- Volume 60 , Number 2
- Page: 234–43
Polymerase chain reaction amplifying DNA coding for species-specific rRNA of Mycobacterium leprae
ABSTRACT
The sensitivity of the polymerase chain reaction (PCR) on the DNA coding for the species-specific fragment of 16S rRNA of Mycobacterium leprae studied on mouse foot pad harvests and human skin biopsies varies widely between 1 and 3 × 104 organisms. This is probably the result of variations in the proportions of organisms with sufficiently intact DNA suitable for PCR. Preserving human skin biopsies for 3 weeks at an ambient temperature even after boiling for 6 minutes gives rise to a 10-fold decrease in sensitivity. Fixation of tissues in formol 10% or Lowy fixative or preserving in Dubos OAA broth is very harmful to the PCR, mainly due to the enhancement of an inhibitory effect on the PCR reaction. For preservation, the best choice at the moment seems to be alcohol 70%. Sample preparation of five cycles of frcezc-boiling is simple and generally more efficient than proteinase K treatment and DNA extraction.RÉSUMÉ
La sensibilité de la réaction de polymerase en chaîne (PCR) sur le décodage de l'ADN pour le fragment spécifique d'espèce de 16S rRNA de Mycobacterium leprae, étudiée sur des prélèvements du coussinet plantaire de souris et des biopsies cutanées humaines, varie largement entre 1 et 3 × 104 organismes. Ceci est probablement le résultat de variations dans les proportions d'organismes avec un ADN suffisamment intact pour la PCR. La conservation des biopsies cutanées humaines pendant 3 semaines à température ambiante, même après ebullition pendant 6 minutes, diminue la sensibilité d'un facteur de 10. La fixation des tissus dans du formol à 10% ou le fixateur de Lowy ou leur conservation dans le liquide de Dubos OAA est très mauvaise pour la PCR, principalement par la stimulation de l'effet inhibiteur sur la réaction de PCR. Pour la conservation, le meilleur choix pour le moment paraît être l'alcool à 70%. La préparation de l'échantillon en cinq cycles d'ébullition-réfrigération est simple et généralement plus efficiente que le traitement à la proteinase K et l'extraction de l'ADN.RESUMEN
La sensibilidad de la reacción en cadena de la RNA polímera sa (PCR) sobre cl DNA que codifica para cl fragmento de RNA 16S específico del Mycobacterium leprae (aislado de la almohadilla plantar del ratón y de biopsias de piel humana), varía ampliamente entre 1 y 3 × 104 organismos. Esto probablemente se debe a la variación en la proporción de organismos con DNA lo suficientemente intacto como para permitir el análisis por PCR. Si las biopsias de piel humana se mantienen durante 3 semanas a temperatura ambiente, la sensibilidad disminuye unas 10 veces aún después de hervir el material durante 6 min. La fijación de los tejidos en formol al 10% o en fijador de Lowy, o su preservación en caldo Dubos con OAA, también resultan muy perjudiciales para el PCR porque se aumenta el efecto inhibitorio sobre la reacción. Hasta el momento, la mejor forma de preservar el tejido parece ser su inclusión en alcohol al 70%. La preparación del material por cinco ciclos de congelación y ebullición, es simple y generalmente más eficiente que el tratamiento con proteinasa K y extracción del DNA.Cox, et al. (4) recently sequenced the DN A coding for the variable, species-specific fragment of rRNA of Mycobacterium leprae, thus providing a specific tool for the detection of M. leprae with the polymerase chain reaction (PCR). We have tested the sensitivity of this application of the PCR on mouse foot pad harvests (MFPH) and human biopsies maintained in different conditions.
MATERIALS AND METHODS
MFPH, in phosphate-buffered saline (PBS) with 0.5% bovine albumin fraction V, of several strains of M. leprae maintained in the laboratory were stored at - 20ºC for several weeks before being processed for PCR.
A group of human skin biopsies from active multibacillary (MB) leprosy patients- never treated before or relapsing after treatment-taken in Bamako, Mali, were put into dry tubes and transported on wet ice to Antwerp, Belgium, where they arrived 30 hr later. Suspensions were prepared and kept at - 20ºC until further processing. Biopsies were eventually divided into two fragments and processed (see Results).
