- Volume 60 , Number 2
- Page: 244–9
Isolation of cultivable Mycobacteria f rom feces and lungs of armadillos infected with Mycobacterium leprae
ABSTRACT
In the past, no cultivable mycobacteria were isolated f rom armadillos captured in the state of Florida, U.S.A. But recent findings of acid-fast bacilli (AFB) in the lungs of armadillos infected with Mycobacterium leprae prompted us to undertake this study to determine the correlation between systemic leprosy infection and the occurrence of cultivable mycobacteria in the lungs and stools of these animals. No AFB could be isolated f rom noninfected animals. Seventy percent of the infected animals developed disseminated infection, but no cultivable mycobacteria were isolated f rom their livers and spleens. However, cultivable mycobacteria were isolated f rom the lungs and stools of a large number of armadillos showing disseminated infection. The most common among these were M. gordonae, M. fortuitum, and M. avium. There was a close correlation between the development of disseminated leprosy infection and the occurrence of cultivable mycobacteria in their lungs and stools, perhaps due to the decline in the immune system in these animals in the later stages of infection.RÉSUMÉ
Dans le passé, aucune mycobactérie cultivable n'a été isolée de tatous capturés dans l'état de Floride, U.S.A. Mais les découvertes récentes de bacilles acidorésislants (BAR) dans les poumons de tatous infectés par Mycobacterium leprae nous a amenés à entreprendre cette étude pour déterminer la relation entre l'infection lépreuses systémique et la présence de mycobactéries cultivables dans les poumons et les selles de ces animaux. Aucun bacille acido-resistant n'a pu être isolé d'animaux non infectés. Septante pourcents des animaux infectes ont développé une infection disséminée, mais aucune mycobactérie cultivable n'a été isolée de leur foie et rate. Cependant, des mycobactéries cultivables ont été isolées des poumons et selles d'un grand nombre de tatous présentant une infection disséminée. Les plus communes parmi celles-ci étaient M. gordonae, M.fortuitum. et M. avium. Il y avait une relation étroite entre le développement d'une infection lépreuse disséminée et la présence de mycobactéries cultivables dans leurs poumons et selles; ceci était peutêtre dû au déclin du système immunitaire chez ces animaux dans les dernières phases de l'infection.RESUMEN
En el pasado no se habian aislado micobacterias cultivables de los armadillos capturados en el estado de Florida, USA. Sin embargo, los recientes hallazgos de bacilos ácido resistentes (AFB) en los pulmones de armadillos infectados con Mycobacterium leprae nos impulsaron a emprender este estudio para determinar la correlación entre la infección leprosa sistémica y la ocurrencia de micobacterias cultivables en los pulmones y excrementos de estos animales. No se aislaron AFB de los animales no infectados. Setenta por ciento de los animales infectados desarrollaron una infección diseminada pero no se aislaron micobacterias cultivables de sus hígados y bazos. En cambio se pudieron aislar micobacterias cultivables de pulmones y excrementos de un gran número do armadillos con infección diseminada. Las micobacterias cultivables más comunes fueron M. gordonae, M.fortuitum, y M. avium. Hubo una estrecha correlación entre el desarrollo de la infección leprosa diseminada y la ocurrencia de micobacterias cultivables en sus pulmones y excrementos, quizá esto se debió a la depresión de la función inmune que ocurre en las etapas tardías de la enfermedad en estos animales.Since the discovery of Mycobacterium leprae by Hansen in 1873 (5), there has been a long search for an animal model for leprosy. It has involved almost 30 species of animals, and almost as many protocols as researchers (6). Until 1960, the only host susceptible to leprosy was human.
In 1960, Shepard developed the mouse foot pad model (19) which has been used since then to determine viability and drug sensitivity of M. leprae obtained from leprosy patients. In 1972, Kirchheimer and Storrs (8) reported a disseminated infection in nine-banded armadillos (Dasypus novemcinctus Linn.) inoculated earlier with human-derived M. leprae. The histopathology of lesions was similar to that of human leprosy infection, and the organisms recovered demonstrated all of the characteristics of M. leprae isolated from human patients (9). The major organs involved were the liver, spleen, and lymph nodes. However, unlike human leprosy, M. leprae could also be found in the lungs, heart, brain, and kidneys, although the number of organisms recovered was far less (10). Subsequently, natural, leprosy-like infection has been reported in armadillos captured in the wild from the states of Texas and Louisiana in the United States (20, 23, 26). Also there have been reports of rapid-growing, cultivable mycobacteria isolated from armadillos captured in the wild (11). But, neither M. leprae nor other rapid-growing mycobacteria has been observed in wild armadillos from the state of Florida.
