- Volume 59 , Number 3
- Page: 405–15
IgM antibodies to native phenolic glycotipid-I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy
ABSTRACT
In a randomized, double-blind vaccine trial in Venezuela, about 29,000 contacts of leprosy patients have been vaccinated with either a mixture of heat-killed Mycobacterium leprae and BCG or BCG alone, and are being resurveyed annually to detect new cases of leprosy. All contacts had a serum sample collected at the time of entry into the trial, and 13,020 of these sera have been analyzed for antibodies to phenolic glyco-lipid-I (PGL-I). Antibody levels have been related to various characteristics of the contacts and to their risk of developing leprosy in the following 4 years. A strong association was found between PGL-I antibody level and the risk of developing leprosy, in spite of possible modification of the incidence rate induced by vaccination. Antibody levels were higher in females than in males, and declined progressively with age. Household contacts had higher levels than did nonhousehold contacts, and levels were higher in individuals f rom the state in Venezuela which has the highest incidence of the disease. No substantial differences were found in antibody levels between contacts of multibacillary and paucibacillary patients, which may in part reflect the influence of treatment, and there was no clear association with the presence of BCG or lepromin scars or with skin-test responses to PPD and leprosy soluble antigen.The assay of antibodies to PGL-I seems unlikely to provide a sensitive or specific test for infection with M. leprae, and measuring PGL-I antibody levels as a screening procedure to identify those at high risk of developing leprosy is unlikely to be particularly useful in most leprosy control programs. Such assays may be useful for the epidemiological monitoring of changes in the intensity of infection with M. leprae in a community and for the study of carefully defined groups of contacts during some phases of control programs.
RÉSUMÉ
Dans le cadre d'un essai randomisé en double aveugle d'un vaccin au Venezuela, environ 29.000 contacts de malades de la lèpre ont été vaccinés avec soit un mélange de Mycobacterium leprae tués par la chaleur et de BCG, soit avec du BCG seul, et sont actuellement contrôlés chaque année pour détecter les nouveaux cas de lèpre. Tous les contacts ont eu un échantillon de sérum récolté au moment d'être intégrés dans l'expérimentation, et 13.020 de ces sérums ont été analysés pour la recherche d'anticorps vis-à-vis du glycolipide phénolique-I (PGL-I). Les taux d'anticorps ont été étudiés en relation avec diverses caractéristiques des contacts, et avec leur risque de développer la lèpre au cours des 4 années suivantes. Une forte association a été trouvée entre le taux d'anticorps anti-PGL-I et le risque de développer la lèpre, en dépit d'une modification possible du taux d'incidence induite par la vaccination. Les taux d'anticorps étaient plus élevés chez les femmes que chez les hommes, et diminuaient progressivement avec l'âge. Les contacts domiciliaires avaient des taux plus élevés que les contacts extra-domiciliaires, et les taux étaient plus élevés chez les personnes provenant de l'État présentant l'incidence la plus élevée au Venezuela. On n'a pas trouvé de différence substantielle dans les taux d'anticorps entre les contacts de patients multibacillaires, et ceux de patients paucibacillaircs, ce qui pourrait être dû en partie à l'influence du traitement; on n'a pas trouvé d'association évidente avec la présence de cicatrice du BCG ou de lépromine, ni avec la réponse aux tests cutanés au PPD et avec l'antigène soluble.Le titrage des anticorps anli-PGL-I semble peu apte à fournir un test sensible ou spécifique pour l'infection avec M. leprae, et il est peu probable que la mesure des taux d'anticorps anti-PGL-I comme procédé de dépistage pour identifier les personnes à risque élevé de développer la lèpre soit particulièrement utile dans la majorité des programmes de lutte contre la lèpre. De tels titrages pourraient être utiles pour la surveillance épidémiologique, dans une communauté, des modifications dans l'intensité de l'infection par M. leprae, et pour l'étude de groupes soigneusement définis de contacts durant certaines phases de programmes de lutte contre la lèpre.