The bacteria were counted by standard procedures (12). The limit for bacterial counts was 104 ml. Suspensions with < 104/ml bacilli, but showing acid-fast bacilli (AFB) in a thick smear, are indicated as < 104/ml thick. Smear negative suspensions are presented as "negative."
The instruments used to handle tissues possibly infected with M. leprae were sterilized by boiling in water for 20 min, scrubbed with soap under tap water, cleaned with 1 N HC1, and boiled in a second waterbath for 15 min before the next use.
For the PCR, tissue suspensions were either submitted to five cycles of freezing and boiling (FB) or treated with proteinase K (PK) followed by DNA extraction.
In the FB procedure, samples in 100- µ l volumes were immersed alternately for 2 min in liquid nitrogen and 2 min in a boiling waterbath, 10 µ l being used in the PCR.
For the PK procedure, a 100- µ 1 sample was incubated for 3 hr at 37ºC with 40 of proteinase K in 0.01 M Tris HC1, pH 7.8, 5 mM EDTA, 0.5% (w/v) sodium dodecylsulfate. After two successive extractions with phenol: chloroform : isoamyl alcohol (25/ 24/1) followed by two extractions with chloroform : isoamyl alcohol (24/1), nucleic acids were precipitated twice with ethanol, washed with 80% (v/v) ethanol, dried, and dissolved in 6 µ l of distilled water; 5 µ l was used in the PCR and 1 µ 1 for making 10-fold dilutions.
PCR reactions were performed in 50- µ l volumes containing 10 µ l of the FB sample or 5 µ l of the PK-treated sample; 10 pmoles of each primer; 0.2 mM each of dATP, dCTP, dGPT, dTTP; 1 unit of Taq polymerase (The Cetus Corporation, Emoryville, California, U.S.A.); 50 mM of KC1; 10 mM Tris HC1, pH 8.3; 2.5 mM MgCL and 100 Mg/ml of bovine serum albumin. In every PCR run external negative (distilled water) and positive controls (a M. lep raopositive mouse foot pad harvest) were included. The amplification (Hybaid; Hybaid Ltd, Teddington, Middlesex, U.K.) cycle was as follows: denaturation for 2 min at 94ºC, hybridization at 55ºC for 2 min, extension at 72ºC for 2 min (35 cycles), and a final extension step at 72ºC for 10 min.
The primers used were those described by Cox, et al. (4) except that the sequence of primer cv 3' was based on the 16S rRNA sequence of M. leprae instead of M. bovis.
primer 1:
5' AAACCCAGACCTTCGTCGATG 3'
primer 2:
5' CGGAAAGGTCTCTAAAAAATCTT 3'
The aplicon produced consists of 405 base pairs (bp).
Ten µ l of the reaction mixture was electrophoresed in 1.5% agarose gels at 5 V/cm in TBE buffer (0.09 M Tris-borate, 0.002 M EDTA), and the reaction products were visualized by ethidium bromide fluorescence.
Internal control (IC). An M. leprae amplicon obtained from a suspension of armadillo liver, inoculated with M. leprae from human origin, was eluted from an agarose gel and ligated into the Eco RV site of plasmid pBR322, cleaving the tetracycline R gene. The ligation mixtures were transformed into Escherichia coli JM 83 and transformants selected as ampicillin R, tetracycline S colonies. The cloned amplicons, containing unique Apal and Xhl sites, were cut with these enzymes and purified by agarose gel electrophoresis. The cut ends were made blunt with T4 DNA polymerase followed by ligation. About 10% of the transformants contained the right construct with the shortened amplicon of 268 bp. Plasmids were extracted from a positive clone and linearized by treatment with ECO RI, for which the plasmids have a unique site. This improves the detection limit 1000-fold (13).
RESULTS
Influence of the presence of IC on the sensitivity of the reaction for detection of M. leprae. Since the presence of the internal control (IC) might affect the sensitivity of the assay due to competition between two templates for the primers, a titration experiment was made in which dilutions of a FB suspension of M. leprae were mixed with dilutions of an IC preparation. The Figure shows the results. With high amounts of M. leprae DN A there is inhibition of the IC (lanes A, B, D, G). With the lowest amount of M. leprae DN A tested, there is inhibition of the target DN A (lane I). Therefore in all further experiments a dilution of 10-2 of this preparation of IC was used.
