- Volume 59 , Number 3
- Page: 385–91
A clinical, immunological, and histological study of neuritic leprosy patients
ABSTRACT
An assessment has been made of 108 neuritic leprosy patients to find out if the number of affected nerves and the clinical presentations of these patients give any indication of the underlying severity (classification) of the disease. Detailed clinical recordings, skin smears, lepromin testing with Dharmendra antigen, and a leukocyte migration inhibition test (LMIT) using sonicated Mycobacterium leprae antigens were done in these patients. Nerve biopsies of available affected nerves were taken in 39 patients. The results show that neuritic leprosy patients also have a spectrum. However, none of the clinical parameters, including the number and distribution of affected nerves, the immune response and the nerve histology, were found to be inter-related. Further, even though ail of the patients were skin-smear négative, a significant proportion showed lepromatous histology and nearly two thirds had a moderate-to-heavy bacterial load within the nerves.RÉSUMÉ
On a réalisé une évaluation de 108 malades présentant une forme nerveuse de la lèpre pour étudier dans quelle mesure le nombre de nerfs affectés ainsi que la présentation clinique donnaient certaines indications de la sévérité sous-jacente (c'est-à-dire le type) de la maladie. Des examens cliniques détaillés, des frottis cutanés, un test à la lépromine avec l'antigène de Dharmendra, et un test d'inhibition de la migration leucocytaire (LMIT) in utilisant des antigènes soniqués de Mycobactcrium leprae onx été réalisés chez ces patients. Des biopsies des nerfs affectés ont été réalisées chez 39 patients. Les résultats montrent que les patients présentant une lèpre nerveuse sont aussi distribués le long d'un spectre. Cependant, on n'a pas trouvé de corrélation entre aucun des paramètres cliniques, y compris le nombre et la distribution des nerfs atteints, la réponse immunitaire et l'histologie des nerfs. De plus, bien que tous ces patients étaient au frottis cutané, une proportion non négligeable d'entre eux ont montré une histologie de type lépromateux, et près de deux-tiers d'entre eux avaient une charge bactérienne modérée à importante à l'intérieur des nerfs.RESUMEN
Se hizo un estudio de 108 pacientes con lepra neurítica para determinar si el número de nervios afectados y las características clínicas de estos pacientes dan alguna indicación de la severidad subyacente (clasificación) de la enfermedad. En estos pacientes se hicieron registros clínicos detallados, extendidos de linfa cutánea, pruebas con lepromina con el antigeno de Dharmendra, y pruebas de la inhibición de la migración de leucocitos usando como antígeno sonicados del Mycobacterium leprae. Se tomaron biópsias de los nervios afectados en 39 pacientes. Los resultados mostraron que los pacientes con lepra neurítica también tienen un espectro. Sin embargo, ninguno de los parámetros clínicos, incluyendo el número y la distribución de los nervios afectados, la respuesta inmune y la histología de los nervios, se encontraron interrelacionados. Además, aún cuando todos los pacientes fueron negativos en los extendidos de linfa cutánea, una significante proporción de ellos mostró una histología lepromatosa y casi 2 tercios tuvieron una importante carga bacteriana dentro de los nervios.Nerve affection, whether clinical or microscopic, is an important feature of leprosy and occurs in practically all types of disease in association with skin involvement. However, in leprosy there can also be nerve involvement without any primary skin change whatsoever. This form of disease has been called neuritic leprosy (4,6,11,14) (pure neural, primary neural, pure neuritic, primary neuritic). Although such patients constitute a significant proportion of all cases, this category of patients has not been thoroughly investigated. In the Ridley-Jopling classification (13), this category of patients has been considered to belong to the spectrum and are placed across it on the basis of the histological findings in the nerves. Since obtaining tissue to study the histology of the nerve is very often not feasible, many of these cases are simply considered to belong to the borderline tuberculoid (BT) group because several of these patients are lepromin positive and, on nerve biopsy, have shown a tuberculoid picture (5,10).
For purposes of field management, presently all leprosy patients are divided into paucibacillary (PB) and multibacillary (MB) groups, depending upon their classification (based on skin lesions) and their bacterial index (BI) of skin smears (16,17). Since all of the patients with neuritic leprosy are skin-smear negative for acid-fast bacilli (AFB), they are considered part of the PB group even though several cases on histology have shown borderline (3) and even lepromatous (LL) features and have had large numbers of bacilli within the nerves (2,8,12). Thus, it seemed reasonable to examine neuritic patients more closely to determine whether or not they are a homogeneous group.
