- Volume 60 , Number 2
- Page: 255–68
A quantitative basis for sustainable anti-Mycobacterium leprae chemotherapy in leprosy control programs
Editorial opinions expressed are those of the writers.
Leprosy is feared largely because it can cause permanent physical damage. The elimination of leprosy as a public health problem, therefore, involves the reduction of the risk of disability due to leprosy to acceptably low levels. The inhibition of Mycobacterium leprae is sufficient to avert one class of disabilities-those due to unrestrained bacterial proliferation. However, host responses to M . leprae and its constituents can cause disability even among patients on effective anti- M. leprae treatment.1-3 Anti- M. leprae chemotherapy (AMC) forms only one part of the measures needed to prevent disability. Sufficiently early diagnosis, expert monitoring to detect insidious nerve damage, 2, 3 and timely antiinflammatory treatment are among the other requirements.
It has been hoped that AMC with increasingly effective drugs would reduce the transmission of M . leprae so that the other measures to prevent disability would become superfluous. Leprosy control programs could then be dismantled, and the resultant savings used partly for even more expensive and effective anti- M. leprae drugs. There is a flaw in that view. The decline in the number of patients on AMC has been largely cosmetic, a natural accompaniment of the reduced duration of AMC. A reduction in the underlying transmission of M. leprae and the number of patients newly recognized is less certain, at least within India.
The socioeconomic context of leprosy deserves some attention. Nine out often newly detected cases of leprosy are estimated now to come from India. Material want is common in India. Leprosy seems sustainable in Indian conditions, and leprosy control efforts (including AMC) must pay some attention to cost-effectiveness. There is room for improvement in the promptness of case detection, the quality of monitoring for nerve damage, and the effective use of antiinflammatory drugs. The more cost-effective is AMC, the better the chances of mustering funds and enthusiasm for other important components of leprosy control programs.
Initiatives for more rational AMC often fall back on theory. This is partly because trials of new regimens take inconveniently long. Theory can help formulate new regimens, predict efficacy and cost to some extent and, hopefully, steer innovations between excessive caution and recklessness. The quantitative treatment of the relevant evidence puts some constraints on subjective preferences. Even so, "results" of calculations must be viewed as being no better than signposts whose validity must be tested.
This editorial summarizes one attempt4 to fit relevant evidence from scientific publications into an internally consistent framework of quantitative theory. The following questions are addressed: a) What regimens of AMC are likely to prove effective and affordable? and b) Which persons should receive such cost-effective regimens of AMC?
MINIMUM REQUIRED DURATION OF AMC
There is a case to be made for life-long treatment of many leprosy patients. India abounds in sources of infection, and many patients remain susceptible to re-infection. However, the economic pressures in India are obvious. Life-long treatment with AMC for Indian patients could well damage other components of the leprosy control program. Therefore, a limited duration of AMC followed by surveillance and re-treatment, where necessary, seems a reasonable compromise. Incidentally, re-treatment should be able to deal not only with re-infection, but also with "persisters" 5, 6 that begin to proliferate.
The minimum duration of AMC theoretically required to eliminate viable (nonpersister) M. leprae from a previously untreated patient depends partly on the: a) rate of decline in viable drug-susceptible bacteria; b) equilibrium frequency of drug-resistant bacteria; c) relative fitness of drugresistant bacteria; and d) maximum initial number of viable M. leprae.
Rate of decline in viable drug-susceptible bacteria
Serial dilutions of M. leprae have been used in mouse experiments. These permit estimates of the rate of decline in viable bacteria during treatment.4, 7 Figure 1 shows the results of calculations based on such experiments; confidence limits are likely to be slightly wider than those shown 4 (sources of data are shown in Table 1).
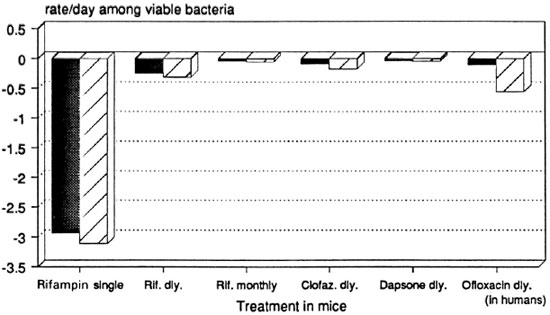
Fig. 1. Net growth rate of viable M. leprae in mice during treatment with single drugs: 99% confidence limits based on "infectious dose 50's." Dosages = rifampin 10 mg/kg by gavage; clofazimine 0.01 g% w/w in diet; dapsone 0.01 g% w/w in diet; ofloxacin (in humans) 400 mg/day orally. Data sources: See Table 1.

The single initial dose of rifampin seems remarkably more effective than daily rifampin. This may be due to pharmacokinetics, the induction of altered bacterial metabolism, or other mechanisms. Monthly rifampin, on the other hand, is even less effective than clofazimine mixed in diet, and possibly of the same order of efficacy as dapsone given in diet. Daily ofloxacin was tested in human beings, 13 and it is at least as effective as clofazimine in the diet and possibly more effective than daily rifampin.
Interactions between drugs have been disregarded in the absence of suitable data from serial dilution experiments.