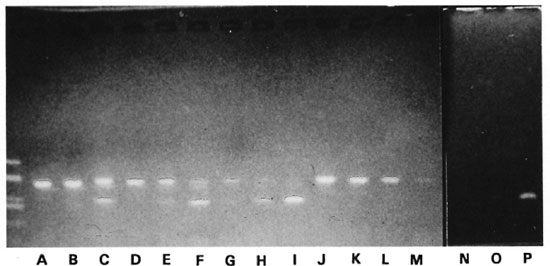
The figure. Effect of the IC on the sensitivity of the PCR for an M. leprae suspension from a freezc-boiled human biopsy. M. leprae 3.6 x 103: lanes A-C and J; Ml 3.6 x 102: lanes D-F and K; Ml 36: lanes G-I and L; Ml 3.6: lanes M and N-P, with an IC preparation diluted 10-3 added in lanes A, D, N; IC diluted 10 - in lanes B, E, H, O; IC diluted 102 in lanes C, F, I and P (no IC added in lanes J-M); unmarked lane: Haelll digest of plasmid PUC19.
PCR on mouse foot pad harvests. As shown in Table 1, three MFPH negative for M. leprae were negative in the PCR, both after FB and PK. The five MFPH with < 104 M. leprae were positive after FB; three of these tested after PK were also positive. All positive PCR results after PK were on undiluted samples only; whereas after FB, all but one sample gave a positive result in dilutions of 10-1, 10-2 and even 10-3. The next series ofMFPH in Table 1, with countable numbers of M. leprae, allow the calculation of the lower limit of detectability of this PCR. For the FB procedure, this varies from 1.5 to 2.2 x 103 bacilli. However, all of the lower sensitivities were observed in harvests with at least 106 M. leprae per ml. The mean value for the 14 lowest figures is 36.5 bacilli detected. The lowest limit of detectability after PK is 210 and goes up to 103 and 104. Clearly, the PK procedure is much less sensitive than FB.
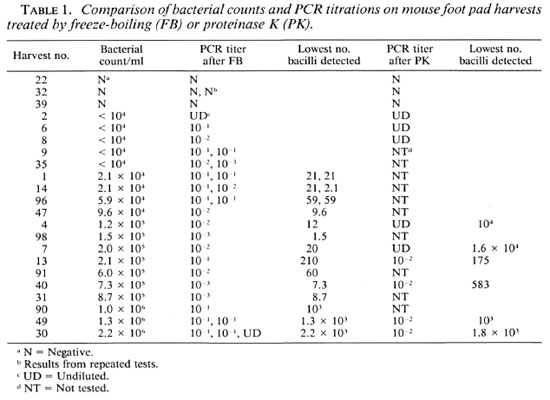
Some PCRs were repeated involving FB of an aliquot of the original suspension. As shown in Table 1 there may be a variation ofone dilution between two successive tests. The use of the IC did not reveal much interference with inhibitors. In some cases however, the control signal obtained with the undiluted specimen was less intense than with the diluted specimens.
Influence of fixatives or Dubos OAA broth on PCR results on MFPH. These influences were studied in two experiments on MFPH from two strains of M. leprae. From each group of harvests, one MFPH (experiment 2) or two MFPH (experiment 1) were kept at -20ºC until tested in PCR; while others were kept for 2 weeks at room temperature, either in formol 10%, alcohol 70%, Lowy fixative, or Dubos OAA broth. All suspensions were PCR tested after FB. Table 2 shows the results.
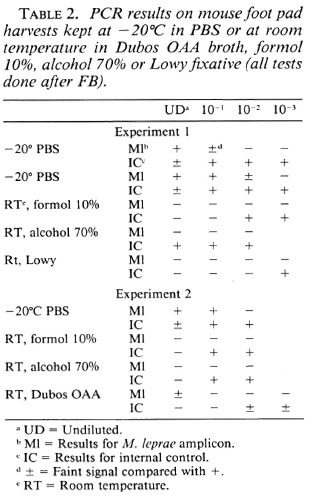
In experiment 1, the frozen samples were positive at dilutions of 10-1 and 10-2, respectively. In both cases the signal ofthe IC was weaker in the undiluted sample, pointing to some inhibition in the test.