During the last 2 years, we have noticed the occurrence of large numbers of cultivable mycobacteria in the lungs of armadillos in our colony. This occurrence was only in armadillos infected with M. leprae and showing signs of disseminated leprosy and was not found in uninoculated animals or in those inoculated animals that failed to develop disseminated disease. The role of atypical mycobacteria, such as M. avium and M. intracellulare, in developing fatal opportunistic infections in AIDS patients has been well established (18, 27). The clinical consequences of an infection with atypical mycobacteria in AIDS patients remain unknown. It has been suggested that the gastrointestinal tract is a portal of entry for M. avium-intracellulare-complex organisms, since the isolation of these organisms from stools of AIDS patients has been reported (21).
The present study was undertaken to isolate cultivable mycobacteria from the lungs of armadillos infected with M. leprae and to determine the relationship, if any, with the fecal excretion of cultivable mycobacteria in these animals.
MATERIALS AND METHODS
Armadillos. A total of 187 nine-banded armadillos (Dasypus novemcinctus Linn.), all captured in central Florida, were examined. Of these, 99 animals were uninfected and 88 were infected earlier with human- or armadillo-derived M. leprae.
Armadillos are routinely treated for intestinal parasites as soon as they adapt to eating food in captivity. The treatment of choice is thiabendazole/piperazine, given daily on the food for 3 days. All 32 uninfected animals were in the colony for 2-3 months before they were sacrificed and the livers and spleens removed. Stools from these animals were obtained from their co
Ions. The remaining 67 uninfected animals also in the colony for at least 3 months were not sacrificed. Fecal samples from these animals were collected either late in the day or first thing in the morning. The animals that were infected with M. leprae (intravenously with 1 x 108 organisms) were also in the colony for at least 3 months before being inoculated.
All of the infected animals were sacrificed 2-3 years postinoculation, and smears were made from the livers, spleens, and lungs and stained by the Ziehl-Neelsen (ZN) method. Stools were collected from the colons of these animals.
When the animals were sacrificed and smears from their livers and spleens were examined after ZN staining, if the bacterial index (BI) was 2+ or higher (16), the animal was designated as having developed systemic infection. All other Bis (1+ or 0) were considered as not having developed systemic infection.
Bacterial examinations. Suspensions were prepared from the livers, spleens, and lungs in 0.05 M phosphate buffer, pH 7.0; these were then decontaminated with 4% sodium hydroxide and saturated acid potassium phosphate. Aliquots of these suspensions were then inoculated onto Lowenstein-Jensen and Middlebrook 7H10 slants and incubated at 32ºC and 37ºC for up to 8 weeks.
To isolate acid-fast bacilli (AFB) from fecal samples, the method of Portaels, et al. (15) was adopted. The fecal samples were mixed well in trypticase soy broth and centrifuged for 5 min at 1000 rpm. The supernates were incubated at 37ºC for 5 hr to allow spore-forming bacteria to germinate, and these were then killed by adding malachite green and cyclohexamide. The suspensions were then decontaminated using sodium hydroxide and hydrochloric acid. After washing with phosphate buffer several times, the sedimented material was inoculated onto Lowenstein-Jensen and Middlebrook 7H10 slants and incubated at 32ºC and 37ºC for up to 8 weeks.
The organisms isolated on slants were determined to be AFB by ZN staining, and further identified using standard biochemical methods, such as nitrate reduction, Tween 80 hydrolysis, aryl sulfatase, urease, tellurite reduction, and catalase (17).
RESULTS
Among the 99 uninfected animals, 32 animals were sacrificed. The ZN-stained smears, as well as cultures, from the livers, spleens, and lungs from all of these animals failed to show AFB. Similarly, the fecal samples from the remaining 67 uninfected animals showed no AFB either under direct microscopic examination or on any of the mycobacterial culture media. Thus, the lung and fecal samples of all of the uninfected animals were free of detectable, cultivable mycobacteria.