Equilibrium frequency of drug-resistant M. leprae
The equilibrium frequency of drug-resistant mutant bacteria is given by the ratio of the rates (per generation time of typical untreated bacteria) of mutation to and from resistance. 14 A cautious working hypothesis is adopted here: that drug resistance in M. leprae is not rapidly reversible. The biological basis of drug resistance in M. leprae is discussed elsewhere.4
The equilibrium frequency of dapsoneresistant mutants can fortunately be calculated from data using a few reasonable assumptions.4 Rees, in the early 1960s, tested high concentrations of dapsone (> 0.01 g% w/w in mouse diet, equivalent to > 750 ng/ ml in serum or over 100 mg/day in a human adult) against previously untreated M. leprae; 15 82% (± 8%, 95% c.i.) of "over 100" mouse foot pads failed to show growth of M. leprae. Therefore, as discussed fully elsewhere, 4 the equilibrium frequency of mutants resistant to high concentrations of dapsone has probably always been between 1 in 10,000 and 1 in 100.
The observed equilibrium frequency of "full-dose" dapsone-resistant, mutant M. leprae limits the practical importance of the hypothesis of step-wise mutation. If stepwise mutation occurs at all, it is unlikely to be more than a negligible accompaniment of single-step mutation to resistance against high concentrations of dapsone.
Appropriate data for the other anti- M. leprae drugs is not yet available in the literature. Future attempts to collect such data will hopefully remain alert to the danger that an insufficient duration of the experiment can mask the slowed growth of drugresistant mutants during monotherapy. For now, the equilibrium frequency of mutants resistant to a drug other than dapsone is assumed to be between 1 in 108 and 1 in 103.
Relative fitness of drug-resistant mutants during monotherapy
The alterations in metabolism which permit survival of drug-resistant mutants during treatment are generally accompanied by "pleiotropic costs"16 which reduce the relative fitness of drug-resistant mutants-even during monotherapy. "Relative fitness," a standard measure in population biology, 14 compares the birth rate of treated drug-resistant organisms to that of typical untreated organisms. The relative fitness of bacteria takes a value between 0.5 and l.0. 4
The relative fitness of dapsone-resistant mutants during treatment with dapsone is apparently well below 1.0. "Full-dose" dapsone-resistant mutants are sufficiently frequent to occur in every patient before treatment.4, 15 A single such mutant doubling every 11 days should yield 1012 viable M. leprae within a year and a half. Dapsoneresistant failure of treatment, if it occurs at all, is typically delayed well beyond a year or two of monotherapy. Therefore, the relative fitness of "full-dose," dapsone-resistant, mutant M . leprae must be importantly lowered by dapsone monotherapy. The low cumulative probability of dapsone-resistant failure of treatment among patients ever smear-positive in India, under 10%, 4, 17, 18 is consistent with this view.
Clofazimine-resistant mutants also appear to grow more slowly than do typical untreated M. leprae, at least in mice treated with clofazimine monotherapy. 19, 20 Evidence for rifampin and ofloxacin is awaited.
M. leprae exhibits a remarkable peculiarity which makes the birth rate and relative fitness of drug-resistant M. leprae important both biologically and economically. M. leprae appears to have an important rate of natural death. During logarithmic proliferation of M . leprae in untreated "nude" mice, the proportion of acid-fast M . leprae which stain as "solids" (the "solid ratio") converges on only about 10%. 21 The solid ratio is demonstrably correlated with the proportion viable, 22, 23 and at least one attempt to demonstrate the physical integrity of nonsolids was unsuccessful.24 Other evidence for the viability of nonsolids is open to the following objection: Sampling error may have led to the inclusion of a "solid" in inocula intended to exclude "solids."4 The calculation of such sampling error is given elsewhere. 4
If an important proportion of acid-fast M. leprae are nonviable during logarithmic proliferation, the natural death rate of M. leprae must be importantly greater than zero, 21, 25, 26 and its generation time must be shorter than its doubling time. The generation time is defined as the average interval between successive cell divisions of typical untreated bacteria. The doubling time is the time taken for acid-fast M. leprae (both viable and nonviable) to double in number. A previous calculation yielded a generation time of about 1 day21 which is consistent with the following general relationship.
During logarithmic proliferation of bacteria, when nonviable bacteria disappear at a negligible rate, the generation time is given by the product of the doubling time and the limit of the proportion viable.4 For M . leprae, doubling time 11 days27 x proportion viable 10% = 1 day generation time.
M. leprae could, therefore, well prove to have a birth rate of about 0.63 per day and a natural death rate of about 0.57 per day. If so, a very small decrease in the birth rate will ensure the slowed growth (or even decline) of viable M . leprae. Moreover, the accumulation of slowly proliferating M. leprae will only be possible if host responses are sufficiently small. Therefore, the presence of drug-resistant M. leprae in relatively large numbers at the start of treatment is no guarantee that they will proliferate or accumulate during monotherapy.
The lowered relative fitness of dapsoneor clofazimine-resistant mutants during monotherapy with the respective drugs suggests that these drugs should be useful in the retreatment of patients with M . leprae simultaneously resistant to multiple drugs. The theoretical risk of multidrug resistance with MD T (multidrug therapy) is discussed later.
Maximum initial number of viable M. leprae
Lepromatous leprosy (LL)28 patients are presumed to harbor no more than 4.5 x 1012 acid-fast M . leprae, enough to occupy about 30% of the total body volume in an adult weighing 70 kg.4Ten percent of these bacilli may reasonably be presumed viable before treatment, 4.5 x 1011 viable M. leprae. Untreated borderline lepromatous (BL) patients 28 are presumed to have a hundredth as many viable M . leprae as found in LL patients.