RESUMEN
En un estudio al azar y en doble ciego en Venezuela, se han vacunado cerca de 29,000 contactos de pacientes con lepra con una mezcla de Mycobacterium leprae inactivado por calor y BCG o con BCG sólo. Los contactos se han examinado anualmente para descubrir los nuevos casos de lepra. De todos los cantados se tomó una muestra de suero al inicio del estudio, y 13,020 de estos sueros han sido analizados para la búsqueda de anticuerpos contra el glicolipido fenólico-1 (PGL-I). Los niveles de anticuerpo se han relacionado con varias características de los contactos y con su riesgo de desarrollar lepra en los siguientes 4 años. Se encontró una fuerte asociación entre los niveles de anticuerpo contra PGL-I y el riesgo de desarrollar la enfermedad, no obstante la posible modificación del grado de incidencia inducido por la vacunación. Los niveles de anticuerpo fueron mayores en las mujeres que en los hombres y declinaron progresivamente con la edad. Los contactos convivientes tuvieron niveles más elevados que los no convivientes, y los niveles fueron más altos en los individuos del estado de Venezuela con mayor incidencia de la enfermedad. No se encontraron diferencias substanciales en los niveles de anticuerpo entre los contactos de pacientes con lepra multibacilar y aquellos con lepra paucibacilar (lo cual podría ser el reflejo de la influencia del tratamiento) y no hubo una clara asociación con la presencia de cicatrices por BCG o lepromina ni con la reactividad en piel al PPD o a antigenos solubles del bacilo de la lepra.De lo anterior se deduce que es poco probable que el ensayo de anticuerpos contra el PGL-I sea una prueba sensible o especifica de la infección por el M. leprae y también, que es poco probable que la medición de los niveles de PGL-I, como un medio de descubrir los casos de alto riesgo, resulte de alguna utilidad en la mayoría de los programas de control contra la lepra. Tales ensayos pueden ser útiles para el seguimiento epidemiológico de los cambios en la intensidad de la infección con M. leprae en una comunidad y para el estudio de grupos cuidadosamente definidos de contactos, durante algunas fases de los programas de control.
Phenolic glycolipid-I (PGL-I), characterized by Brennan and coworkers in 19801982 (6, 19, 20), is a highly specific antigen of Mycobacterium leprae. Serological assays have been developed to detect the presence of antibodies, principally of the IgM class, which react with the native antigen or with neoconjugates containing the carbohydrate component toward which the antibody response is directed (2,9). Tests for antibodies based upon an enzyme-linked immunosorbent assay (ELISA) have been carried out in serological surveys, many of which have been cross-sectional studies in which antibody levels have been compared among multibacillary (MB) and paucibacillary (PB) patients, contacts of such patients, and healthy individuals from leprosy-endemic and nonendemic areas. The findings in a selection of these studies are summarized in Table 1. The criteria used to define positive antibody levels have varied in the different studies, and this probably accounts for some of the variability apparent in Table 1.
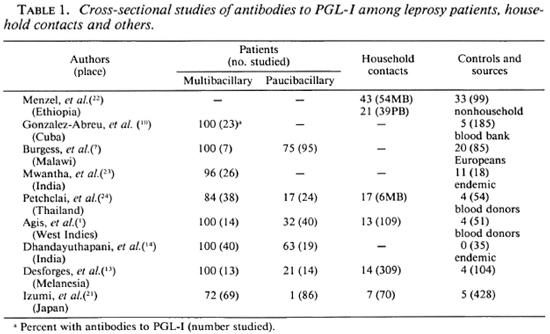
Most patients with MB leprosy have high levels of antibodies to PGL-I. Some treated patients have been included in this category, and it is possible that the lower levels of antibodies in some studies (21) may reflect this fact, since there is evidence that antibody levels correlate with bacillary load and decline with effective therapy (3, 17). The findings among PB patients have been more variable, but a substantial proportion of these patients do not have elevated antibody levels. Household contacts of leprosy cases usually have higher levels of antibodies than controls. The proportion of healthy individuals from leprosy-endemic areas with a positive PGL-I response has varied from 0% in one study to 33% in another, but in general the level of positive responses among controls has been of the order of 5% to 10%. These findings suggest that measuring antibodies to PGL-I may be a sensitive test for MB leprosy, but the test is much less sensitive for PB cases.
It is difficult to determine to what extent the test measures infection with M. leprae. The higher levels of antibody found among household contacts suggest that it may be of some value in this respect but the sensitivity may be poor, as suggested by the findings among PB patients.
In spite of the limitations noted above, tests based upon the detection of antibodies to PGL-I have been advocated as being of potential value in leprosy control programs and, in particular, it has been suggested that the screening of sera of apparently healthy individuals may permit the identification of subclinical disease. If such cases could be detected and treated, it is theoretically possible that their infectivity for others would be curtailed, with a significant impact on the overall incidence of leprosy.
