data not shown). Fresh and EGTA-treated serum increased the uptake of M . bovis BCG and M. leprae. Phagocytosis of mycobacteria in the presence of heat-inactivated or EDTA-treated scrum was comparable to phagocytosis without sera.
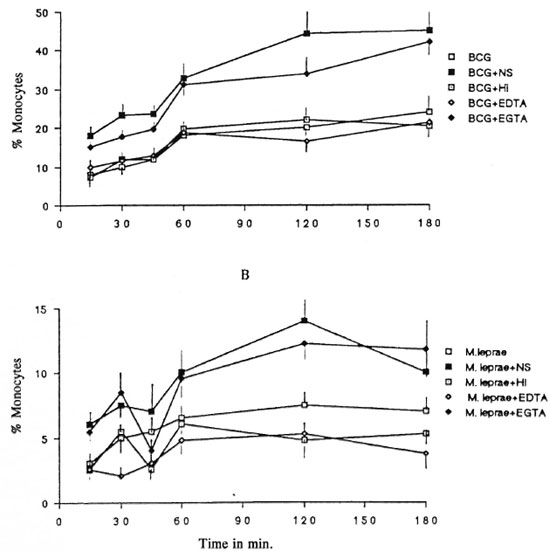
Fig. 4. Kineties of the effects of fresh (NS), heat-inactivated (HI), EDTA- and EGTA-treated sera from multibacillary patients on the phagocytosis of BCG (A) and M. leprae (B). Sera from paucibacillary leprosy patients and from normal controls gave the same pattern of response (data not shown). Results expressed in percentages of monocytes with at least one mycobacterium (mean ± S.D., N = 5).
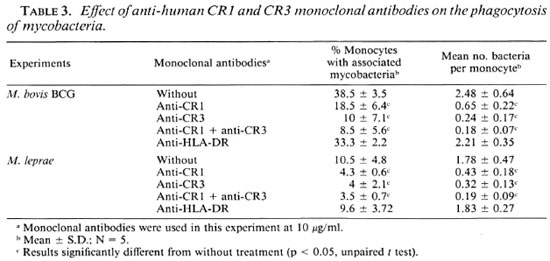
Effects of mAb anti-human CR1 and CR3 on phagocytosis of mycobacteria. The role of receptors for complement in the phagocytosis of M. leprae and M. bovis BCG was then studied. We incubated monocytes with mAb against CR1 and CR3 in the presence of 1% serum and mycobacteria. Monocyte viability was evaluated by Trypan blue exclusion. The presence of monoclonal antibodies (alone or in combination) did not impair the monocyte viability. As shown in Table 3, at a concentration of 10 µ g/ml antihuman CR1 and CR3 mAbs, or a combination of both, significantly reduced the percentage of human monocytes with associated M. bovis BCG or M. leprae and also reduced the mean number of bacteria per monocyte. Monoclonal antibody against HLA-DR (control antibody) did not inhibit BCG or M. leprae adherence.
DISCUSSION
The aim of this study was to determine the role of serum components in the phagocytosis of mycobacteria in vitro. We demonstrated that complement components play an essential role in the phagocytosis of M. bovis BCG, a mycobacterium with reduced pathogenicity, and M . leprae, the mycobacterium responsible for leprosy. For this, we have measured two parts of the phagocytosis reaction: a) the chemiluminescence (CL) response associated with the oxidative respiratory burst of the phagocyte, and b) the uptake of mycobacteria by healthy human monocytes.
As previously described (9-12), M. leprae in contrast to BCG is a poor stimulant of oxygen-free radical production by human monocytes. The CL response to M . bovis BCG was increased in the presence of fresh serum. However, in agreement with a previous study (10), the same fresh serum had no effect on the CL response to M. leprae. The role of complement components in this phenomenon was assessed. Results obtained after heat inactivation or treatment with EDTA or EGTA showed that the activation of the alternative pathway of complement by M . bovis BCG was essential. EGTA blocks only the classical complement pathway and EDTA blocks both the classical and the alternative complement pathways. The same results were observed in phagocytosis assays: activation of the alternative complement pathway is needed for uptake of M . bovis BCG. However, in these experiments we do not know if calcium chelation by EDTA might affect the oxidative burst independently of its effects on the complement cascade. The use of EDTAtreated complement-deficient serum might be useful to evaluate the direct effect of calcium chelation on the respiratory burst.