Predicted course of viable M. leprae during treatment
Figures 2 to 6 show the predicted course of viable nonpersister M. leprae during treatment with various regimens. The most optimistic and most pessimistic set of assumptions are used, in turn, for each regimen. The minimum plausible rate of bacterial decline and the maximum plausible frequency of drug-resistant bacteria form the most pessimistic assumptions. The reverse is true for the most optimistic assumptions.
Fig. 2. Predicted course of viable M, leprae during WHO-MDT in an LL patient according to the most pessimistic assumptions used.
Important parameters disregarded in these simplified calculations include interactions between drugs, re-infection or persisters, variations dependent on bacterial density, mutants simultaneously resistant to three drugs, and the removal of viable M . leprae by host responses.
WHO-MDT, LL patient, pessimistic prediction. The WH O Study Group regimen for multibacillary leprosy29 should (most pessimistically) eliminate viable nonpersister M . leprae from LL patients within 18 months. Figure 2 suggests that daily clofazimine could prove superfluous beyond 9 months, by which time all surviving M . leprae are predicted to be clofazimine-resistant. M. leprae simultaneously resistant to clofazimine and dapsone are predicted to be eliminated by 1 year of treatment, and rifampin could prove superfluous beyond that point. Dapsone alone may well prove sufficient for the remaining months of treatment.
Similar calculations4 show that 100 initial daily doses of rifampin could suffice to shorten AMC in LL patients; only 8 months of daily clofazimine and 14 months of dapsone may be required. This prediction, too, is based on the most pessimistic assumptions.
RCD-MDT, BL or LL patient, optimistic prediction. Rifampin, clofazimine, and dapsone (RCD) regimens could eventually be shortened to a few months. BL patients may require no more than 4 months of RCD including only one dose of rifampin, .while LL patients (Fig. 3) may require no more than 5 months (again with a single initial dose of rifampin). Rifampin is expected to be indispensable beyond the initial dose only if mutants simultaneously resistant to clofazimine and dapsone occur before treatment. The most optimistic assumptions were used.
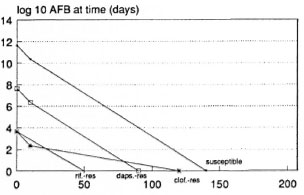
Fig. 3. Predicted course of viable M. leprae during treatment of an LL patients with a single rifampin dose followed by clofazimine and dapsone (single R. CD) according to the most optimistic assumptions used.
The use of daily rifampin could shorten RCD regimens even further. Three months of daily RCD treatment could prove sufficient for LL patients, according to the most optimistic assumptions.4
Clofazimine is not universally acceptable. RD (rifampin-dapsone) and OR D (ofloxacin + RD) regimens are among the alternatives which are clofazimine-free.
RD regimen. Rifampin and dapsone alone are insufficient in the identifiable minority of patients who show a negligible rate of bacterial clearance. Viable M. leprae, like acid-fast M. leprae, are probably removed at differing rates by various groups of lepromatous (L)30 patients.31 "Slow responders" to AMC may be defined pragmatically (until more refined criteria become available) as those with a remarkably high bacterial index (BI)32 before treatment (e.g., 3 + or more at any site) and/or a relatively slow decrease in the BI during treatment (e.g., less than 0.75 log10 units per year). Such patients form a very small minority of those newly detected to be infected with M . leprae in India (and the world). RD regimens, because of their low cost and freedom from the side effects of clofazimine, may prove useful in all patients except "slow responders."
Rifampin-resistant mutants are expected to be relatively spared during RD treatment.4, 33 "Slow responders" on RD regimens are therefore expected to suffer an important risk of relapse with rifampin-resistant M . leprae. The selective pressure exerted by rifampin may be minimized, e.g., by confining rifampin to a single initial dose. The selective advantage of rifampin-resistant mutants may be minimized by maintaining adequate concentrations of dapsone, e.g., by monthly injection.34 (The avoidance of monthly dapsone injections is unlikely to lower the risk of HIV infection in India, where injections are widely demanded and used.) These presentations against the selection of rifampin-resistant M. leprae are likely to be futile only among "slow responders."
Before discussing RD regimens among leprosy patients other than "slow responders," we should consider dapsone monotherapy. Figure 4 shows that dapsone-susceptible M. leprae could be eliminated by 3 years of regular dapsone in adequate dosage. The birth rate of resistant mutants need only be reduced from 0.63/day to 0.55/day to ensure their elimination within 3 years, even if 1 in 100 viable M. leprae are "full-dose" dapsone-resistant before treatment. An even smaller reduction in fitness of resistant mutants will suffice to ensure their decline if host responses clear viable M. leprae.
Fig. 4. Predicted course of viable M. leprae during dapsone monotherapy of an LL patient according to the relative fitness (RF)of drug-resistant mutants during monotherapy.
Three-year dapsone monotherapy for lepromatous patients has seldom been tried. It is yet to be found wanting, possibly because it is yet to be used in "slow responders." Lowe35 was unable to convincingly show bacterial proliferation following the withdrawal of dapsone monotherapy within 41 months among 139 L patients followed up for an average of 22 months after cessation of monotherapy. Dietrich, et al. 36 have yet to publish their final report, but they could not demonstrate any advantage from the addition of daily rifampin or other drugs to dapsone given daily for only 3 years. "Slow responders," who have a disproportionately high risk of drug-resistant failure with any regimen, were probably excluded from the study of Dietrich, et al. since "dapsone-resistant" patients were excluded from dapsone monotherapy.