Among the 88 animals infected earlier with M. leprae (Table 1), 62 animals (70%) developed systemic leprosy infection (AFB in livers, spleens, and lungs), while 26 animals (30%) did not show any signs of systematic infection 2-3 years after inoculation. Cultivable AFB were not found in the livers or spleens of any of these 88 animals. Among the 62 animals showing systemic infection, lung smears from 52 animals (84%) and fecal smears from 36 animals (58%) were positive for AFB. The corresponding figures for animals not showing systemic infection were 4 (15.4%) and 3 (11.5%), respectively. A total of 27 animals (5 from systemic infection group and 22 from the other group) did not show any AFB in either the lungs or the fecal samples.
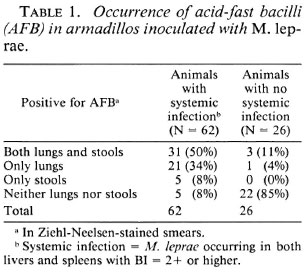
As seen in Table 2, 56 of the 88 infected animals were positive for AFB in the smears of their lungs; of these, 93% had developed systemic infection. On the other hand, of the 32 animals who did not show any AFB in their lung smears, only 32% had developed systemic infection. Furthermore, of the 56 animals with lung smears positive for AFB, cultivable mycobacteria were isolated from the lungs of 34 animals (61%).
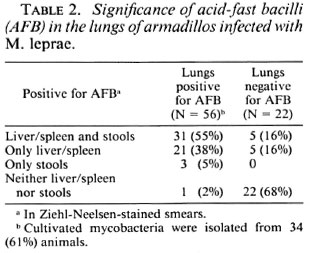
The data in Table 3 indicate that among the 39 of the infected animals that had shown AFB in their fecal smears, 36 (92%) had developed systemic infection; the corresponding number for the animals with fecal smears negative for AFB was 26 (53%). Only 3 out of 39 animals showing fecal smears positive for AFB did not develop systemic infection; but in these three animals, lung smears were positive for AFB. The fecal samples of all 39 animals with smears positive for AFB were also positive for cultivable mycobacteria.
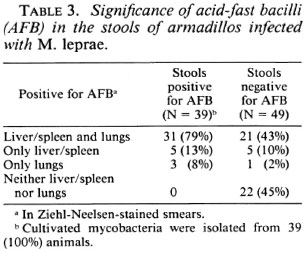
To summarize the above results, among the 56 infected animals with lung smears positive for AFB, 34 (61%) showed cultivable mycobacteria in their lungs. All of the 39 animals with fecal smears positive for AFB were also positive for cultivable mycobacteria in the lung smears. Among these 39 animals, cultivable mycobacteria were isolated with both lung and fecal samples of 34 animals.
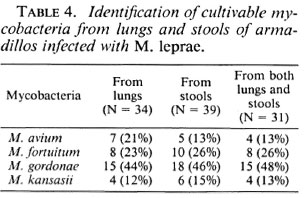
Table 4 summarizes the results on the identification of mycobacteria from the lungs and feces of armadillos based on the biochemical tests performed. The most common mycobacteria observed in both lung and fecal samples was M. gordonae, followed by M. fortuitum. Only 7 out of 34 (21%) lung samples showed the presence of M. avium; the corresponding number for the fecal samples was 5 out of 39 (13%).
DISCUSSION
This is perhaps the first account of the occurrence of AFB in the stools of armadillos infected with M. leprae. Mycobacteria have been isolated from various organs of armadillos captured in the wild (10), although we could not find such organisms earlier in armadillos captured in Florida. In the present study, we observed the presence of AFB in fecal samples of armadillos infected with M. leprae and showing signs of systemic infection. Noninfected armadillos, as well as those M. leprae -infected armadillos not showing signs of infection, did not shown any AFB in their fecal samples. Another observation was that among the 39 animals from which cultivable mycobacteria were isolated from fecal samples, 34 of these animals showed cultivable mycobacteria in their lungs also. Thus, there appears to be a good correlation between occurrence of cultivable mycobacteria in stools and systemic leprosy infection in armadillos.