The present work has been undertaken to see if the number and distribution of clinically affected nerves, the lepromin status, and other immunological tests give any clue as to the type of underlying nerve pathology.
MATERIALS AND METHODS
The study included 108 consecutive, untreated leprosy patients from an endemic area who had no skin manifestations but had definite nerve thickening with some nerve deficit. Nerve thickening was ascertained by comparison with the same nerve on the contralateral side. Patients with evidence of any skin patch(es) and infiltration or having a past history of any skin lesion(s) were excluded from the study, as were those whose skin smears showed AFB. A detailed history was taken and a physical examination was made to exclude patients with other types of polyneuropathy. Other diseases with thickening of the nerves, such as primary amyloidosis of nerves and progressive hypertrophic neuropathy, were excluded on the basis of intact deep tendon reflexes, lack of involvement of other organ systems, nerve biopsy (done in a proportion of the patients), the absence of a family history, and their rarity.
In each patient, a detailed examination was done to record the number and distribution of the affected nerves. Sensory impairment, motor deficit and disability/deformity status were assessed using standard methods. In brief, test tubes containing hot and cold water were used for thermal sensation testing, and nylon filaments and pin prick were used for assessing touch and pain perception, respectively. Individual muscle power was graded as per the method described by the Medical Research Council of London (3). Disability was recorded using the standard World Health Organization (WHO) grading scheme.
Skin smears were taken from three/four sites. In each case, the smears were taken from both earlobes and one or two skin sites with sensory deficit. The cell-mediated immune response of each patient was assessed by an intradermal injection of 0.1 ml of standard Dharmendra lepromin (15) and by the leukocyte migration inhibition test (LMIT) employing sonicated Mycobacterium leprae as antigen. A positive lepromin was defined as an erythema of > 10 mm in diameter 24-48 hr after injection. For the LMIT, a slightly modified technique described by Hudson and Hay (7) was employed. In brief, 10 jul capillaries were filled with washed human leukocytes adjusted to 5 x 107 cells/ml. After sealing the lower ends with paraffin, the capillaries were cen-trifuged at 150 x g x 5 min. Each capillary was cut at the upper edge of the cell pack and placed in a migration chamber filled with tissue culture medium with and without antigen (0.1 ml containing the sonicate of 106 killed M. leprae). The chambers were covered with cover slips and incubated for 24 hr at 37ºC. The area of migration was recorded by photographing the migrating leukocyte "fans" and projecting the image of the migration onto graph paper. A migration inhibition of 20% of control (area of migration in wells without antigen) was regarded as positive.
A nerve biopsy was done in all patients in whom thickened or palpable sensory nerves were available. Nerve biopsies were not done (for ethical reasons) in those patients who had only a partial loss of nerve function, in particular motor function. A skin biopsy from the region of the sensory deficit was also done. Both skin and nerve biopsies were collected in Formol-Zenker fixative, and paraffin blocks were made. Five-μm-thick sections were cut and stained with hematoxylin and eosin (H&E) and Fite's modification of the Ziehl-Neelsen stain.
RESULTS
The maximum number of clinically affected nerves in a single patient was found to be 10. Depending upon the clinical extent of the disease, the patients have been grouped into four categories: Group I -only one nerve thickened (49 patients); Group II-two or more nerves thickened but localized to only one limb (32 patients); Group III -two or more distant nerves thickened with asymmetrical distribution (nerve thickening involving another limb on the same or opposite side of the body) (10 patients); Group IV-two or more nerves thickened and symmetrical in distribution (17 patients).
The age of the patients ranged from 8 to 65 years (mean ± S.D. = 37.19 ± 11.3). Ninety-five percent of the patients (102 of 108) were males. The duration of symptoms ranged from 2 to 96 months (mean ± S.D. = 28.9 ± 31.1). Table 1 shows that about half of the patients presented within 1 year of the onset of the disease; 34% of the patients had disease of > 2 years' duration.