The addition of a single, initial, large dose of rifampin to dapsone regimens is likely to reduce costs without inflicting a high risk of rifampin-resistant failure of treatment. Calculations suggest that 2 years of regular fulldose dapsone (e.g., by monthly injection) following a single, large, initial dose of rifampin (e.g., 1200 mg in an adult) could prove sufficient for the elimination of nonpersister viable M. leprae from all patients except "slow responders." This compares with 3 years of dapsone monotherapy (Fig. 4) if rifampin is omitted. It must be emphasized that these calculations are based on the assumption that mutants simultaneously resistant to rifampin and dapsone will have a lowered fitness in the presence of dapsone, much as dapsone-resistant mutants appear to have.
The most optimistic calculations suggest that 10 weeks of daily rifampin and 4 months of dapsone could eliminate viable nonpersister M. leprae from a borderline lepromatous (BL) patient.4 This compares with 4 months of daily clofazimine and dapsone following a single initial dose of rifampin in a BL patient.
It may well be found that RD regimens, particularly single rifampin and monthly injections of dapsone, offer the optimal balance between anti- M. leprae effect and cost in all leprosy patients except "slow responders."
ORD regimens (with ofloxacin). "Slow responders" are likely to fare relatively poorly on any anti- M. leprae regimen. The use of relatively expensive drugs such as ofloxacin might be justifiable in such patients, even in India. In affluent countries, ofloxacin might well provide an alternative to clofazimine.
Figure 5 shows that, even by the most pessimistic assumptions, OR D regimens might not need to be continued beyond 7 months, including only 4 months of daily rifampin. All surviving M. leprae could prove resistant to rifampin by the end of 4 months.
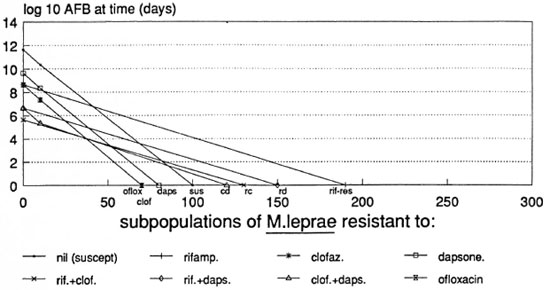
Fig. 5. Predicted course of viable M. leprae in an LL patient treated with ofloxacin, rifampin and dapsone (ORD) according to the most pessimistic assumptions used.
The most optimistic assumptions suggest that as little as 7 weeks of ORD could prove sufficient in BL patients (Fig. 6). The omission of rifampin after the initial dose is unlikely to alter the outcome of treatment; by this prediction, ofloxacin-resistant M. leprae should be sufficiently inhibited by dapsone.
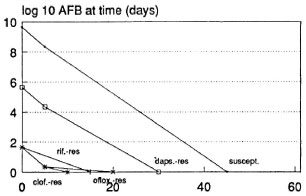
Fig. 6. Predicted course of viable M. leprae in a BL patient treated with ofloxacin, rifampin and dapsone (short ORD) according to the most optimistic assumptions used.
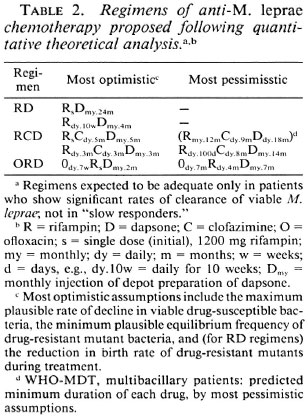
Table 2 summarizes the regimens suggested by the calculations. Many would argue that trials of newly proposed regimens should start with the most pessimistic assumptions and gradually move toward the most optimistic regimens. The small group of "slow responders" seems to deserve a special place in trials of new regimens; they should be included but should be dealt with separately in the analysis.
MDT and multidrug resistance
Trials of MD T so far are likely to underestimate the risks associated with MDT. First, most "slow responders" were probably excluded from such trials on the grounds of dapsone resistance. Yet newly detected lepromatous patients will not exclude such "slow responders." Second, patients already treated with dapsone monotherapy were included, artificially lowering the risk of relapse and drug-resistant failure. Figure 1 shows that daily dapsone approaches monthly rifampin in efficacy. Therefore, dapsone-treated patients are unlikely to be representative of newly detected patients.
The risk of multidrug resistance as a consequence of MDT has seldom been emphasized. This is short-sighted since MD T with drugs no more effective than rifampin or ofloxacin cannot be relied upon to stop cell divisions; nor can multiple-resistant M. leprae be presumed absent from patients at the start of treatment; nor can patients be relied upon to take all self-administered doses of a regimen. The reasons for these claims are as follows.
The measured rate of bacterial decline during treatment is made up of several components, including the natural death rate of bacteria. Since the natural death rate of M. leprae could well be 0.57 per day, as discussed above, only the single initial dose of rifampin can be assumed to reduce the birth rate of M. leprae to zero. Only the initial dose of rifampin produces a net rate of decline in viable bacteria of more than 0.57 per day. The remaining treatments may be merely prolonging the interval between cell division and exposing the effect of natural bacterial death. If cells divide, mutations cannot be ruled out. Since there are millions of patients and up to a million times a million viable M. leprae per patient, multiple-resistant mutants could arise during well-implemented MDT and be selected by well-implemented MDT.