There have been few prospective studies in which antibody levels to PGL-I have been related to the subsequent risk of leprosy and it has been impossible to evaluate what role serological testing might have in early detection of leprosy through large-scale surveys. An opportunity to partially evaluate the serological test in this way arose in the context of a large field trial, which is being conducted in Venezuela, to evaluate the protective efficacy against leprosy of a combined vaccine containing heat-killed, armadillo-derived M. leprae bacilli and BCG. Since the study has been carried out in a population subsequently subjected to vaccination procedures which might be expected to modify the immune response and the expression of disease, the observations reported here with regard to risk must be evaluated with caution within the context of the trial. Nevertheless, the epidemiological observations reflect studies on a large population, and the data on risk appear to reflect trends which are relevant to unvac-cinated populations.
MATERIALS AND METHODS
Population studied. Convit, et al. (11,12) have described the use of a mixture of heat-killed M. leprae and live BCG in the immunotherapy of MB forms of leprosy and have reported the development of cell-mediated skin test reactivity in persistently Mitsuda-negative contacts and patients with indeterminate leprosy following vaccination (10, 11). These observations have provided the basis for a controlled, double-blind field trial of the immunoprophylactic efficacy of the combined vaccine among the close contacts of leprosy patients in Venezuela (12).
The population eligible for entry into the trial consisted of about 65,000 household and other close contacts of prevalent leprosy cases in three states in western Venezuela. These contacts were skin tested with M. leprae soluble antigen and PPD, and the resulting indurations were measured at 48 hr. All of those with negative reactions (< 10 mm) to M. leprae soluble antigen and a random sample of those with positive reactions of 10 mm or more were admitted to the trial. The final trial population consisted of about 29,000 contacts who were distributed in a randomized, double-blind selection to be vaccinated with either a mixture of 6 x 108 killed M. leprae and BCG or with BCG alone. The dose of BCG administered, with or without M. leprae, was varied according to the skin test response to PPD. Individuals with reactions of < 10 mm were given 0.2 mg and those with larger reactions were given 0.04 mg of BCG.
At the time the skin tests were read, blood samples were taken in capillary tubes from all those admitted to the trial. After entry into the trial, the participants have been examined clinically every year to detect new cases of leprosy. About 13,000 of the scrum samples collected before vaccination have been analyzed for antibodies to PGL-I. The samples were not selected in any way other than chronological entry of the participants into the trial.
Serum samples and PGL-I antibody assay. The blood samples were refrigerated for a maximum of 2 weeks before shipment under refrigeration to the Institute of Bio-medicine in Caracas, Venezuela, for serum separation and storage. Serum was separated by centrifugation at 450 x g for 15 min. The tubes were cut at the serum-coagulum interface, and the portion of the tube containing the serum was sealed with clay and stored in small vials or in envelopes at -20ºC until analysis.
ELISA testing for antibodies to PGL-I was conducted using native PGL-I. Two different assay procedures were used. The method used for the first 3475 serum samples (Method 1) proved to be laborious, technically deficient, and of very low sensitivity; a modified and simplified technique (Method 2) was used for the assay of the other 9545 samples.
Native PGL-I, generously supplied by Dr. Patrick Brennan and by Dr. R. J. W. Rees, was dissolved in 1% deoxycholate buffer at a concentration of 2 mg/ml and stored at - 20ºC. For the serological tests, this preparation was uniformly suspended by agitation in an ultrasonic water bath for 7 min, then diluted to a concentration of 2 µg/ml in phosphate-buffered saline (PBS), pH 7.2, containing 1% deoxycholate (Method 1) or PBS (Method 2). Wells in polystyrene microliter plates were sensitized for 16 hr at 37ºC with 50 µl of the PGL-I solution. After four washes with PBS, the plates were blocked for 1 hr at 37ºC with 100 µl of PBS containing bovine serum albumin (PBS-BSA); the blocking solution was decanted and the plates were treated as follows:
Method 1. Each serum sample was diluted 1:300 in PBS-BSA and 50 µl was dispensed into two wells containing antigen and a third control well without antigen. After incubation for 1 hr at 37ºC and five washes with PBS, 50 µl of a 1:5000 dilution of anti-human IgM labeled with peroxidase (Diagnostics Pasteur, Marne-La-Coquette, France) was added to each well and incubated for 1 hr at 37ºC. After five washes, 50 µl of substrate containing 0.003% H2O2 and 0.008% o-dianisidine in phosphate buffer, pH 6.0, was added to each well. The plates were incubated for 20 min at room temperature in the dark; the reactions were stopped with 1 N HC1 and read in a microELISA reader (Dynatech Laboratories, Chantilly, Virginia, U.S.A) at 405 nm. The optical density (OD) readings for the wells containing antigen were averaged, and the OD reading of the control well was subtracted from the average. Positive and negative control sera were included in each plate.
