- Volume 60 , Number 2
- Page: 280–3
Avidin-biotin immunoblotting studies on reactivity of leprosy dera with Mycobacterium leprae antigen
To the Editor:
Mycobacterium leprae, the causative agent of leprosy, has the most complex structure of all the mycobacteria (3). Fewer antigenic components in the M . leprae extracts have been shown in comparison with other cultivable mycobacteria by gradient gel electrophoresis (5). Crossed-immunoelectrophoresis of M. leprae sonicate developed using sera from leprosy patients has shown about seven or eight antigenic bands (7). However, by immunodiffusion about 11 to 12 antigenic components could be detected (11). Immunoblotting studies using sera from pooled lepromatous leprosy (LL) patients have revealed about five antigenic bands in the cell-free extract of M. leprae (1). In this communication, we report an avidin-biotin immunoblotting for analyzing the M. leprae antigenic components using sera of leprosy patients.
Collection of serum. Blood samples for the study were collected from untreated leprosy patients attending Central JALMA Institute for Leprosy (CJIL), Agra, India. Patients were clinically classified on the criteria of Ridley and Jopling (9). Blood samples from healthy laboratory volunteers served as controls. Sera were separated and stored at - 70ºC. Normal healthy sera from a nonendemic region for leprosy were kindly provided by Dr. H. D. Engers, World Health Organization, Switzerland.
M. leprae antigen. The cell-free extract of armadillo-derived, purified M. leprae (2) was kindly provided by Dr. R. J. W. Rees from the IMMLEP (WHO) bank, London.
SDS-Polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE of the M . leprae antigens was carried out according to the method of Laemmli (8) in 12% homogenous gel along with molecular weight markers (Pharmacia, Sweden) at a constant current in a discontinuous buffer system using a Pharmacia PAGE apparatus.
Immunoblotting. Immunoblotting was done as per the method of Towbin, et al. (12). Here the SDS was removed from the electrophoresed gel by soaking it into blotting buffer for ½ hr with two changes. The gel containing M. leprae proteins was later blotted onto nitrocellulose paper (S & S, Germany) using a Pharmacia electroblotting apparatus.
Avidin-biotin system. Biotin is covalently coupled to free amino groups of anti-IgG/ IgM, and avidin is conjugated to horseradish peroxidase. To each molecule of biotin many molecules of avidin bind by strong noncovalent interaction, thus making the avidin-biotin interaction a convenient and highly sensitive system (4).
Enzyme reaction. Strips of about 3-mm breadth were cut from the antigen-containing nitrocellulose paper, and the free sites were blocked with 5% fat-free casein solution [or 3% bovine serum albumin (BSA) solution] in phosphate buffered saline (PBS), pH 7.4. The blocked nitrocellulose strips were then incubated for 2 hr at room temperature in 2 ml casein/BSA solution with 20 µl of different test sera on a horizontal shaker (Rockomat, Switzerland). Strips were then washed five times with PBS and reincubated for 2 hr in 2 ml of casein/PBS solution with 1:1000 antihuman biotinylated IgG (Sigma, U.S.A.). The strips were washed again five times, and were incubated for 2 hr more in 2 ml of casein/PBS with peroxidase-conjugated avidin (1:1000). The strips were washed again five times, and were incubated in α -4 chloronaphthol (Sigma, U.S.A.) substrate solution for about hr, or until the contrasting reaction was observed. The strips were then rinsed in deionized water, dried, and photographed immediately. An enzyme reaction was also developed with peroxidase-conjugated antihuman IgG (conventional system) under similar conditions.
In the avidin-biotin system the number of antigens detected in the cell-free extracts of M. leprae using sera from different types of leprosy were as follows: 12-14 antigenic bands by using lepromatous (LL) sera; 12 using borderline lepromatous (BL) sera; 10 using midborderline (BB) sera; 5 using borderline tuberculoid (BT) sera; and 1 using tuberculoid (TT) sera (Fig. 1).
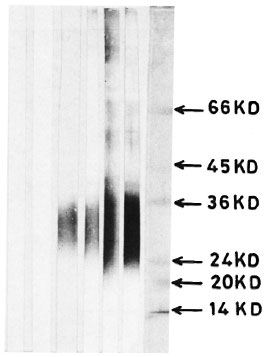
Fig. 1. Immunoblotting of M. leprae antigens on nitrocellulose paper and reaction with scrum samples using the conventional developing technique: Lane 1 = control scrum; lane 2 = TT serum; lane 3 = BT serum; lane 4 = BB serum: lane 5 = BL serum; lane 6 = LL serum; lane 7 = molecular weight markers.
In a conventional system (without avidin-biotin), the number of antigens detected using sera from different types of leprosy were as follows: 10 using LL, 10 using BL and 4 using BB sera. There was no reactivity using TT sera; only one antigenic component could be detected using BT sera (Fig. 2).