Fixation of MFPH in both formol 10% and Lowy fixative increased very considerably the inhibitory effect in the reactions. This was not the case after preservation in alcohol, but the M. leprae amplicon failed nevertheless.
In experiment 2, formol 10% enhanced inhibition ofthe reaction less drastically than in the first experiment; Dubos OAA broth provoked a considerable inhibitory effect although the M. leprae amplicon was produced in the undiluted sample. Alcohol enhanced the inhibition only in the undiluted sample, but once more the M. leprae amplicon failed.
The conclusion from these experiments is that from the different preservation methods tested, alcohol 70% has the least effect on the inhibitors, but still reduces the sensitivity of the test.
PCR results on fresh human biopsies. Fresh human biopsies were divided into two halves; one was examined as a fresh specimen (after some time at - 20ºC) while the other was transferred into cither alcohol (70%) or Dubos OAA broth and kept at room temperature for 1 week. They were then minced and treated by FB or PK for PCR. The results appear in Table 3. Once more there may be a difference of one dilution upon repeated testing.
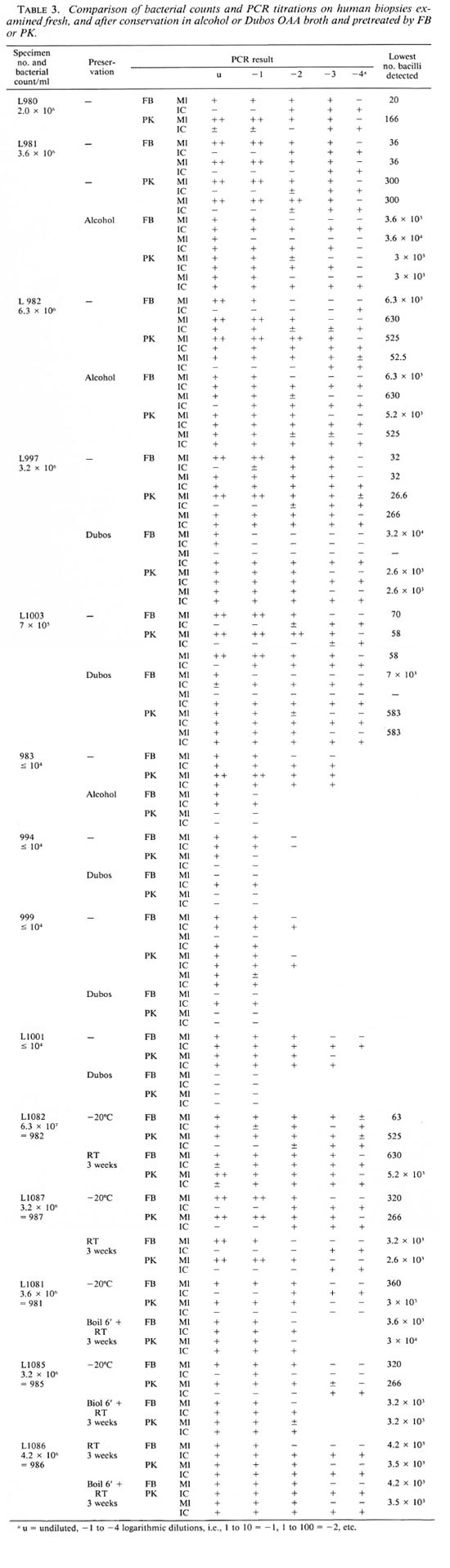
The results of the first five fresh biopsies show that after FB the lower limit of detectability varies between 20 (L980-) and 6300 bacilli (L982-). The result of 6.3 x 103 obtained once on sample L982-FB should be regarded as an outlier and could be disregarded in the interpretation of the results, the more so since in that particular test inhibitors were detected up to a dilution 10-3. On this basis, the mean lower limit of sensitivity for the FB procedure is 122.2 bacilli compared with 194.6 bacilli after the PK treatment, a nonsignificant difference. However, if the results on sample L982 are not considered, the results after FB are much more uniform and vary between 20 and 70 bacilli, with a mean of 37.6 bacilli, and the FB procedure is 5 times more sensitive. On this basis, the number of M. leprae in biopsies L983, L994, L999 (with counts below 104 bacilli/ml) was estimated at 3.1 x 104 ml, not significantly different from 104, with LI001 as an unexplained exception since it should have contained 3.1 x 105 M. leprae per ml.
