- Volume 60 , Number 2
- Page: 283–5
Autoradiographic evidence of reduced epidermal cell proliferation in leprosy patients
To the Editor:
Leprosy is an infectious disease having unique peculiarities among which are an early and preferential peripheral nerve impairment and a continuous spectrum of clinical manifestations between two poles, tuberculoid and lepromatous. The changes in nerves (2, 4-7, 9, 16, 22)and the dermal inflammatory process (3, 8, 11, 13, 17, 28) have been studied using ultrastructural, immunocytochemical and cell kinetic methods. However, epidermal changes scarcely have been studied (11, 13, 14, 21). Mitsuda (18, 19) suggested that the differences in the local response to leprosy antigens could be due to the resistance of neuromacular leprosy patients as opposed to nodular leprosy patients. It is now known that it is the state of the cellular immunity of the patient that determines the appearance of a specific clinical manifestation of the disease (10). Thus, at the tuberculoid pole the patient has lesions with a high ratio of T4 (helper)/T8 (suppressor) lymphocytes and with levels of interleukins 1 and 2 and of interferon-gamma which allow him to present an inflammatory response with cells able to kill the bacillus. At the lepromatous pole are those patients whose macrophages are just hosts to the bacillus (13). The number of Langerhans' cells and keratinocytes as well as the Ia+ phenotypic expression by keratinocytes also correlate with the clinical and morphologic spectrum of leprosy (10, 12-15, 26, 27)
One of the most important clinical features of this disease is the occurrence of trophic ulcers. Although it has been suggested that they might be caused by cutaneous anesthesia, decreased epidermal proliferation could also be their cause.
In the present communication, preliminary quantitative data on epidermal cell proliferation determined by biopsies of cutaneous lesions of leprosy patients, using tritiated thymidine as a marker, are reported.
Leprosy patients, newly diagnosed and untreated, 10 to 60 years old, of either sex, clinically classified as tuberculoid (TT), borderline tuberculoid (BT), indeterminate (Ind), borderline lepromatous (BL), and lepromatous (LL), received clear and careful explanations about the purpose of the study and the procedure to be used. After giving their written consent, they received a 0.1 ml 3H-thymidine (100 µCI/local, Sp. Act. 6.7 Ci/mol; Dupont, U.S.A.) intradermal injection at a site showing a cutaneous anesthetic lesion and at an unafTected contralateral site. The injection sites were either on the trunk or limbs. One hr later, 4.0-mm punch biopsies were made at both injection sites. The whole procedure was always performed during the morning.
The biopsy fragments were fixed in 10% formaldehyde, containing 10% glycerine, for 24 hr. Paraffin sections, 4 µm- thick, were taken either for autoradiography or for staining with hematoxylin and eosin (H&E) or Fite-Faraco. For autoradiography every fifth section was taken so as to avoid counting the same cell twice. The sections were covered with Kodak AR10 stripping film according to Pelc (23), exposed for 28 days, developed, and stained with H&E.
The histopathologic classification of the patients was made according to Ridley and Jopling. (25) The index of labeled cells was determined as the ratio between the number of basal and suprabasal labeled cells and the total number of basal cells counted. A preliminary cumulative index established by us showed that at least five silver grains were considered to be labeled. Areas with artifacts or high background were excluded from counting.
Statistical analysis of the data was done using the Mann-Whitney U test, with a level of significance set at 5%.
Our data (The Table) show that in the cutaneous lesions of leprosy patients epidermal cell proliferation, expressed by the index of labeled cells as compared to that of contralateral areas without lesions, is reduced significantly. This reduction is particularly striking in lepromatous patients.
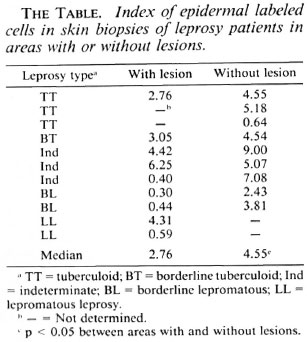
Our results suggest that the impaired epidermal repair which occurs in leprosy patients could be due to a reduced epidermal cell proliferation. Our data concur with those of Kaplan, et al. (14, 15)- which showed that in PPD-induced dermal reactions epidermal thickening and changes from Ia- to Ia+ expression in keratinocytes occurred in tuberculoid patients but not in lepromatous patients. In such patients, whose lesions have reduced levels of IL-2 (11), the intradermal injection of recombinant IL-2 leads to extensive epidermal thickening (11). Epidermal keratinocyte proliferation in mice is dependent upon IL-1 (26). It is thus probable that the decreased epidermal cell proliferation, very striking in our lepromatous leprosy patients, could be due to a lack or deficiency of lymphokines.
- Maria Heloisa Rached Palmero, M.D.
Sérgio Zucoloto, M.D., Ph.D.
Professor
Department of Pathology
Faculty of Medicine
University of São Paulo
14.049 Ribeirão Preto, SP, Brazil
- Ithamar Vugman, M.D., Ph.D.
Professor
Department of Histology
Institute of Biomedical Science
University of São Paulo
São Paulo, Brazil
- Raul Negrão Fleury, M.D., Ph.D.
Department of Pathology
Faculty of Odontology
University of São Paulo
Bauru, Brazil
- Emilia Simões Trad, M.D., Ph.D.
Department of Medicine
Dermatology Section
University of São Paulo
14.049 Ribeirão Preto, SP, Brazil
Acknowledgment. The authors would like to thank Mrs. Márcia Aparecida Oliva Destido for typing the manuscript. Financial support was provided by CNPq and FAPESP.
REFERENCES
1. ARNOLDI, J., GERDES, J. and FLAD, H.-D. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am. J. Pathol. 137(1990)749-753.