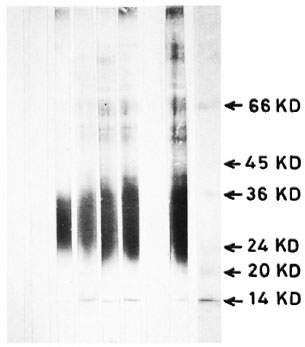
Fig. 2. Immunoblotting of M. leprae antigens on nitrocellulose paper and reaction with serum samples using the avidin-biotin technique: Lane 1 = control scrum; lane 2 = TT scrum; lane 3 = BT scrum; lane 4 = BB scrum; lane 5 = BL serum; lane 6 = LL serum; lane 7 = LL pooled scrum absorbed with M. leprae ; lane 8 = pooled LL scrum absorbed with Escherichia coli; lane 9 = molecular weight markers.
Reactivity to the 65-kDa component was observed using BT, BB, BL and LL types of leprosy sera as seen by the avidin-biotin assay. However, it could hardly be detected in TT, BT, and BB sera by conventional blotting procedures. Reactivity to the 60kDa and 48-kDa proteins was observed in only BB, BL and LL sera by the avidinbiotin assay, and in only BL and LL sera by the conventional assay.
Reactivity to the 30-40-kDa component was observed in all of the leprosy patients by the avidin-biotin assay. However, by the conventional assay the reactivity was not seen with TT leprosy sera.
In the present study as many as 12-14 antigen-reacting components could be seen using LL sera by the avidin-biotin assay in comparison with five components in pooled LL sera as shown by autoradiography (1) and about 10 components by conventional immunoblotting. Klatser, et al. (6) could not identify M. leprae antigen using sera from tuberculoid cases. In the present study using the avidin-biotin system, BT sera could identify as many as five antigenic components. By the SDS-polyacrylamide gel. electrophoresis immunoperoxidase technique (SGIP) about 12 antigenic components are reported in the cell-free extract of M. leprae, but the assay is cumbersome, delicate, and difficult to reproduce.
In the present study the identification of the number of antigens was higher, as expected, by LL sera than by BT sera. However, minor variations in the identification of a number of antigens were observed within the same groups. The reactivity to the 65-kDa component of M . leprae was noted in most of the leprosy sera tested by the avidin-biotin system. This protein has been reported to be ubiquitous since it could be found in all of the bacteria (10).
The detection of a higher number of antigen-reacting components in the present study could be attributed to the augmented sensitivity of the assay using the avidin-biotin system.
- Shripad A. Patil, Ph.D.
Assistant Research Officer
- Lalita Shivraj, Ph.D.
Research Officer
- Bhawncshwar K. Girdhar, M.D.
Deputy Director
- Utpal Sengupta, Ph.D.
Deputy Director
Central JALMA Institute for Leprosy
P.O. Box 31
Taj Ganj, Agra 282001, India
REFERENCES
1. CHAKRABORTY, A. K., MAIRE, M. and LAMBERT. P. H. SDS-PAGE analysis of M. leprae protein antigens reacting with antibodies from the sera from lepromatous patients and infected armadillos. Clin. Exp. Immunol. 49(1982)523-531.
2. DRAPER, P. Protocol 1/79. Purification of. M. leprae. In: Report of the Enlarged SC Meeting, Geneva, 7-8 February 1979. Annex 1. Geneva: World Health Organization, 1980. TDR/IMMLEP-SWG
3. GRANGE, J. M. Mycobacterial Diseases. Current Topics in Infection No. 1. London: Edward Arnold (Publishers), 1980.
4. GUESDON, J. L., TERNYNCK, T. and AVRAMEAS, S. The use of avidin-biotin interaction in immunoenzymatic techniques. J. Histochem. Cytochem. 27(1979)1131-1139.
5. HARBOE, M., CLOSS, O., BJORVATN, B., KRONVALL, G. and AXELSON, N. H. Antibody response in rabbits to immunization with Mycobacterium leprae. Infect. Immun. 18(1977)792-805.
6. KLATSER, P. R., VON RENS, M. M. and EGGELTE, T. A. Immunochemical characterization of Mycobacterium leprae antigens by the SDS-polyacrylamide gel electrophoresis immunoperoxidase technique (SGIP) using patients' sera. Clin. Exp. Immunol. 56(1984)537-544.
7. KRONVALL, G., BJUNE, G., STANFORD, J. L., MENZEL, S. and SAMUEL, D. Mycobacterial antigens in antibody responses of leprosy patients. Int. J. Lepr. 43(1975)299-306.
8. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227(1970)680-685.
9. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
10. SHINNICK, T. M., VODKIN, M. H. and WILLIAMS, J. C. The Mycobacterium tuberculosis 65-kiIodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli Groel protein. Infect. Immun. 56(1988)446-451.
11. STANFORD, J. L., ROOK, G. A. W., CONVIT, J., GODAL, T., KRONVALL, G., REES, R. J. W. and WALSH, G. P. Preliminary taxonomic studies on the leprosy bacillus. Br. J. Exp. Pathol. 56(1976)579-585.
12. TOWBIN, H., STAEHAELIA, T. and GODON, G. Electrophoretic transfer of proteins from polyacrylamide gel to nitrocellulose sheets, procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76(1979)4350-4354.
Reprint requests to Dr. Patil at his present address: Department of Microbiology, NIMHANS, P.O. Box 2900, Bangalore 560029, India.
Dr. Shivraj's present address: Cancer Research Institute, San Diego, California, U.S.A.