Method 2. Each sample was diluted as above and 50 µl was dispensed in a single well. Positive and negative controls were included in each plate. The procedure was as described above, except that the substrate was 0.012% H2O2 and 0.04% o-phenylene-diamine-2HCl in citrate buffer, pH 5.0; the reactions were stopped with 2.5 NH2SO4 and read at 488 nm. If a test serum gave an OD of 37% or higher of the positive control, the sample was re-tested using two antigen-sensitized wells and one control well and the reagents described for Method 2.
The positive control sera for these studies consisted of pools of sera from six patients with lepromatous or borderline leproma-tous leprosy with known high levels of antibody to PGL-I in ELISA tests. The negative controls were pools of six sera from students at the Vargas Medical School in Caracas who had not lived in leprosy-endemic areas.
Analysis. Since two different methods were used to assay PGL-I antibody levels, it was not possible to combine the results based on all of the data, and the findings for the two methods are presented separately. Relatively few results are reported using Method 1 because of the extremely low sensitivity of the method used; in this method, optical density differences (ODDs) were obtained for each sample, including the positive and negative controls. Using Method 2, optical densities (ODs) were obtained for all sera and ODDs were obtained only for those repeated using Method 1 because of high OD values. The ODD values of this second group arc not analyzed in detail since they were available for only a selected sample of the group.
There are two simple ways of presenting the results for individual sera relative to the results for positive and negative control sera. Some investigators have reported the ratio for each serum sample to that for a positive control (i.e., OD/OD+ or ODD/ODD+). Since sera from negative controls were also included in the assays, a reasonable alternative measure would be (OD-OD-/OD+-OD-) or (ODD-ODD-/ODD+-ODD-). The results were analyzed using both measures and the findings were very similar. To facilitate comparison with other studies, the analyses presented are those based on comparison with the positive control sera only.
Means and 95% confidence limits are shown in the figures. Analysis of variance methods were applied as indicated in the text.
RESULTS
Method 1 was used to analyze 3475 sera and 9545 were analyzed by Method 2. Since the technical procedure used for the first 3475 sera was very insensitive, as reflected in the much lower readings in the corresponding tables and figures, the findings in the second group are presented in more detail.
Figure 1 shows the distribution of PGL-I antibody levels measured for the samples analyzed by Method 2. The results (OD readings) for each individual serum sample are expressed as a proportion of the value observed for the positive control sample on each microtiter plate. The distribution is unimodal, with the great majority of antibody levels being less than 50% of the positive control values. Figure 2 shows the corresponding distribution of ratios of ODDs for the sera analyzed by Method 1.
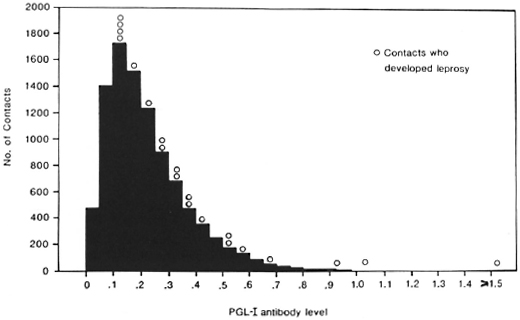
Fig. 1. Distribution of PGL-I antibody assays among 9545 contacts ofleprosy patients (Method 2). O = Assays for the 20 contacts who developed leprosy during the following 4 years; data are expressed as OD/OD+ ratios.
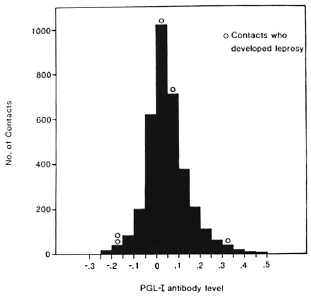
Fig. 2. Distribution of PGL-I antibody assays among 3475 contacts of leprosy patients (Method 1). O = Cases in the 4-year follow-up; data represent ODD/ ODD+ ratios.
