- Volume 59 , Number 3
- Page: 416–25
A seroepidemiological study of leprosy in high- and low-endemic Indonesian Villages
ABSTRACT
A seroepidemiological study was performed in three different leprosy-endemic areas in Indonesia, including two isolated villages with high endemicity in South Sulawesi (Kaluarang and Hulo) and an area with low endemicity in Java (Jepara). A total of 2430 serum samples were collected f rom 2672 individuals in these locations. The prevalence of leprosy in these three areas, as determined during this study, was 29/1000, 11/1000, and 7/1000 in Kaluarang, Hulo and Jepara, respectively.Two serological assays were employed in this study to detect antibodies against Mycobacterium leprae. One is an enzyme-linked immunosorbent assay (ELISA) based on the detection of antibodies to the species-specific epitope of phenolic glycolipid-I (PGL-I) of M. leprae. The second test, using inhibition of an ELISA reaction (ELISA-INH) detects antibodies to a species-specific epitope on the 36-kDa protein antigen of M. leprae. In comparison with clinical findings, the specificity of both serological tests was calculated to be 91%. The sensitivity of the ELISA was 97.6% for multibacillary (MB) cases and 56.8% for paucibacillary (PB) cases; for the ELISA-INH, it was 97.6% and 81.8% for MB and PB cases, respectively.
Seropositivity rates were shown to be unrelated to sex, to Mitsuda skin-test reactivity, or to BCG vaccination status. The pattern of seropositivity was, however, clearly age-related, with high seropositivity in the age group 10-19 years and deceasing rates of positivity in the older age groups. Age-standardized seropositivity ratios were not correlated to the prevalence of leprosy when comparing the three areas. Therefore, it is not yet clear whether or not seropositivity reflects infection. If it does, other, as yet unidentified, factors may play a role in the natural history of the disease.
RÉSUMÉ
Une étude séro-épidémiologique a été réalisée dans trois régions endémiques pour la lèpre en Indonésie: deux villages isolés à haute endémicité à Sud-Sulawesi (Kaluarang et Hulo), et une région à basse endémicité à Java (Jepara). Un total de 2430 sérums ont été récoltés parmi 2672 personnes. La prevalence de la lèpre dans ces 3 zones, comme cela fut déterminé au cours de 1' étude, était de 29/1000, 11/1000 et 7/1000 respectivement à Kaluarang, Hulo et Jepara.Deux épreuves sérologiqucs ont été utilisées au cours de l'étude pour la recherche d'anticorps vis-à-vis du Mycobacterium leprae. L'un est un test enzymatique (ELISA) basé sur la détection des anticorps spécifiques vis-à-vis de l'épitope glycolipide phénolique-I (PGL-I) de M. leprae. Le second test, basé sur l'inhibition d'une réaction ELISA (ELISA-INH), détecte les anticorps spécifiques de l'antigène protéique de 36 kDA de M. leprae. La spécificité des deux tests sérologiqucs a été estimée à 91% par rapport à l'examen clinique. La sensibilité de l'ELISA était de 97, 6% pour les cas multibacillaires (MB), et 56, 8% pour les paucibacillai-res (PB); pour le test ELISA-INH, la sensibilité était de 97, 6% pour les multibacillaires et de 81, 8% pour les paucibacillaires.
Les taux de séropositivité n'étaient pas liés au sexe, à la réactivité au test cutané de Mitsuda, ni au statut vaccinal vis-à-vis BCG. La séropositivité était cependant clairement associée à l'âge, avec une séropositivité élevée pour le groupe d'âge 10-19 ans, et des taux décroissants dans les groupes d'âge plus élevés. Des ratios de séropositivité standardisés pour l'âge ont été trouvés, pour les deux tests, córreles à la prévalence de la lèpre dans les régions situées à Sulawezi. Cependant, quand Jepara fut inclus dans l'analyse, on ne put démontrer une telle corrélation. Il n'est donc pas encore clair si la séropositivité reflète ou non l'infection. Si c'est le cas, d'autres facteurs, encore non identifiés, pourraient jouer un rôle dans l'histoire naturelle de la maladie.
RESUMEN
Se efectuo un estudio seroepidemiológico en 3 áreas con lepra endémica en Indonesia. Las áreas incluyeron 2 poblaciones aisladas con alta endemicidad en Sulawesi del sur (Kaluarang y Hulo) y un área con baja endemicidad en Java (Jepara). De 2672 individuos se colectaron un total de 2430 muestras de suero. La prevalência de lepra en estas tres áreas fue de 29/1000 (Kaluarang), 11/1000 (Hulo), y 7/1000 (Jepara).Para detectar anticuerpos contra el Mycobacterium leprae se emplearon 2 ensayos serológicos. Uno fue un ELISA para la detección de anticuerpos contra el gli-colípido fenólico-I (PGL-I) del M. leprae, el otro fue una inhibición del ELISA (ELISA-INH) para detectar anticuerpos contra un epitope de la proteína de 36-kDa del M. leprae. En comparación con los hallazgos clínicos, la especificidad de ambos ensayos serológicos fue del 91%. La sensibilidad del ELISA fue del 97.6% para los casos multibacilarcs (MB) y del 56.8% para los casos paucibacilares (PB); para el ELISA-INH, la sensibilidad fue del 97.6% para los casos MB y del 81.8% para los casos PB.