Influence of fixatives or Dubos OAA broth on PCR on human biopsies. After preservation in alcohol, the sensitivity in the case of sample L981 is 100 to 1000 times lower than on fresh material. A much smaller difference was noted for L982, but for this particular biopsy the sensitivity for the fresh specimen was very low. The sensitivity of the PCR was also considerably lower after preservation of specimens L997 and LI 003 in Dubos OAA broth.
For suspensions with < 104 bacilli/ml calculations on the effect of the preservation media were not possible. However, as can be seen in the second part of Table 3, compared with the results on fresh tissues the titers after preservation in alcohol are 10 times lower, and after preservation in Dubos OAA broth they are 10 to 1000 times lower.
Table 3 also shows that in many instances inhibitory factors are distributed irregularly and are more frequent than in MFPH. They are not necessarily eliminated by PK treatment. A strong specific signal after PCR on an undiluted specimen may "overcome" inhibitors responsible for a weaker or even negative IC signal.
Effect of preservation of skin biopsies at different temperatures. Two skin biopsies were taken out of the freezer and divided into two parts: one was kept at room temperature for 3 weeks, the other was put back at - 20ºC for 3 weeks when the suspensions were prepared and PCR performed after FB and PK treatment. Two other skin biopsies were thawed and divided in halves: one was put back at -20ºC; to the other, 1 ml PBS was added and the tube held in a boiling waterbath for 6 min. It was then kept at room temperature for 3 weeks. PCR was performed 3 weeks later on the 6 specimens.
A last biopsy was divided into two parts: one halfwas kept fresh at room temperature for 3 weeks, the other half was boiled for 6 min and kept at room temperature for 3 weeks when PCR was performed.
Table 4 shows the results. Again, in 5 out of 10 cases the PK procedure was less sensitive than FB. Preservation of biopsies at room temperature after boiling for 6 min or not, reduced the sensitivity of the test 10fold. In the last experiment, a higher titer was obtained when the biopsy was boiled rather than kept fresh for 3 weeks at room temperature.
DISCUSSION
For PCR on M. leprae, genes coding for proteins have been amplified, such as those coding for the 18-kDa (2, 14)-or the 36-kDa proteins (6). Since at least some ofthese proteins may be heat-shock proteins the amplification of genes coding for the speciesspecific rRNA, existing in all living cells, should by definition be more specific. This region has recently been identified by Cox, et al. (4). We used the primers they proposed in the present work, the aim of which was to investigate the sensitivity not the specificity of the test.
Since PCR is difficult to quantify, we performed the reaction on decimal dilutions of the specimens with the result that there frequently was a variation ofone dilution upon repeated testing. This could probably be overcome by performing the PCR on 4-6 tubes per dilution, as done in other instances of biological measurements, allowing calculations of an end point and statistical evaluation This was not done at present because of the workload and cost of reagents involved.
As found previously (5, 15), on the whole, FB was superior to PK. treatment for sample preparation.
The addition of an internal control (IC) as used in the present investigations does inhibit the production of amplicon from the target DNA if the latter is present in amounts near the limit of dctectability. Therefore, the IC should be added in a carefully defined concentration to minimize this inhibitory effect. This shortcoming could be circumvented by performing two reactions in two separate tubes: one with and one without an IC.
In contrast with MFPH, inhibitors of the PCR were frequently present in human skin biopsies. This may be due to the presence of traces of hemoglobin known to be a potent inhibitor of Taq polymerase (7). The IC was therefore particularly useful in tests on human skin biopsies. In some cases, when in the undiluted specimen inhibitors are present together with a large amount of target DNA, a specific M. leprae amplicon may be produced in the absence of, or with a weak IC amplicon; in subsequent dilutions the specific amplicon then disappears, while the IC amplicon appears (Tables 2 and 3).