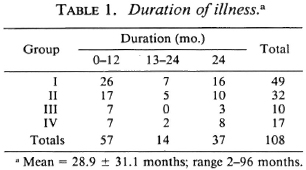
Table 2 shows that the predominant presenting symptom was sensory impairment. A fourth of the patients had paresthesia. Eighteen patients (16%) already had deformities at the time of their first visit to the clinic. Five came because of neuritic pain. In each of the four groups, several patients had more than one presenting symptom.

Sensory assessments showed that Group I and Group II patients had predominantly localized and/or regional sensory impairment limited to the distributions of nerve trunk(s). A large proportion of the patients in Group III and Group IV had the glove-and-stocking type of sensory deficit (Table 3). Loss of sensation on the extremities was not complete, since in several limbs there were small areas with intact sensation in distal parts. In a majority of the patients there were predominantly thermal deficits, although some showed only insensibility to pin prick. Furthermore, the loss/impairment of thermal sensitivity was usually slightly more extensive. No particular chronological pattern of sensory deficit was observed.
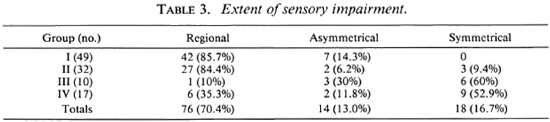
The ulnar nerve was the most commonly affected nerve. Among the 49 patients who had just one nerve affected, 31 had ulnar nerve thickening. Of the remaining 18 patients in Group I, 13 had thickening of the common peroneal (lateral popliteal) nerve, 3 had thickening of the superficial branch of the radial nerve at the wrist, 1 had posterior tibial nerve thickening, and 1 had enlargement of the superficial peroneal nerve. Among the patients with multiple nerve thickening, the ulnar nerve was again the most commonly affected nerve.
As mentioned earlier, although only 18 patients presented with complaints of deformities, 34 patients were found to have some degree of motor deficit. Out of these 34, 20 patients had disability of grade II or more. Table 4 gives the details of voluntary muscle testing and deformities. In all, 41 limbs of 34 patients showed motor deficit; 34 hands had ulnar paresis/palsy, 3 had both ulnar and median deficit, and 4 had common peroneal motor deficit. Two patients had trophic ulcers on their soles, and one had absorption of distal parts of the little and ring fingers. In all, eight patients had more than one limb deformed.
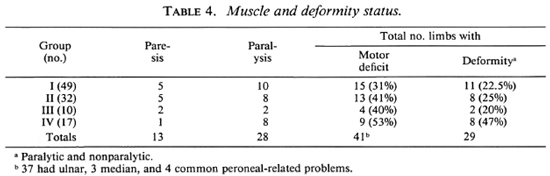
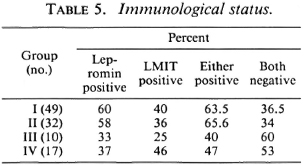
Skin smears were negative for AFB in all of the patients. The results of the immunological tests (lepromin and LMIT) are presented in Table 5 which shows that among the patients of Groups I and II about 60% were lepromin positive; positivity was lower in Groups III and IV. The results of the LMIT were variable; patients with multiple symmetrical nerve involvement (Group IV) showed levels of inhibition in the LMIT similar to that of Groups I and II.
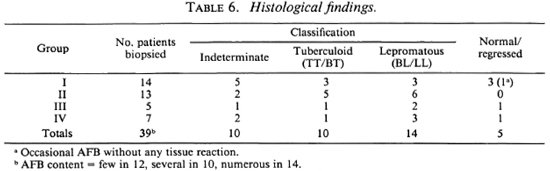
Nerve biopsies could be done in 39 patients. In the others, nerve biopsies were not done because the only nerves which were thickened and likely to contain pathology were mixed, i.e., contained both sensory and motor fibers, and we did not wish to risk creating motor deficits by biopsying them. The 34 patients in whom a nerve biopsy was done were similar to those in the other group where nerve biopsy could not be taken. The only difference was that the former group had thickened, pure sensory nerves, whereas those in the other group had either sensory nerves supplying palms or soles or mixed nerves only. Table 6 shows that 14 of these patients who had nerve biopsies belonged to Group I, 13 to Group II, 5 to Group III, and 7 to Group IV. On the basis of histology, 34 nerves were classified into definite types of leprosy. In addition, two biopsies show AFB within the nerve without any significant infiltrate. The remaining three nerves, although clinically thickened, showed no histological abnormality. Table 6 also shows that a significant proportion of the patients in each group showed histology of the BL/LL type. AFB were seen in all of the 34 patients showing the histological features of leprosy; 24 specimens showed high counts. No significant pathology was found in the analgesic skin of any of these patients.