In the 4 years following the collection of the serum samples, 25 of the 13,020 contacts included in this study developed leprosy. The PGL-I antibody assays for these patients at the time they entered the trial are also shown in Figures 1 and 2. The antibody levels of the five patients whose sera were analyzed by Method 1 arc not markedly different from those of contacts who did not develop leprosy (Fig. 2), but the 20 patients whose sera were analyzed by Method 2 tended to have higher antibody levels than the other contacts (Fig. 1). The results for these 20 patients are summarized in Table 2, which also shows their age, sex, and the type of leprosy which was diagnosed. The relationship between PGL-I antibody levels and the subsequent risk of developing leprosy can be seen more clearly in Table 3, in which the antibody levels have been grouped. There is a strong association between the risk of developing leprosy and the level of antibodies against PGL-I in these sera analyzed by Method 2. Contacts whose serum sample showed a PGL-I antibody level of 75% or more than that of the positive control were about 40 times more likely to develop leprosy in the following 4 years than contacts with an antibody level 25% or less than that of the positive control (3/ 80 vs 6/6349).
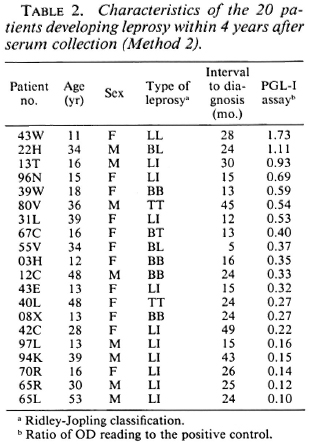
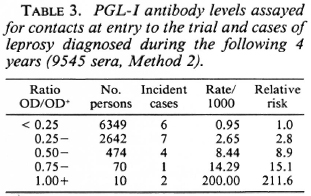
These results must be interpreted with some caution, since a specific search was made for leprosy in about 30 contacts with especially high PGL-I antibody assays and two of the cases shown in Table 3 were identified in this way. Even if these two cases are excluded from the analyses, there is still a statistically highly significant association between the risk of developing leprosy and the PGL-I antibody level [x2 (trend) = 16.7; p < 0.001]. For contacts whose sera were analyzed using Method 1, the association between the risk of developing leprosy and the PGL-I antibody level was not statistically significant (data not shown).
In Table 4, mean PGL-I antibody levels are shown according to various characteristics of the contacts studied. There was a decline in the mean antibody level with increasing age of the subjects studied which was statistically highly significant. The mean anti-PGL-I level for males was also significantly lower than that for females. This difference was manifest at all ages, as shown in Figure 3. Household contacts had a higher PGL-I antibody level than other contacts, which was highly significant for the sera analyzed by Method 2. The mean antibody level was significantly and markedly higher in those contacts resident in the state of Apure compared to those from the states of Tachira and Merida.
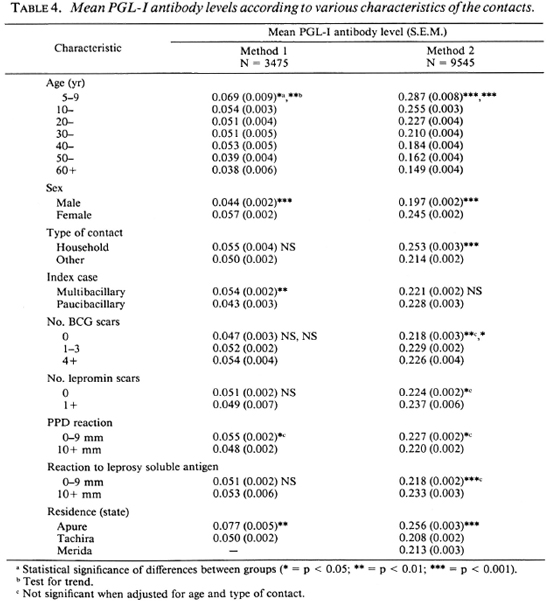
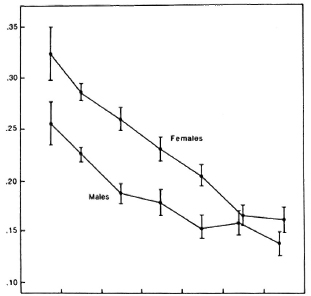
Fig. 3. Mean PGL-I antibody levels of the 9545 sera, assayed by Method 2, according to age and sex. Bars = 95% confidence limits.