2. BODDINGIUS, J. Ultrastructural and histophysiological studies on the blood-nerve barrier and perineural barrier in leprosy neuropathy. Acta Neuropathol. 64(1984)282-296.
3. BURCHARD, G.D. and BIERTHER, M. An electron microscopic study on macrophages and lymphocytes in lepromatous and borderline leprosy. Int. J. Lepr. 53(1985)53-64.
4. CHANDI, S.M. and CHAKO, C. J. C. An ultrastructure study of dermal nerves in early human leprosy. Int. J. Lepr. 55(1987)515-520.
5. DASTUR, D. K., RAMAMOHAN, Y. and SHAH, J. S. Ultrastructurc of lepromatous nerves. Neural pathogenesis in leprosy. Int. J. Lepr. 41(1973)47-80.
6. DASTUR, D. K. and RAZZAK, Z. Degeneration and regeneration in teased nerve fibers. I-Leprous neuritis. Acta Neuropathol. 18(1971)286-298.
7. FLEURY, R. N. and BACCHI, C. E. S-100 protein and immunopcroxidasc technique as an aid in the histopathologic diagnosis of leprosy. Int. J. Lepr. 55 (1987) 338-344.
8. GIMENES, M. F., GIOLI, I. and TAUSK, F. A. Differential expression of Langerhans cells in the epidermis of patients with leprosy. Br. J. Dermatol. 121(1989)19-26.
9. JOB, C. K. Pathology of peripheral nerve lesions in lepromatous leprosy; a light and electron microscopic study. Int. J. Lepr. 39(1971)251-268.
10. KAPLAN, G. and COHN, Z. A. The immunobiology of leprosy. Int. Rev. Exp. Pathol. 28(1986)45-78.
11. KAPLAN, G., KIESSLING, R., TEKLEMARIAM, S., HANCOCK, G., SHEFTEL, G., JOB, C. K., CONVERSE, P., OTTENHOFF, T. H. M., BECX BLEU MINK, M., DIETZ, M. and COHN, Z. A. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J. Exp. Med. 169(1989)893-907.
12. KAPLAN, G., MATHUR, N. K., JOB, C. K., NATH, I. and COHN, Z. A. Effect of multiple interferon gamma injections on the disposal of Mycobacterium leprae. Proc. Natl. Acad. Sci. U.S.A. 86(1989)8073-8077.
13. KAPLAN, G., NUSRAT, A., SARNO, E. N., JOB, C. K., MCELRATH, J., PORTO, J. A., NATHAN, C. F. and COHN, Z. A. A cellular response to the intradermal injection of recombinant human gamma-interferon in lepromatous leprosy patients. Am. J. Pathol. 128(1987)345-353.
14. KAPLAN, G., NUSRAT, A., WITMER, M. D., NATH, I. and COHN, Z. A. Distribution and turnover of Langerhans cells during delayed immune responses in human skin. J. Exp. Med. 165(1987)763-776.
15. KAPLAN, G., WITMER, M. D., NATH, I., STEINMAN, R. M., LAAL, S., PRASAD, H. K., SARNO, E. N., ELVERS, U . and COHN, Z. A. Influence of delayed immune reactions on human epidermal keratinocytes. Proc. Natl. Acad. Sci. U.S.A. 83(1986)3469-3473.
16. KARANTH, S. S., SPRINGALL, D. R., LUCAS, S., LEVY, D., ASHBY, P., LEVENE, M. M. and POLAK, J. M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 157(1989)15-26.
17. MISTRY, N. F., BIRDI, T. J. and ANTIA, N. H. Leprae phagocytosis and its association with membrane changes in macrophages from leprosy patients. Parasite Immunol. 8(1986)129-138.
18. MITSUDA, K. On the value of a skin reaction to suspension of leprosy nodules. Jpn. Dermatol. 19(1919)697-708 (in SEHGAL, et al., 1989).
19. MITSUDA, K. Proceedings of Third International Congress of Leprology. Strasburg: International Congress of Leprosy, 1923 (in SEHGAL, et al, 1989).
20. NARAYANAN, R. B., BHUTANI, L. K., SHARMA, A. K. and NATH, I. Normal numbers of T6 positive epidermal Langerhans cells across the leprosy spectrum. Lepr. Rev. 55(1984)301-308.
21. OKHANDIAR, R. P., SINHA, E., SINHA, R. R. and MISHRA, A. D. Morphometric study of stratum corncum in leprosy. Indian J. Lepr. 61(1989)49-53.
22. PEARSON, J. M. H. and Ross, W. F. Nerve involvement in leprosy pathology; differential diagnosis and principle of management. Lepr. Rev. 46(1975)199-212.
23. PELC, S. R. the stripping film technique of autoradiography. Int. J. Appl. Radiol. Isotopes 1(1956)172-178.
24. REA, T. H., SHEN, J. Y. and MODLIN, R. L. Epidermal keratinocyte la expression, Langerhans cells hyperplasia and lymphocytic infiltration in skin lesion of leprosy. Clin. Exp. Immunol. 65(1986)253-259.
25. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
26. RISTOW, H.J. A major factor contributing to epidermal proliferation in inflammatory skin diseases appears to be interleukin 1 or a related protein. Proc. Natl. Acad. Sci. U.S.A. 84(1987)1940-1944.
27. SEHGAL. V. N., JOGINDER and SHARMA, V. K. Immunology of leprosy. A comprehensive survey. Int. J. Dermatol. 28(1989)574-584 (159 ref.).
28. WALLACH, D., FLAGEUL, B., BACH, M. A., and COTENOTT, B. The cellular content of dermal leprous granulomas; an immunological approach. Int. J. Lepr. 52(1984)318-326.
Reprint requests to Professor S. Zucoloto.