The frequency of mutants should not be confused with the rate of mutation, as discussed above. For example, a mutation rate of 10-13 per cell division together with a rate of reverse mutation no greater than 10-11 implies that the equilibrium frequency of mutants is 1 in 100.4, 14 Dapsone-resistant mutants have been readily demonstrated, but mutants resistant to other drugs may well be missed unless sufficient time is allowed for their expectedly slow growth during monotherapy to be detected. Mutants simultaneously resistant to rifampin, clofazimine and dapsone cannot safely be presumed absent from the worldwide M. leprae population, which could number about (106 patients x 1011 viable M. leprae) viable 1017 M. leprae. The more widespread the use of multiple drugs, the greater the risk that multiple-drug resistance will pose a practical problem.
Noncompliance with prescribed treatment may be viewed as one aspect of a general human trait. Once the novelty of new regimens has worn off, it is unlikely that compliance with treatment will be much better than it was with older regimens. Rifampin-resistant M. leprae are relatively spared by RCD regimens,33 and this is even more certain if dapsone and clofazimine are omitted by the patient. Monthly injections of dapsone represent an important advance in the struggle against noncompliance. Monthly rifampin is unlikely to be sufficiently effective.37
In short, the greater the selective pressure exerted by drugs, the sooner multiple-drug resistance is expected to pose a practical problem. In the particular case of M. leprae, one way to delay multiple-drug resistance would be to restrict AMC to the minimum number of drugs and doses required to produce the desired therapeutic effect. Overtreatment could prove equivalent to overselection of drug-resistant M. leprae.
Multiple-drug resistance, when it eventually emerges, is predicted to occur disproportionately among "slow responders;" in the remaining patients, host immune responses should play the part of an additional "drug," that also a "drug" free from the risk of resistance. It is not too early to start a special worldwide effort to monitor "slow responders," including those who have optimistically been released from AMC after relatively long periods of dapsone monotherapy or MDT.
WHO SHOULD RECEIVE AMC?
The benefits of AMC are so obvious that the risks associated with anti-M. leprae treatment are rarely discussed, even in the case of persons with self-healing infection. As many as 75% of all persons with newly detected tuberculoid30 signs of infection healed themselves without residual damage in the pre-dapsone era. 38, 41 This claim could not be shown inconsistent with the observed prevalence of signs and disabilities of leprosy at the introduction of dapsone, according to one recent mathematical model.4
The distinction between self-healing and other patients with tuberculoid signs depends on valid prognostic signs. Unfortunately, very few attempts have been made (and none published in sufficient detail) to test the validity of well-known prognostic signs, such as the following: 42, 43 1) more than two lesions or lesions on more than one part of the body; 2) lesions that are poorly demarcated; 3) lesions which yield acid-fast bacilli; 4) anesthesia in areas distant from the skin patch; and 5) a negative response to lepromin.
A recent report suggests that the regularity of AMC is positively associated with the risk of future disability.1 This possible increase in risk of disability is only clearly justifiable in patients who rely on AMC to inhibit M. leprae.
The problem is to confine AMC to those infected persons in whom the benefits outweigh the associated risks. If about 25% of those with tuberculoid signs deteriorate without AMC, this 25% should not escape the "net" of unfavorable prognostic signs. Those without a single unfavorable prognostic sign might then be regarded as "lowrisk" tuberculoid patients. While "high-risk" tuberculoid patients might justifiably be put on routine AMC, surveillance and antiinflammatory therapy (when needed, together with anti- M. leprae cover) might prove more effective in "low-risk" tuberculoid patients.
The relative importance of antiinflammatory and anti- M. leprae chemotherapy in "low-risk" tuberculoid patients deserves some attention. A proposal has been made for a trial in which two groups of patients with "low-risk" tuberculoid signs would be closely monitored and would be withdrawn from the trial at the first suspicion of impending adverse outcome.4 The outcome of interest would therefore be "unfavorable withdrawal" rather than deterioration of leprosy. AMC would be freely used after "unfavorable withdrawal." Before that, one group in this double-blind trial would receive a placebo instead of AMC. A more detailed discussion of the issues involved will be found elsewhere.4
Superfluous or harmful AMC among persons with "low-risk" tuberculoid signs may be criticized on other grounds than those mentioned already. Not only do such persons claim an important proportion of scarce resources, but also they are likely to form an increasing proportion of newly detected cases as surveillance is improved. However, improved surveillance is necessary to detect progressive forms of leprosy before disability develops. Therefore prognostic signs among persons with tuberculoid signs deserve emphasis. The criteria quoted above may be regarded as a useful point of departure for better prognostic signs.
CLASSIFICATION OF LEPROSY FOR AMC POLICIES
There are many well-considered classifications of leprosy in use.28, 30 However, they seem to underemphasize important heterogeneities at the two extremes of the spectrum: the subdivision of the tuberculoid30 group into "high-risk" and "low-risk" groups and the subdivision of the lepromatous30 group into "slow responders" and others. "Low-risk" tuberculoid patients could prove to be harmed rather than benefitted by AMC.1 "Slow responders" may well require a prolongation of AMC and a life-long surveillance for re-infection (or endogenous relapse).
All leprosy patients apart from these two extreme groups ("low-risk" tuberculoid and "slow responder" lepromatous) could be tried on the same regimen of AMC. Such a policy could guard against under-treatment of atypical "paucibacillary"29 patients and against over-treatment of "multibacillary"29 patients who are not "slow responders."