Some significant differences in the mean anti-PGL-I levels were apparent according to whether the type of leprosy in the index case was multibacillary or paucibacillary, according to the number of BCG scars, whether or not there was a lepromin scar, and according to the skin-test reactions to PPD and leprosy soluble antigen but, in general, the differences were either small or not consistently different in the analyses based on Methods 1 and 2; in most instances the differences were not significant if adjustment was made for age and household or nonhousehold contact. The findings for Method 2 are shown graphically in Figure 4. In summary, the most marked differences in mean antibody levels were observed with respect to age, sex, whether or not the subjects were household contacts of a leprosy case, and according to their place of residence. The last two differences persisted when the age and sex differences were adjusted for using analysis of variance methods.
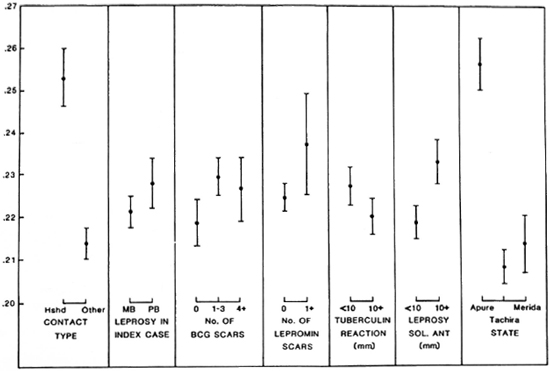
Fig.4. Mean PGL-I antibody levels of the 9545 sera, analyzed by Method 2, according to various characteristics. Bars = 95% confidence limits.
DISCUSSION
Our understanding of the epidemiology of leprosy would be greatly advanced if it were possible to identify infection by M. leprae. Control of the disease would be facilitated if it were possible to detect individuals with subclinical infection who might be expected to develop clinical disease within the next few years because of the lack of an appropriate cell-mediated response. Much of the interest in the development of serological tests for leprosy has been carried out with these two objectives in mind and, in particular, attempts have been made to evaluate the usefulness of measuring antibodies to PGL-I for these purposes.
The present study is one of the few in which it has been possible to partially evaluate the predictive value of PGL-I assays for future risk of developing leprosy. We have shown a strong association between the PGL-I antibody level and the risk of developing leprosy in the 4 years following the serological test; those with high antibody levels were much more likely to develop the disease than those with low levels. It is particularly important to emphasize that all of the persons included in this study were vaccinated with either BCG alone or a mixture of BCG plus M. leprae at the time that blood was taken for the PGL-I assay. It is probable that this vaccination will have reduced the risk of leprosy in the study groups, since a retrospective study has shown that BCG alone confers protection against leprosy in Venezuela (Convit, et a!., manuscript in preparation). We do not know to what extent any protective effect in either the incidence or type of leprosy is expressed in relation to the PGL-I antibody level at the time of entry into the trial.
Despite the finding of a strong association between antibody level and the subsequent risk of developing leprosy, it seems unlikely that screening for anti-PGL-I levels would be a particularly useful way of detecting persons at high risk of developing leprosy in most control programs, even if the necessary infrastructure for performing the tests were available at acceptable cost. Although two cases of multibacillary leprosy were detected among the ten contacts with the highest antibody levels to PGL-I, it was necessary to screen over 9500 sera to identify those ten persons. The majority of those who developed leprosy (65%) did not have an elevated antibody level. Since most of the cases detected during the course of this study were of paucibacillary leprosy, this finding is not particularly surprising. Such narrowing of the risk group is likely to be of very limited value in the context of most control programs.
There have been only a few other prospective studies in which antibody levels to PGL-I have been related to the subsequent risk of leprosy, and none has been carried out within the context of a vaccination trial. Bagshawe, et al. (4) assayed antibody levels to PGL-I in 877 residents in a village in Papua New Guinea with a high leprosy incidence rate. Sixteen developed leprosy during the following 2 years, but no association was found between antibody levels and the risk of developing leprosy. The interpretation of this finding is complicated by the fact that even patients with prevalent leprosy did not have elevated PGL-I antibody
levels. In a study in The Philippines, Douglas, el al. (5) found that leprosy developed in 3 of 36 household contacts with elevated PGL-I antibody levels and in only 1 of 285 household contacts whose antibody levels were not elevated during a 2-year period of observation. Chanteau, et al. (8) followed 724 household contacts of leprosy cases on Tahiti Island for 2 years after measuring antibodies to PGL-I. Three cases of paucibacillary leprosy developed among the 631 contacts with negative serology and one multibacillary case developed among the 93 contacts with positive serology, this one case being among the eight with the highest antibody levels recorded.