Since M. leprae together with scrum, treated or not, did not induce oxygen-free radical production, we cannot assume that complement plays any role in the CL response to M . leprae. However, fresh and EGTA-treated sera increased the uptake of M . leprae by human mononuclear cells. Therefore, the phagocytosis of M. leprae needs the activation of the alternative pathway of complement.
The role ofantibodies on the CL response to the mycobacteria and the phagocytosis of the mycobacteria was, in our opinion, minor. Serum with specific antibodies to mycobacteria gave the same pattern of response as did serum without specific antibodies. However, some authors have reported that specific antibodies against lipids can inhibit the uptake of mycobacteria by Schwann cells (6), and others have found that specific rabbit anti-sera against total M. leprae antigens reversed the M. leprae- induced inhibition of phagosome-lysosome fusion (8).
Monocytes express the complement receptors CR1 and CR3. CR1 is specific for C3b, and CR3 may bind to C3bi. We analyzed the role of monoclonal antibody against CR1 and CR3 and observed that each monoclonal antibody, alone or in combination, inhibited the CL phenomenon and the phagocytosis of the mycobacteria. We conclude that CR1 and CR3 are receptors for M. bovis BCG and M. leprae, and that this route ofpenetration into the phagocytes is functional for destruction of mycobacteria. However, our study is in contrast with other results which showed that entry via the CR pathway protects the pathogen from the phagocyte oxidative burst (21). Although M . leprae may, indeed, enter this way without oxygen-free radical production, M. bovis BCG produced a strong activation of the oxidative burst. The mechanism of the failure of M. leprae to trigger an efficient oxidative response has been attributed to phenol glycolipid-I (PGL-I) and lipoarabinomannan which act as oxygen-scavenger species (4, 5). But, as shown in a previous study (11), the weak phagocytosis of M . leprae as compared with M . bovis BCG may also contribute to this phenomenon. In our present experiments M. leprae and M. bovis BCG were killed by different techniques (irradiation versus heat-killing), and this might also explain some of the differences in the results. However, our previous study (12) has shown that live and heat-killed and -irradiated M. leprae all failed to stimulate any CL response.
CR receptors are needed in the phagocytosis of intracellular pathogens such as Legionella pneumophila (13), Histoplasma capsulatum (3), and Leishmania (20). The importance of CR receptors in mediating phagocytosis of mycobacteria, particularly M. tuberculosis and M. leprae, has been described previously (16-18), but other receptors, such as fibronectin receptors and mannosyl fucosyl receptors, have been implicated in the phagocytosis of bacteria. We did not analyze these receptors, but it has been demonstrated that these receptors play little or no role in the phagocytosis of my cobacteria by human monocytes (17, 18). However, Ezekowitz, et al. have demonstrated that Pneumocysti carinii enter into pulmonary macrophages by mannosyl fucosyl receptors (7). The importance of such receptors in the phagocytosis of mycobac teria must be studied. If different receptors could play a role in the phagocytosis of mycobacteria in pulmonary macrophages, we should attempt specific immunomanipulation of the interaction between the pathogen and its receptor ligand. Indeed, interferon-gamma modulating the complement-receptor function of human macrophages is certainly one of the most powerful immunomodulators to be used in mycobacterial infections (18).
Acknowledgment. The authors acknowledge B. Diouf for his skillful assistance and C. Diouf for typing the manuscript. This work was supported by an Association Raoul Follcrcau (France) grant.
REFERENCES
1. BACH, M.-A., WALLACH, D., FLAGEUL, B., HOFFENBACH, A. and COTTENOT, F. Antibodies to phenolic glycolipid I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. BLOOM, B. R. and GODAL, T. Selective primary health care: strategics for control of diseases in the developing world. V. Leprosy. Rev. Infect. Dis. 5(1983)765-771.
3. BULLOCK, W. E. and WRIGHT, S. D. Role of the adherence-promoting receptors, CR3, LFA-1 and pi50,95 in binding Histoplasma capsulatum by human macrophages. J. Exp. Med. 165(1987)195-210.
4. CHAN, J., FUJIWARA, T., BRENNAN, P., MCNEIL, M., TURCO, S., SIBILE, J. C, SNAPPER, M., AISEN, P. and BLOOM, B. R. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc. Natl. Acad. Sei. U.S.A. 86(1989)2453-2457.
5. CHAN, J., FAN, X. D., HUNTER, S. W., BRENNAN, P. and BLOOM, B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect. Immun. 59(1991)1755-1761.