In short, the existing classifications of leprosy28, 30 are adequate provided due importance is given to the "low-risk" tuberculoid group and the "slow responders" among lepromatous patients.
RECURRENT VARIABLE COST OF AMC
The cost of AMC has been one of the most neglected areas of leprosy research. This is unfortunate. The enthusiasm and prosperity of donors can be relied upon only in the relatively short term. Unrestrained expenditure for AMC cannot be maintained without eventual damage to other components of the leprosy control program, and even the eradication of leprosy services on grounds of cost.
New regimens involve differing expenditures. Predictions of cost might eventually be overtaken by circumstances, but they could well be an improvement over neglect of cost. Those expenditures which are likely to vary in the medium term (two decades) according to the regimen used are of most interest. Further, the shorter AMC is, the fewer the patients receiving AMC at any given time. Once the backlog of patients has been released from AMC of fixed duration, a steady-state is attained, dependent on the incidence rate of new infections. Predictions will be confined to recurrent variable costs in the steady-state, assuming that AMC will continue to form one component of leprosy services.
The conceptual model for calculations has been fully described elsewhere.4 Briefly, calculations depend not only on the cost of drugs (including details such as transportation, freight and insurance for imported drugs) but also on the cost of drug delivery. A wide range of assumptions are used to allow for prevailing uncertainties. For each regimen considered, a variety of estimates are obtained for the minimum and maximum predicted costs, respectively.
Costs were analyzed to arrive at predictions for four regimens: a) long-term dapsone monotherapy, b) WHO-MDT,29 c) -year dapsone monotherapy (2 years for all except "slow responders"), and d) RCD-MDT lasting 5 months or less. Pricing policies for ofloxacin were not known at the time of these calculations. Also, the cost of imported drugs has escalated since these calculations because of the marked devaluation of the Indian rupee in recent times.
Figures 7 and 8 both show that for the entire range of assumptions used, the WHO Study Group regimens29 (WHO-MDT) are far more expensive in the steady-state than even long-term dapsone monotherapy. The reduction in duration of the WHO-MD T MB regimen to only 2 years is insufficient to lower the cost of the WHO-MDT regimens to the level of long-term dapsone monotherapy. Newly suggested short-term regimens are even more economical than long-term dapsone monotherapy. The contrast between the costs of WHO-MDT and single-rifampin plus 2-year dapsone regimens is likely to be striking. RCD regimens lasting 5 months or less could be made as economical as single rifampin with 2-year dapsone (1 RD), provided extra costs (such as cash bonuses to staff, blister packs, etc.) were kept to a minimum.
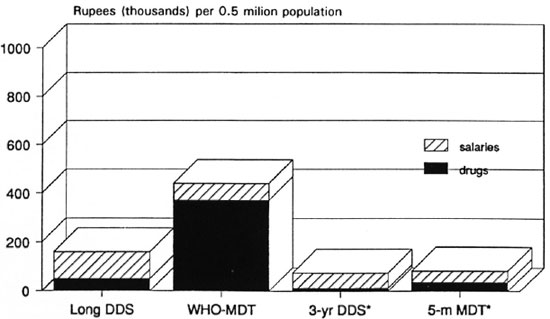
Fig. 7. Recurrent variable cost of anti-M. leprae chemotherapy predicted for 4 regimens: minimum steady-state cost in high-incidence area of India, with drug delivery by "vertical" program [5-m MDT = single rifampin, daily clofazimine and dapsone (DDS) for 5 months].
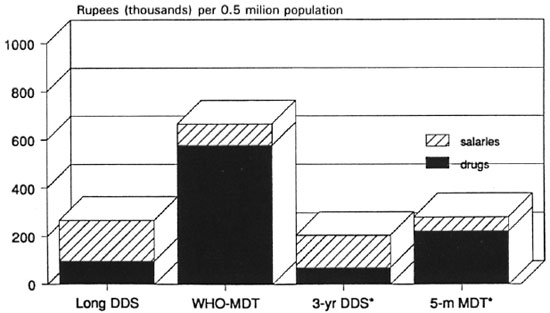
Fig 8. Recurrent variable cost of anti-. M. leprae chemotherapy predicted for 4 regimens: maximum steadystate cost in high-incidence area of India, with drug delivery by "vertical" program [5-m MDT = single rifampin, daily clofazimine, and dapsone (DDS) for 5 months].
Single rifampin plus 2-year dapsone (1 RD) regimens deserve a fair trial, being both biologically rational and economical. Five-month or shorter RCD regimens are for further into the future, unless trials of 14- or 18-month RCD regimens are omitted. Five-month regimens are likely to be useful only at the extremes of population density (e.g., urban or mountainous areas) where fewer patients would entail less time spent in reaching the patients. In moderately populated areas, a small number of patients scattered over a wide area might well require the same amount of travelling time from the leprosy staff as do far more patients.
Leprosy services, particularly important components other than AMC, are likely to be strengthened if AMC is made shorter as well as less costly. An unrestrained expenditure on AMC, on the other hand, could weaken other components of the leprosy services or even lead to their abolition.
CONCLUSIONS
AMC (anti- M. leprae chemotherapy) is arguably only one component of leprosy services whose chief mandate is to prevent disability. Cost-effective and biologically rational regimens of AMC, such as the following, deserve trial in the order mentioned.
Rupees (thousands) per 0.5 milion population
1. 1RD (single initial rifampin 1200 mg plus monthly injections of dapsone for 24 months).