In the studies described above, antibody levels were graded as either positive or negative. The findings in our study suggest that such a dichotomy, which attempts to divide the population into infected and noninfect-ed groups, may not be an appropriate model, since we found that the risk of developing leprosy increased progressively with increasing antibody levels.
In summary, our findings are consistent with those of some other studies in finding that PGL-I antibody levels do give an indication of the risk of developing leprosy in the next few years, even in a vaccinated population. Used for this purpose within the context of the vaccination trial, the test has both low sensitivity and low specificity; it would appear to be of very limited use as a screening test in leprosy control programs except in very carefully defined groups.
The findings in cross-sectional studies suggest that the sensitivity and specificity of the tests for antibodies to PGL-I as indicators of past infection with M. leprae may be poor. In some populations, it has been possible to use a skin test with tuberculin to distinguish between those persons who have been infected previously with tubercle bacilli and those who have not. In such populations, the distribution of the sizes of skin test responses is bimodal and a cut-off point can be chosen to distinguish between infected and noninfected individuals (25). There is no evidence of such a bimodal distribution for the PGL-I antibody assays in this study nor in others conducted in Malawi (7) and in a high-prevalence village in Papua New Guinea (5). The failure to find a bimodal distribution clearly suggests that it is inappropriate to use the measurement of antibody levels to PGL-I as a simple test to determine if an individual has been infected with M. leprae or not. The antibody level may reflect the intensity of exposure, with higher levels induced by repeated exposure. Some support for this possibility is provided by the observation of higher levels among household contacts and in contacts living in the state of Apure which has the highest incidence of leprosy in Venezuela. Assuming that antibody levels correlate with the extent of exposure, we might have expected that the contacts of multibacillary cases would have higher levels of antibodies than the contacts of paucibacillary cases, but this was not the case. A possible explanation is that the great majority of the index cases had received treatment for many years, and this is likely to have influenced the extent to which they would have been sources of infection for their current contacts.
While measuring antibodies to PGL-I may not be a particularly useful test of individual risk of leprosy, it is possible that it would be more useful in population studies. It might be of interest to determine if the average level of PGL-I antibodies shows a parallel decline in areas where the incidence of leprosy is decreasing.
The marked decline in antibody levels with increasing age and the higher levels observed among females in all age groups were among the most striking findings in this study. Similar sex and age differences have been described for persons living in an endemic area of Malawi (7,16). The significance of these observations is not clear. In the whole group of 29,000 contacts included in the trial, the incidence of leprosy has been similar in males and females (29 and 26 cases, respectively). Higher incidence rates of leprosy have been reported among young children than in adults, but among adults there has been no evidence of a decreasing incidence rate with increasing age.
Acknowledgment. This investigation received support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases and from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICIT) of Venezuela. We wish to thank Migdalia Marcanoand OliuskaOporte de Caraballo for excellent technical assistance and the many field workers who made this study possible. We arc grateful for helpful advice and support from Howard Engers.
REFERENCES
1. Agis, F., Schlich, P., Cartel, J.-L., Guidi, C. and Bach, M.-A. Use of anti-M. leprae phenolic gly-colipid-I antibody detection for early diagnosis and prognosis of leprosy. Int. J. Lepr. 56(1988)527-536.
2. Anonymous. Serological tests for leprosy. (Editorial) Lancet 1(1986)533-535.
3. Bach, M.-A., Wallach, D., Flageul, B., Hoffenbach, A. and Cottenot, F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients: evolution during therapy. Int. J. Lepr. 54(1986)256-267.
4. Bagshawe, A. F., Garsia, R. J., Baumgart, K. and Astbury, L. IgM scrum antibodies to phenolic glycolipid-I and clinical leprosy: two years' observation in a community with hypcrendemic leprosy. Int. J. Lepr. 58(1990)25-30.
5. Baumgart, K., Britton, W., Basten, A. and Bagshawe, A. Use of phenolic glycolipid I for serodiagnosis of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81(1987)1030-1032.
6. Brennan, P. J. and Barrow, W. W. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int. J. Lepr. 48(1980)382-387.
7.Burgess, P. J., Fine P. E. M., Ponnighaus, J. M. and Draper, C. Serological tests in leprosy. The sensitivity, specificity and predictive value of ELISA tests based on phenolic glycolipid antigens, and the implications for their use in epidemiological studies. Epidcm. Infect. 101(1988)159-171.
8. Chanteau, S., Cartel, J.-L., Guidi, C, Plich-art, R. and Bach, M.-A. Seroepidemiological study on 724 household contacts of leprosy patients in French Polynesia using disaccharidc-oc-tyl-BSA as antigen. Int. j. Lepr. 55(1987)626632.