In the presence of inhibitors both IC and specific amplicon are present in dilutions. However, a small amount of target DNA may be missed if the reaction is inhibited in the undiluted sample and the target DNA is diluted out in the dilutions. For all these reasons the use of an IC is superior to the procedure of blind dilution of the sample as done previously (5).
The quantitative aspects of the present work were based on microscopic counts of bacilli without determination of their viability. Theoretically, the PCR can be positive for dead organisms if the portion of DNA to be amplified is intact, which is most probably a question of chance. The results with MFPH show an important difference between harvests containing more or fewer than 106 bacilli per ml. For the 14 tests performed on 11 harvests with counts between 2.1 x 104 and 8.7 x 105, the mean sensitivity was 36.5 bacilli, range 1.5 to 210; whereas for 6 tests on 3 harvests with counts above 106 bacilli per ml the sensitivity was 103 to 2.2 x 103 Thus, the sensitivity decreases 10 to 1000-fold with increasing numbers of bacilli in the MFPH. This may reasonably be ascribed to an increasing proportion of dead bacilli during the late logarithmic and plateau phases of the growth curve (11).
The sensitivity of the PCR on suspensions of fresh, human multibacillary leprosy biopsies, kept frozen at -20ºC for 4 to 6 weeks, varied widely from 3 to 3 x 103 bacilli; the mean for the 6 lowest values after FB treatment was 37.6. The great variation in sensitivity may, in this case, also be due to variable proportions of viable bacilli in the suspensions. Unfortunately, the suspensions were not titrated in mice, although it is possible again that there is not necessarily a parallelism between viability as measured by multiplication in the mouse foot pad and the presence of the undamaged fragment of the PCR target DNA.
Paucibacillary leprosy lesions with a bacterial index (BI) of 0 contain a maximum of 103 bacilli per g of tissue (9). A suspension of a biopsy weighing 20 mg in 0.5 ml PBS would contain a maximum of 2 x 103 organisms, and 10 µ l of this suspension used in the PCR would contain 40 bacilli, very close to the mean of the lowest level of detection in the present experiments. With a sensitivity 10 times higher, which can exceptionally be reached with the present technique or more regularly by using a probe to detect the amplicon, the minimum number of bacilli that could be detected would be 104 organisms per g of tissue, provided the DNA is not degraded and there are no inhibitors of the reaction in the sample. Thus, it is very unlikely that the PCR will be able to solve the problems of the diagnosis of indeterminate or minimal tuberculoid leprosy lesions.
The genes coding for ribosomal RNA are present in all bacteria in multiple copies. Unfortunately, in slow-growing mycobacteria and M. leprae only one copy is present (1). Thus, although this DNA is by definition highly specific, a PCR based on it may be somewhat less sensitive than in the case of other bacteria. Whether the sensitivity of the PCR may be increased by amplification of repetitive sequences present in the genome, such as the ones described by ClarkCurtiss and Dochcrty (3) and used by Woods and Cole (15), remains to be investigated.
For the PCR to be useful in practical circumstances, preservation and transportation of specimens at ambient temperature is desirable. However, preservation of biopsies at room temperature resulted in a loss of sensitivity of 1 log. As shown with MFPH, fixation of suspensions in formol 10% or in Lowy fixative was very harmful, and Dubos OAA broth was also deleterious.
Biopsies kept at room temperature after boiling for 6 or 10 min brought no solution. On the whole, alcohol 70º gave the most satisfactory results.
Hsu, et al. compared the effect of formol, buffered formol, and alcohol cither at room temperature or with heating for the detection of the hepatitis-B virus in liver tissue(8). They found heating of tissues in alcohol the best procedure. The loss of sensitivity of the PCR was thought to result from nucleases degrading the DNA, and they concluded that for preservation of specimens a procedure is required that rapidly inactivates these enzymes. This seems to be the reason why heating in a microwave oven was the most efficient procedure. Clearly, a comparably rapid-acting fixation technique for leprosy biopsies has to be found.