2. RCD ( 100 daily doses of rifampin 600 mg plus clofazimine 50 mg daily for 8 months plus monthly dapsone injections for 14 months).
These regimens should be tried in all classes of patients except "slow responder" lepromatous patients (who might need prolongation of AMC and life-long surveillance) and "low-risk" tuberculoid patients (in whom AMC could prove harmful). Should these regimens, based on pessimistic assumptions, prove adequate, more adventurous regimens such as the following might be approached by a gradual reduction in the duration of AMC.
3. 1RCD (single initial rifampin 1200mg plus clofazimine 50 mg daily for 5 months plus dapsone monthly injections for 5 months).
4. 70RD (70 doses of daily rifampin 600 mg plus monthly injections of dapsone for 4 months) in non-LL patients who find clofazimine unacceptable.
5. Short RCD (daily rifampin 600 mg for 3 months plus daily clofazimine 50 mg for 3 months plus monthly injections of dapsone for 3 months).
Ofloxacin may find some use in a minority of Indian patients, those "slow responders" who relapse after RCD regimens or who refuse clofazimine. Affluent countries or individuals may wish to experiment with regimens such as the following.
6. ORD (daily ofloxacin 400 mg for 7 months plus rifampin 600 mg daily for 4 months plus monthly injections of dapsone for 7 months). This regimen is based on pessimistic assumptions. The most optimistic assumptions permit the following OR D regimen for non-LL patients.
7. Short OR D (ofloxacin 400 mg daily for 7 weeks plus single initial rifampin 1200 mg plus monthly injections of dapsone for 2 months).
"Low-risk" tuberculoid patients (without even one of the suggested unfavorable prognostic signs) deserve a controlled trial with close monitoring and withdrawal from the trial prior to deterioration of leprosy to evaluate the claim that AMC increases the risk of disability in such patients.
"Slow responder" lepromatous patients are probably identifiable by simple criteria, such as their BI before treatment and the rate of decrease in their BI during effective treatment. These patients run a much higher-than-average risk of re-infection, endogenous relapse and failure of treatment with multiple-resistant mutant M . leprae. The exclusion of such patients from trials of new regimens (e.g., on grounds of dapsone resistance) or failure to analyze their results separately would underestimate their risk of treatment failure and relapse/re-infection. "Slow responders" may well require prolonged treatment and life-long surveillance.
Well-implemented MD T with drugs no more effective than rifampin and ofloxacin cannot be relied upon to prevent multipledrug resistance of M. leprae.
WHO-MD T is among the least cost-effective options for multiple-drug therapy. It is unlikely to prove sustainable in India (where most new cases arise) except at the cost of other components of leprosy services, or at the cost of the leprosy program itself.
- Joel G. Almeida, M.B.B.S., Ph.D. (Lond.)
Consultant in Infectious Diseases
P.O. Box 25
Kodaikanal 624 101, India
1. Radhakrishna, S. and Nair, N. G. K. Association between regularity in dapsone (DDS) treatment and development of deformity. Int. J. Lepr. 55(1987)425-434.
2. Karat, S., Rao, P. S. S. and Karat, A. B. A. Prevalence of deformities and disabilities among leprosy patients in an endemic area. Part II. Nerve involvement in the limbs. Int. J. Lepr. 40(1972)265-270.
3. Srinivasan, H., Rao, K. S. and Shanmugham, N. Steroid therapy in recent "quiet nerve paralysis" in leprosy-report of a study of twenty-five patients. Lepr. India 54(1982)412-419.
4. Almeida, J. G. Leprosy: short-term treatment. A quantitative scientific basis for anti-microbial chemotherapy in control programmes. Kettering, U.K.: Plateau, 1960, p. 106.
5. Waters, M. F. R., Rees, R. J. W., McDougall, A. C. and Weddell, A. G. M. Ten years of dapsone in leprosy; a clinical, bacteriological and histological assessment and the finding of viable leprosy bacilli. Lepr. Rev. 45(1974)288-298.
6. Toman, K. Bacterial persistence in leprosy. Int. J. Lepr. 49(1981)205-217.
7. Finney, D. J. Assays based on quantal responses. In: Statistical Method in Biological Assay. 3rd cdn. London: Charles Griffin and Co. Ltd., 1978, pp. 394-401.
8. McDermott-Lancaster, R. D., Ito, T., Kohsaka, K., Guelpa-Lauras, C. C. and Grossct, J. H. Multiplication of M. leprae in the nude mouse, and some applications of nude mice to experimental leprosy. Int. J. Lepr. 55(1987)889-895.
9. Ji, B., Chen, J.. Lu, X., Wang, S., Ni, G., Hou, Y., Zhou, D. and Tang, Q. Antimycobacterial activities of two newer ansamycins, R-76-1 and DL 473. Int. J. Lepr. 54(1986)563-577.
10. Grosset. J. H. and Guelpas-Lauras, C.-C. Activity of rifampin in infections of normal mice with M. leprae. Int. J. Lepr. 55(1987)847-851.
11. Colston, M. J., Hilson, G. R. F. and Banerjee, D. K. The "proportional bactericidal test": a method for assessing bactericidal activity of drugs against M. leprae in mice. Lepr. Rev. 49(1978)7-15.
12. Colston, M. J., Hilson, G. R. F. and Lancaster, R. D. Intermittent chemotherapy of experimental leprosy in mice. Am. J. Trop. Med. Hyg. 29(1980)103-108.