DISCUSSION
Apart from the definitive diagnosis, the main problem with neuritic leprosy is its classification since treatment depends on it. Conventionally, these patients have been considered to belong to the tuberculoid end of the leprosy spectrum since many of them are lepromin (Mitsuda) positive (5,10). Further, since all of these patients are negative on bacilloscopy, they are classed with other patients of the paucibacillary group. However, several recent reports (2,8,12) show that some of these patients have definite lepro-matous features in the nerves with a fair load of bacilli. No clinical distinguishing features of neuritic patients with leproma-tous pathology have been recorded.
The present study has been an attempt to determine if the clinical characteristics, lepromin testing, or immunological investigations can help to discern the likely his-topathology within the nerves, since nerve biopsy is not always feasible. In the absence of a nerve biopsy a definitive diagnosis of neuritic leprosy may not be possible. However, clinical features of thickened nerves with nerve deficit in patients from endemic areas are taken to reflect neuritic leprosy. This is borne out by the findings of the present study in which none of the 39 biopsies showed any other pathology.
The results show that the extent of clinical involvement, as judged by the number and distribution of clinically affected nerves, gives very little information as to the severity of the underlying disease. As seen from Tables 5 and 6, of the 49 patients with single nerve involvement 36.6% were negative for both lepromin and LMIT. Of the three patients in this group who showed lep-romatous histology, one was positive for lepromin and LMIT. Likewise, of all the patients who were either lepromin and/or LMIT positive, and in whom a nerve biopsy was done, 25% showed BL/LL histology with a significant bacterial load within the nerve(s). Of those who were negative for lepromin and/or the LMIT and in whom nerve histology was done, seven showed indeterminate or TT/BT histology. These findings indicate that in neuritic patients the number of clinically affected nerves, the immune response, and the nerve pathology are not interrelated. However, on further analysis of the data for the degree of positivity of the lepromin response, it was observed that the lepromin response was comparatively stronger (14.2 ± 7.1 mm) in patients in Groups I and II as compared to Groups III and IV (11.1 ± 3.3 mm). The difference, however, was not statistically significant. In the present study, Dharmendra lepromin has been used for the intradermal testing and M. leprae sonicate for the LMIT. Even when the same antigens have been used for the lepromin response and the LMIT, the correlation between the two was only about 60%. This might have been because of the variation in the amount of antigens. It is possible that a better correlation may be found between the Mitsuda lepromin response and the LMIT in neuritic patients using whole M. leprae as antigen, as indicated by Pannikar, et al. (12).
With regard to other parameters, it was observed that sensory impairment was the single most important presenting feature of neuritic leprosy, irrespective of the number and distribution of the nerves affected. A slightly lower figure in Group III possibly could be due to a relatively short duration of disease in these subjects (Table 1). It was observed that among the patients with multiple nerve involvement significantly more patients had symmetrical, glove-and-stock-ing type impairment of sensations, suggesting that, together with involvement of peripheral nerve trunks, dermal nerves also get damaged in these patients.
Patients in all four groups had paralytic deformities, and no correlation was found with either the number of nerves affected, the immunological status, or the histology. Equal numbers of patients with or without deformities or motor deficit had positive or negative lepromins, indicating that the intraneural inflammatory cellular response does not parallel the skin reaction to M. leprae antigens.
The presence of AFB in the nerves in a majority of the patients, even in those in whom the histology was tuberculoid or indeterminate, is a significant finding. In 24 of the 39 biopsies, the AFB content was moderate to high; another 12 had few bacilli. These findings are similar to observations made by other workers (12,13). This raises the question, Would it not be better from a treatment point of view to include these patients with the multibacillary rather than the paucibacillary group? An argument against this could be that the affected nerve constitutes only a fraction of the body mass and, therefore, the total count may still be less than 106. However, no definite information is available as to the nature and extent of the involvement in the clinically un-involved nerves. Preliminary work done by Antia, et al. (1) in this regard has indicated that distant uninvolved nerves, such as the index branch of the radial cutaneous nerve, do show evidence of disease.