9. Cho, S.-N., Yanaoihara, D. L., Huntlr, S. W., Gelber, R. H. and Urennan, P. J. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
10. Convit, J., Aranzazu, N., Pinardi, M. E. and Ulrich, M. Immunological changes observed in indeterminate and lepromatous patients and Mit-suda-negative contacts after inoculation of a mixture of Mycobacterium leprae and BCG. Clin. Exp. Immunol. 36(1979)214-220.
11. Convit, J., Aranzazu, N., Ulrich, M., Pinardi, M. E., Reyes, O. and Alvarado, J. Immunotherapy with a mixture of Mycobacterium leprae and UCG in different forms of leprosy and in Mit-suda-negative contacts. Int. j. Lepr. 50(1982)415424.
12. Convit, J., Ulrich, M., Aranzazu, N., Castellanos, P. L., Pinardi, M. E. and Reyes, O. The development of a vaccination model using two microorganisms and its application in leprosy and leishmaniasis. Lepr. Rev. 57 Suppl. 2(1986)263273.
13. Desforges, S., Bohin. P., Brethes, B., Huerre, M., Moreau, J.-P. and Bach, M.-A. Specific anti-M. leprae PGL-I antibodies and Mitsuda reactions in the management of household contacts in New Caledonia. Int. J. Lepr. 57(1989)794-800.
14. Dhandayuthapani, S., Anandan, D., Vasanthi, li. and Bhatia, V. N. Use of eluates of filter paper blood spots in ELISA for the serodiagnosis of leprosy. Indian J. Med. Res. 89(1989)150-157.
15. Douglas. J. T., Celona, R. V., Abalos. R. M., Madarang, M. G. and Fajardo, T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Cebu, The Philippines. Int. J. Lepr. 55(1987)718-721.
16. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. and Draper, C. C. Seroepidemiological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using glycoconjugate antigen. Int. J. Lepr. 56(1988)243254.
17. Gelber, R. H., Li, F., Cho, S.-N.. Byrd. S., Ra-jagopalan, K. and Brennan, P. J. Serum antibodies to defined carbohydrate antigens during the course of treated leprosy. Int. J. Lepr. 57(1989)744-751.
18. Gonzalez-Abreu, E. and Gonzalez, A. Sero-reactivity against the Mycobacterium leprae phenolic glycolipid-I in mycobacteria infected or stimulated groups of individuals. Lepr. Rev. 58(1987)149-154.
19. Hunter, S. W. and Brennan. P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bactcriol. 147(1981)728-735.
20. Hunter, S. W., Fujiwara, T. and Brennan, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 258(1982)15072-15078.
21. Izumi, S., Fujiwara, T., Ikeda, M., Nishimura, Y., Sugiyama, K. and Kawatsu, K. Novel gelatine particle agglutination test for serodiagnosis of leprosy in the field. J. Clin. Microbiol. 28(1990)525-529.
22. Menzel, S., Harboe, M., Bergsvik, H. and Brennan, P. J. Antibodies to a synthetic analog of phenolic glycolipid-1 of Mycobacterium leprae in healthy household contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
23. Mwantiia, J., Moreno, C, Sengupta, U., Sinha, S. and Ivanyi, J. A comparative evaluation of serological assays for lepromatous leprosy. Lepr. Rev. 59(1988)195-199.
24. Petchclai, B., Khupulsup, K., Hiranras, S., Sampatavanich, S., Sampoonachot, P. and Lee-larusamee, A. A passive hemagglutination test for leprosy using a synthetic disaccharide antigen. Int. j. Lepr. 56(1988)255-258.
25. Styblo, K. Epidemiology of Tuberculosis. Jena: VEB Gustav Fischer Verlag, 1984.
1. Ph.D., Immunologist; Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
2. M.D., Epidemiologist; Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
3. M.D., Epidemiologist; Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
4. M. Centeno, Research Assistant; Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
5. M.D., Dermatologist; Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
6. M.D., Dermatologist; Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
7. M.S., Computation Engineer; Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
8. Director, Instituto de Biomedicina, Apartado Postal 4043, Caracas 1010A, Venezuela.
9. D.Sc, Department of Epidemiology and Population Sciences, London School of Hygiene and Tropical Medicine, Keppel Street, London WC1E 7HT, U.K.
Received for publication on 18 January 1991.
Accepted for publication in revised form on 29 April 1991.