When the animals develop systemic infection, the AFB load in the liver and spleen is in the range of 1-5 x 109/g of tissue. In these studies we did not quantitate the AFB in the lungs. The BI of lungs was usually 2-3 + ; thus, the AFB load can be estimated to be 1-5 x l08/g. We did quantitate the load of cultivated AFB in the fecal samples of infected animals, and it was in the range of 5 x l02-2 x 103/g stool.
In any ecosystem, natural biotopes and living beings co-exist, and the possibility of environmental mycobacteria causing mycobacterioses in man and animals must be considered (7). M. gordonae has been isolated mostly from rivers, fish ponds, ditches, and swamp holes (1, 4, 25). Also, a great number of M. gordonae strains have been recovered from soil and tropical vegetation (14, 22, 25). M. avium has been isolated from different substrates but mainly from soil, which is regarded by some investigators as its normal habitat (13). Similarly, M.fortuitum and M. kansasii have been found in a multitude of biotopes, predominantly in rivers and waste water and in tropical grass (1, 12, 22, 24, 25). Knowing the habitat of armadillos, it would not be surprising if these organisms are already present in these animals in the wild. Another and more serious aspect of this situation is the recent association of M. avium as an opportunistic infection in AIDS patients (18, 27). The possible gastrointestinal origin of M. avium intracellulare infections in AIDS patients is now being discussed (13, 21). The presence of these organisms from stools of AIDS patients, and not from healthy individuals, has been reported.
In the present study, about 20% of M. leprae -infected animals showed M. avium in the lungs or stools or both. Thus, it seems that these organisms enter the host via contaminated food and water, and colonize in the lungs as well as in the gastrointestinal tract. When the animal's immune mechanism is diminished due to disseminated infection with M. leprae, these opportunistic mycobacteria invade the animal. Collins (2) has made a similar suggestion that M. avium-intracellulare possess factors which enable them to attach to intestinal mucosa, colonize the membranes, and invade them when the AIDS virus has depleted the T-cell defenses. The same argument can be put forth with other isolates, such as M. gordonae, M.fortuitum and M. kansasii. All of these organisms are isolated from the abovementioned sources, and all can cause chronic pulmonary disease. The fact that these organisms are not isolated from armadillos not infected with M. leprae, or not developing disseminated infection after inoculation with M. leprae, suggests that these pathogens invade the armadillos toward the end of disseminated leprosy infection, and then are seen in large numbers in the lungs and stools. Since these are potential pathogens, care is suggested in the handling of these animals and their waste.
Acknowledgment. This study received financial support from the German Leprosy Relief Association, Wurzburg, Germany; the National Institutes of Health (AI-52563); the IMMLEP component of the WHO Special Program for Research and Training in Tropical Diseases. The technical assistance of Mr. Anthony Murphy and Ms. Sharon Williams is greatly appreciated.
REFERENCES
1. BEERWORTH, W. Mykobakterien in Viehtranken und Oberflachen-gewassern. 80(1973)398-401.
2. COLLINS, F. M. M. avium complex infections and development of the acquired immunodeficiency syndrome: casual opportunistic or casual cofactor? Int. J. Lepr. 54(1986)458-474.
3. DAMSKER, B. and BOTTONE, E. J. M. avium-M. intracellular from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J. Infect. Dis. 151(1985)179-181.
4. GOSLEE, S. and WOLINSKY, E. Water as a source of potentially pathogenic mycobacteria. Am. Rev. Respir. Dis. 113(1976)287-292.
5. HARBOE, M. Armauer Hansen-the man and his work. Int. J. Lepr. 41(1973)417-424.
6. JOHNSTONE, P. A. S. The search for animal models in leprosy. Int. J. Lepr. 55(1987)535-547.
7. KAZDA, J. F. The principles of the ecology of mycobacteria. In: The Biology of the Mycobacteria, Volume 2; Immunological and Environmental Aspects. London: Academic Press, 1983, pp. 323-341.
8. KIRCHHEIMER, W. F. and STORRS, E. E. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int. J. Lepr. 39(1971)693-702.
9. KIRCHHEIMER, W. F., STORRS, E. E. and BINFORD, C. H. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. II. Histologic and bactcriologic postmortem findings in lepromatoid leprosy in the armadillo. Int. J. Lepr. 40(1972)229-242.