In conclusion, our investigations have shown that not all patients with neuritic leprosy are similar. If biopsies of infected nerves are taken, the histopathologic picture ranges all the way from tuberculoid to leproma-tous. No indication regarding the severity or the histological classification of disease type was obtained from any of the clinical parameters or immunological tests used. A fifth of our neuritic patients with single nerve involvement showed BL/LL histology, and about 42% of our neuritic patients with multiple nerve thickening showed a TT/BT picture.
Acknowledgment. The authors wish to express their thanks to: Dr. H. Srinivasan for his valuable comments, Dr. B. Mishra for his help in collecting material, Sh. A. S. Bhatia for helping with the analysis, and Sh. S. K. Kulshrcstha for secretarial assistance.
REFERENCES
1. Antia, N. H., Mehta, L., Shetty, V. and Irani, P. F. Clinical, electrophysiological, quantitative, histological and ultrastructural studies of the index branch of the radial cutaneous nerve in leprosy. I. Preliminary report. Int. J. Lepr. 43(1975)106-113.
2. Basombrio, G. and Bosq, F. J. Lepra lepromatosa dc comienzo oligoncuritico puro. Lcprologia 5 (1960) 31. English abstract in Int. J. Lepr. 29(1961)535-536.
3. Clain, A., ed. Peripheral nerves. In: Hamilton Bailey's Demonstrations of Physical Signs in Clinical Surgery. Bristol: John Wright & Sons, Ltd., 1973, p. 411.
4. Dharmendra. The pure ncuritic group. In: Leprosy, Volume 1. Bombay: Kothari Medical Publishing House, 1978, Chap. 8, pp. 94-107.
5. Dharmendra, Ramanujam, K. and Ramu, G. Pure polyneuritic leprosy of tuberculoid type. Lepr. India 38(1968)152-158.
6. Hargrave, J. C. and Marion, Rev. Mother. Leprotic involvement of multiple peripheral nerves in the absence of skin lesions (a case report). Lepr. Rev. 35(1964)78-82.
7. Hudson, L. and Hay, F. C, eds. Macrophage migration inhibition. In: Practical Immunology. 2nd ed. Oxford: Blackwell Scientific Publications, 1980, pp. 295-296.
8. Jacob, M. and Mathai, R. Diagnostic efficacy of cutaneous nerve biopsy in primary ncuritic leprosy. Int. J. Lepr. 56(1988)56-60.
9. Jopling, W. H. Borderline (dimorphous) leprosy maintaining a polyneuritic form for eight years: a case report. Trans. R. Soc. Trop. Med. Hyg. 50(1956)478-480.
10. Jopling, W. H. and Morgan-Hughes, J. A. Pure neural tuberculoid leprosy. Br. Med. J. 2(1965)799-800.
11. Noordeen, S. K. Epidemiology of (poly) neuritic type of leprosy. Lepr. India 44(1972)90-96.
12. Pannikar, V. K., Arunthathi, S., Chacko, C. J. G. and Fritschi, E. P. A clinicopathological study of primary ncuritic leprosy. Lepr. India 55(1983)212-221.
13. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
14. Schmitt, J., Schmidt, C, Adam-Goldberg, E. and Floquet, J. La lèpre nerveuse en France Métropolitaine. Ann. Med. Intern. (Paris) 127(1976)247-251. English abstract in Int. J. Lepr. 46(1978)237.
15. Sengupta, U., Ramu, G. and Desikan, K. V. Assessment of Dharmendra antigen. II. Standardization of the antigen. Lepr. India 51(1979)316-320.
16. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988, pp. 14-15. Tech. Rep. Ser. 768.
17. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982, pp. 24-25. Tech. Rep. Ser. 675.
18. World Health Organization. A Guide to Leprosy Control. Geneva: World Health Organization, 1980, p. 78.
1. Final year medical student on an elective period, University Hospital of Wales, Cardiff, U.K.
2. M.D.; Central JALMA Institute of Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
3. M.B.B.S.; Central JALMA Institute of Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
4. M.S.; Central JALMA Institute of Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
5. M.D.; Central JALMA Institute of Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
6. Ph.D., Central JALMA Institute of Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
7. M.D., Central JALMA Institute of Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
Reprint requests to Dr. B. K. Girdhar.
Received for publication on 6 August 1990.
Accepted for publication in revised form on 25 March 1991.