In previous work with PCR, amplifying the gene for the 36-kDa protein, the harmful effect of unbuffered formalin had been ascribed to the interaction of the formalin with nucleic acids (5). The present work shows that the fixatives act by increasing inhibition. It is possible that in some cases, particularly with the FB procedure, the- fixatives themselves, remaining in the specimens, may inhibit the PCR. In the present study, the inhibitors were also not related to antileprosy treatment, since all the human biopsies were from untreated patients.
REFERENCES
1. BERCOVIER, H., KAPRI, O. and SELA, S. Mycobacteria possess a surprisingly small number of ribosomal RNA genes in relation to the size of their genome. Biochem. Biophys. Res. Comm. 136(1986)1136-1141.
2. BOOTH, R. J., HARRIS, D. P., LOVE, J. M. and WATSON, J. D. Antigenic proteins of Mycobacterium leprae: complete sequence of the gene for the 18 kDa protein. J. Immunol. 140(1988)597-601.
3. CLARK-CURTISS, J. E. and DOCHERTY, M. A. A species-specific repetitive sequence in Mycobacterium leprae DNA. J. Infect. Dis. 159(1989)7-15.
4. COX, R. A., KEMPSELL, K., FAIRCLOUGH, L. and COLSTON, M. J. The 16S ribosomal RNA of Mycobacterium leprae contains a unique sequence which can be used for identification by the polymerase chain reaction. J. Med. Microbiol, (in press).
5. DE WIT, M. Y. L., FABER, W. R., KRIEG, S. R., DOUGLAS, J. T., LUCAS, S. B., MONTREEWASUWAT, N., PATTYN, S. R., HUSSAIN, R., PONNIGHAUS, J. M., HARTSKEERL, R. A. and KLATSER, P. R. Application of a polymerase chain reaction for the detection of Mycobacterium lepraein skin tissues. J. Clin. Microbiol. 29(1991)906-910.
6. HARTSKEERL, K. D., DE WIT, M. Y. L. and KLATSER, P. R. Polymerase chain reaction for the detection of, Mycobacterium leprae. J. Gen. Microbiol. 135(1989)2357-2364.
7. HIGUCHI, R. Rapid, efficient DNA extraction for PCR from cells or blood. Amplifications 2(1989)1-3.
8. Hsu, H.-S., PENG, S.-Y. and SHUN, C.-T. High quality of DNA retrieved for Southern blot hybridization from microwave-fixed, paraffin-embedded liver tissues. J. Virol. Methods 31(1991)251-261.
9. SHEPARD, C. C. Recent developments in the chemotherapy of leprosy. Leprologia 19(1974)230-234.
10. SHEPARD, C. C. Statistical analysis of results obtained by two methods for testing drug activity of drugs against Mycobacterium leprae. Int. J. Lepr. 50(1982)96-101.
11. SHEPARD, C. C. and McRae, D. H. Mycobacterium leprae in mice: minimal infectious dose, relationship between staining quality and infectivity and effect of cortisone. J. Bacteriol. 89(1965)365-372.
12. SHEPARD, C. C. and MCRAE, D. H. A method for counting acid-fast bacteria. Int. J. Lepr. 36(1968)78-82.
13. TRIGLIA. T., PETERSON, M. G. and KEMP, D. J. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 16(1988)8186.
14. WILLIAMS, D. L., GILLIS, T. P.. BOOTH, R. J., LOOKER, D. and WATSON, J. D. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J. Infect. Dis. 162(1990)193-200.
15. WOODS, S. A. and COLE, S. T. A rapid method for the detection of potentially viable Mycobacterium leprae in human biopsies: a novel application of PCR. FEMS Microbiol. Lett. 65(1989)305-310.
1. M.D., Professor, Medical Microbiology, Institute of Tropical Medicine and University of Antwerp, Antwerp, Belgium.
2. Laboratory Technician, Institute of Tropical Medicine, Antwerp, Belgium.
3. Sci.D., University Hospital, Antwerp, Belgium. P. Jamet, M.D., Institut Marchoux, Bamako, Mali.
4. Ph.D., University Hospital, Antwerp, Belgium.
5. M.D., Institut Marchoux, Bamako, Mali.
Reprint requests to Prof. Pattyn, Institute for Tropical Medicine, Nationalestraat 155, B-2000 Antwerp, Belgium.
Received for publication on 28 October 1991.
Accepted for publication in revised form on 10 February 1992.