6. CHOUDHURY, A., MISTRY, N. F. and ANTIA, N. H. Blocking of Mycobacterium leprae adherence to dissociated Schwann cells by anti-mycobacterial antibodies. Scand. J. Immunol. 30(1989)505-509.
7. EZEKOWITZ, R. A. B., WILLIAMS, D. J., KOZIEL, H., ARMSTRONG, M. Y. K., WARNER, A., RICHARDS, F. F. and ROSE, R. M. Uptake of Pneumocystis carinii mediated by the macrophage manose receptor. Nature 351(1991)155-158.
8. FREHEL, C. and RASTOGI, N. Mycobacterium leprae surface components intervene in the early phagosome-lysosome fusion inhibition event. Infect. Immun. 55(1987)2916-2921.
9. HOLZER, T. J., KIZLAITIS, L., VACHULA, M, WEAVER, C. W. and ANDERSEN, B. R. Human phagocytic cell responses to Mycobacterium leprae and Mycobacterium bovis bacillus Calmette-Gucrin. An in vitro comparison of leprosy vaccine components. J. Immunol. 141(1988)1701-1708.
10. HOLZER, T. J., NELSON, K. F., SHAUF, V., CRISPEN, G. and ANDERSEN, B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect. Immun. 51 (1986) 514-520.
11. LAUNOIS, P., BLUM, L., DIEYE, A., MILLAN, J., SARTHOU, J. L., MICHEL, J. C. and BACH, M.-A. M. leprae and BCG-induced chemiluminescence response of monocytes from leprosy patients and healthy subjects: effects of interferon-7 and GM-CSF. Int. J. Lepr. 59(1991)582-589.
12. LAUNOIS, P., MAILLERE, B., DIEYE, A., SARTHOU, J. L. and BACH, M.-A. Human phagocyte oxidative burst activation by BCG, M. leprae and atypical mycobacteria: defective activation by M. leprae is not reversed by interferon-7. Cell. Immunol. 124(1989)168-174.
13. PAYNE, N. R. and HORWITZ, M. A. Phagocytosis of Legionella pneumophila by human monocyte complement receptors. J. Exp. Med. 166(1987)1377-1389.
14. RAMANATHAN, V. D., CURTIS, J. and TURK, J. L. Activation of the alternative pathway of complement by mycobacteria and cord factor. Infect. Immun. 29(1980)30-35.
15. RAMANATHAN, V. D., PARKASH, O., TYAGI, P., SENGUPTA, U. and RAMU, G. Activation of the human complement system by phenolic glycolipid 1 of Mycobacterium leprae. Microb. Pathogen. 8(1990)403-410.
16. SCHLESINGER, L. S., BELLINGER-KAWAHARA, C. G., PAYNE, N . R. and HORWITZ, M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144(1990)2771-2780.
17. SCHLESINGER, L. S. and HORWITZ, M. A. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J. Clin. Invest. 85(1990)1304-1314.
18. SCHLESINGER, L. S. and HORWITZ, M. A. Phagocytosis of M. leprae by human monocyte-dcrived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD1 lb/CD 18), and CR4 (CDllc/CD18) and IFN-γ activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 147(1991)1983-1994.
19. SCHWARTZ, R. P., NAAI, D., VOGEL, C. W. and YEAGER, H., JR. Differences in uptake of mycobacteria by human monocytes: a role for the complement. Infect. Immun. 56(1988)2223-2227.
20. WILSON, M. E. and PEARSON, R. D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect. Immun. 56(1988)363-369.
21. WRIGHT, S. D. and SILVERSTEIN, S. C. Receptors for C3b and C3bi promote phagocytosis but not release of toxic oxygen from human phagocytes. J. Exp. Med. 158(1983)2016-2023.
1. M.D.; Immunologic Cellulaire, Institut Pasteur de Dakar, B.P. 220, Dakar, Senegal.
2. Biological Pharmacist; Immunologic Cellulaire, Institut Pasteur de Dakar, B.P. 220, Dakar, Senegal.
3. Biological Pharmacist, Immunologic Cellulaire, Institut Pasteur de Dakar, B.P. 220, Dakar, Senegal.
4. M.D.; Institut de Leprologie Appliquée de Dakar, Dakar, Senegal.
5. M.D., Director, Institut de Leprologie Appliquée de Dakar, Dakar, Senegal.
Received for publication on 18 November 1991.
Accepted for publication in revised form on 25 February 1992.