10. KIRCHHEIMER, W. F. and SANCHEZ, R. M. Quantitative aspects of leprosy in armadillos. Lepr. India 44(1976)84-87.
11. KIRCHHEIMER, W. F. and SANCHEZ, R. M. Examination of North American armadillos for mycobacteriosis. Lepr. India 50(1978)156-160.
12. KUBICA, G. P., BEAM, K. E., PALMER, J. W. and BIGDON, A. C. The isolation of unclassified (atypical) acid-fast bacilli from soil and water samples, collected in the state of Georgia. Am. Rev. Respir. Dis. 84(1961)135-136.
13. MARKS, J. and JENKINS, P. A. The opportunist mycobacteria -a 20-year retrospect. Postgrad. Med. J. 47(1971)705-709.
14. PORTAELS, F. Contribution a l'etude des mycobacteries de l'environnement au Bas-Zaire. Ann. Soc. Belg. Med. Trop. 53(1973)373-387.
15. PORTAELS, F., DE MUYNCK, A. and SYLLA, M. P. Selective isolation of mycobacteria from soil: a statistical analysis approach. J. Gen. Microbiol. 134 (Part 3)(1988)849-855.
16. RIDLEY, D. S. Therapeutic trials in leprosy using serial biopsies. Lepr. Rev. 29(1958)45-52.
17. ROBERTS, G. D., KONEMAN, E. W. and KIM, Y. K. Mycobacterium. In: Manual of Clinical Microbiology. 5th edn. Balows, A., Hausler, W. J., Herrmann, K. L., Isenberg, H. D. and Shadomy, H. J., eds. Washington, D.C.: American Society of Microbiology, 1991, pp. 304-339.
18. SELIK, R. M., STARCHER, E. J. and CURRAN, J. W. Opportunistic diseases reported in AIDS patients: frequencies, associations and trends. AIDS 1(1987)175-177.
19. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exper. Med. 112(1960)445-454.
20. SMITH, J. H., FOLSE, D. S., LONG, E. G., CHRISTIE, J. D., CROUSE, D. T., TEWES, M. E., GATSON, A. M., EHRHADT, R. L., FILE, S. K. and KELLY, M. T. Leprosy in wild armadillos (Dasypus novemcinctus) of the Texas Gulf Coast: epidemiology and mycobactcriology. J. Reticuloendothel. Soc. 34(1983)75-88.
21. STACEY, A. R. Isolation of Mycobacterium aviumintracelllare-scrofulaceum complex from feces of patients with AIDS. Br. Med. J. 293(1986)1194.
22. STANFORD, J. L. and PAUL, R. C. Preliminary report on some studies of environmental mycobacteria from Uganda. Ann. Soc. Belg. Med. Trop. 53(1973)389-393.
23. TRUMAN, R. W., SHANNON, E. J., HAGSTAD, H. V., HUGH-JONES, M. E., WOLFF, A. and HASTINGS, R. C. Evaluation of the origin of Mycobacterium leprae infections in wild armadillo (Dasypus novetn-Cinctus). Am. J. Trop. Med. Hyg. 35(1986)588 -593.
24. VIALLIER, J., LAHNECHE, J. and LANERY, R. Etude Mycobacteria from Armadillos critique de quelques tests utilisables pour l'identification des mycobacteries. Ann. Biol. Clin. (Paris) 25(1967)1169-1197.
25. VIALLIER, J. and VIALLIER, G. Inventaire des mycobacteries de la nature. Ann. Soc. Belg. Med. Trop. 53(1973)361-371.
26. WALSH, G. P., STORRS, E. E., BURCHFIELD, H. P., COTTRELL, E. H., VIDRINE. M. F. and BINFORD, C. H. Leprosy-like disease occurring naturally in armadillos. J. Reticuloendothel. Soc. 18(1975) 347-351.
27. ZAKOWSKI, P., FLIGIEZ, S., BERLIN, O. G. W. and JOHNSON, B. L. Disseminated M. avium-intracellulare infection in homosexual men dying of AIDS. JAMA 248(1986)2980-2982.
1. Ph.D.; Department of Biological Sciences, Florida Institute of Technology, Melbourne, Florida 32901-6988, U.S.A.
2. Department of Biological Sciences, Florida Institute of Technology, Melbourne, Florida 32901-6988, U.S.A.
Received for publication on 18 October 1991.
Accepted for publication in revised form on 5 February 1992.