13. Grosset, J. H., Ji, B., Guelpa-Lauras, C.-C, Perani, E. G. and N'Deli, N. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58 (1990)281-295.
14. Elseth, G. D. and Baumgardncr, K. D. Population Biology. New York: van Nostrand, 1981.
15. Rees, R. J. W. Recent bactériologie, immunologic and pathologic studies on experimental human leprosy in the mouse foot pad. Int. J. Lepr. 33(1965)646-657.
16. Hall, B. G. Evolution of new metabolic functions in laboratory organisms. In: Evolution of Genes and Proteins. Nei, M. and Koehn, R. K., eds. Sunderland, Massachusetts: Sinauer Assoc., Inc., 1983, pp. 234-257.
17. Almeida, J. G., Chacko, C. J. G., Christian, M., Taylor, P. M. and Fritschi, E. P. DDS-resistant infection among leprosy patients in the population of Gudiyatham Taluk, South India. Part 3. Prevalence, incidence, risk factors and interpretation of mouse foot pad test results. Int. J. Lepr. 51(1983)366-373.
18. Almeida, J. G., Christian, M. and Chacko, C. J. G. Results of long-term domiciliary dapsone (DDS) monotherapy for lepromatous leprosy in Gudiyatham Taluk, South India. Int. J. Lepr. 51(1983)385-386.
19. Warndorff-van Diepen, T. Clofazimine-resistant leprosy: a case report. Int. J. Lepr. 50(1982)139-142.
20. Levy, L. Clofazimine-resistant M. leprae. Int. J. Lepr. 54(1986)137-140.
21. Hastings, R. C. and Morales, M. J. Observations, calculations and speculations on the growth and death of M. leprae in vivo. Int. J. Lepr. 50(1982)579-582.
22. Shepard, C. C. and McRae, D. H. Mycobacterium leprae in mice: minimal infectious dose, relationship between staining quality and infectivity, and effect of cortisone. J. Bacteriol. 89(1965)365-372.
23. McRae, D. H. and Shepard, C. C. Relationship between the staining quality of M. leprae and infectivity for mice. Infect. Immun. 3(1971)116-120.
24. Rees, R. J. W. and Valentine, R. C. The appearance of dead leprosy bacilli by light and electron microscopy. Int. J. Lepr. 30(1962)1-9.
25. Wilson, G. S. The proportion of viable bacteria in young cultures with especial reference to the technique employed in counting. J. Bacteriol. 7(1922)405-446.
26. Pirt, S. J. Death of cells in growing cultures. In: Principles of Microbial and Cell Cultivation. Oxford: Blackwell Scientific Publications, 1975, 57-62.
27. Levy, L. Studies of the mouse foot pad technique for cultivation of M. leprae. 2. Doubling time during logarithmic multiplication. Lepr. Rev. 47(1976)237-245.
28. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
29. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982, p. 33. Tech. Rep. Ser. 685.
30. Madrid classification. Report on the Madrid Congress technical resolutions. Int. J. Lepr. 21(1953)504-516.
31. Ridley, D. S. A logarithmic index of bacilli in biopsies. 2. Evaluation. Int. J. Lepr. 35(1967)187-193.
32. Ridley, D. S. Bacterial indices. In: Leprosy in Theory and Practice. 2nd edn. Cochrane, R. G. and Davey, T. F., eds. Bristol: John Wright and Sons, 1964, pp. 620-622.
33. Almeida, J. G. and Chacko, C. J. C. Computerised mathematical model of M. leprae population dynamics during multiple drug therapy. Indian J. Lepr. 57(1985)780-789.
34. Pieters, F. A., Woonink, F. and Merkus, F. W. A field trial among leprosy patients in Nigeria with depot injections of dapsone and monoacetyldapsone. Int. J. Lepr. 56(1988)10-20.
35. Lowe, J. Chemotherapy of leprosy; late results of treatment with sulphonc, and with thiosemicarbazone. Lancet 2(1954)1065-1068.
36. Dietrich, M., Rangaraj, J., Ganapati, R., et al. Combination therapy vs monotherapy in BL and LL patients: a prospective randomised multicente study. In: Abstracts of the XIII International Leprosy Congress. The Hague. 1988. Quad. Coop. Sanit. 9(1988)629(PQ656).
37. Almeida, J. G. How effective is monthly rifampin? (Letter) Int. J. Lepr. 60( 1992)81-82.
38. Cochrane, R. G., deSimon, D. S. and Fernando, A. C. Preliminary observations on childhood leprosy in Ceylon. Int. J. Lepr. 5(1937)61-65.
39. Davcy, T. F. A repeated leprosy survey in southeastern Nigeria; the progress of untreated cases of leprosy. Int. J. Lepr. 9(1941)77-86.
40. Lara, C. B. and Nolasco, J. O. Self-healing, or abortive and residual forms of childhood leprosy and their probable significance. Int. J. Lepr. 24(1956) 245-263.
41. Guinto, R. S., Doull, J. A., Bancroft, H. and Rodriguez, J. N. A field study of leprosy in Cordova, Philippines. resurvey in 1941 after eight years. Int. J. Lepr. 19(1951)117-136.
42. Wade, H. W. and Rodriguez, J. N. A. A description of leprosy, its etiology, pathology, diagnosis and treatment. Manila: Culion Medical Board, Philippine Health Service, 1928.
43. Arnold, H. L. The Madrid classification. (Letter) Int. J. Lepr. 22(1954)473.