El grado de seropositividad no se encontró relacionado con el sexo, con la reactividad en piel (Mitsuda), ni con la vacunación previa con BCG. El patrón de seropositividad estuvo, sin embargo, claramente relacionado con la edad; el grupo de 10-19 años mostró la seropositividad más alta y ésta disminuyó en los grupos de mayor edad. Cuando de compararon las 3 áreas, los índices de seropositividad estandarizados por edad no correlacionaron con la prevalência de la lepra. Por lo tanto, aún no está claro si la seropositividad refleja o nó el estado de infección. Si esto fuera asi, otros factores, aún no identificados, podrían jugar un papel en la historia natural de la enfermedad.
Leprosy is a chronic infectious disease of man caused by the intracellular microorganism Mycobacterium leprae. It affects almost all parts of the body, but primarily the peripheral nerves and skin. This disease is still a public health problem in developing countries. Approximately 1.6 billion people live in areas where the estimated prevalence of the disease is above 1 per 1000 persons, and all of them may be considered to be at risk of contracting the disease (21).
Among the major infectious diseases, leprosy is probably the least well understood as to its epidemiology (7). Our knowledge of the natural history of the disease is also very limited. This is largely due to the fact that the causative microorganism, M. leprae, has not been cultured in vitro. Even the source(s) of infection and the mode(s) of transmission are still subjects of controversy (8). It is generally considered that human beings are the most important source of infection. However, the relative roles of patients with different types of leprosy and, possibly, of subclinically infected individuals in the transmission of infection remains obscure.
It is commonly believed that most individuals will become infected with M. leprae after sufficient contact with leprosy patients, but it seems that in most cases of infection, symptoms fail to develop (11). However, the certain recognition of past or present infection with M. leprae is still a problem that represents one of the greatest challenges facing leprosy research today (7). Until recently, there have been no reliable tools available to ascertain subclinical infection, so that epidemiological studies of leprosy have in the past been based only on the cases with clinical disease (23).
The epidemiological significance of the presumably many people who are infected but without clinical symptoms is still to be investigated, although there has been much speculation about it (8). In addition to improving our understanding of the natural history of leprosy, an ability to recognize infection might ultimately have important implications for leprosy control, particularly if it would permit the definition of targets in the population for immunoprophylactic and (immuno)therapeutic strategies (23).
Several serological tests have been developed to detect infection with M. leprae (reviewed in 19). Results of population studies using any of these tests suggest that infection with At. leprae is far more common than the overt disease (22), and that infection can occur within a short time after initial exposure to the bacillus (6). Many reports on serological tests in leprosy have focused almost entirely on the application of assays to small panels of sera from selected clinical cases and control groups. Only a few examples of the application of such tests in epidemiological studies are available (6,14). Recently, comprehensive studies have been reported by Fine, et al. (9) and Cartel, et al. (4). However, there is still very little known about the seroepidemiology in populations with different endemicities of leprosy. Determining M. leprae infection rates in different populations constitutes a fundamental requirement for unraveling the natural history of leprosy. It is also needed to identify the risk factors for infection and disease as well as other epidemiological indicators that may be important in leprosy control programs, particularly with regard to the use of vaccines currently being investigated. This study aimed to evaluate serological tests as indicators of M. leprae infection by comparing serological responses to M. leprae antigens in three areas in Indonesia with different endemicities of leprosy.
MATERIALS AND METHODS
Selection of study areas. Two areas, one with a high and one with a moderate prevalence of leprosy, were chosen in South Sulawesi: the villages Kaluarang and Hulo. Both are located in Kabupaten Sinjai and represent two isolated locations which share many characteristics in terms of geographical, socioeconomic and cultural conditions. The total population in Kaluarang was 849 and in Hulo 952. The number of registered leprosy cases at the time of the study was 15 in Kaluarang and 6 in Hulo, which corresponds to prevalence rates of 21/1000 and 7/1000, respectively. In both areas, the total population was recruited for the study. For comparison, an area with a low endemicity was selected; this was Troso village, Kabupaten Jepara, Java. It would have been preferable to select an area of low endemicity with similar living conditions to the areas with high endemicities but, unfortunately, these could not be found in South Sulawesi. The total population in Jepara was 12,018, and the number of leprosy patients registered was 26, which corresponds to a prevalence rate of about 2.5 per 1000. Because it was not feasible to investigate the whole population in this area, we used a one stage cluster random sampling method in Troso to get a number of samples comparable with the number from Kaluarang and Hulo. The village of Troso was divided into 70 clusters of 150-200 persons, numbered from 1 to 70. Using random numbers, we selected six clusters for testing.
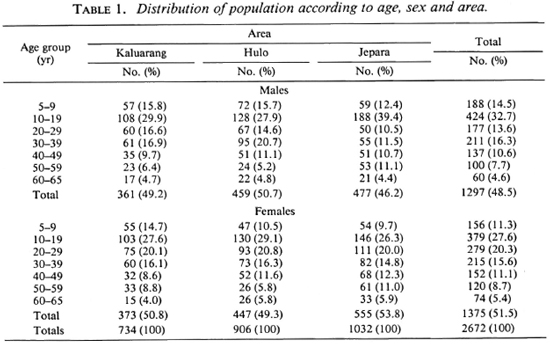
Table 1 shows the distribution of the population in the three areas according to age and sex. There were no differences in sex distribution among the samples from the three areas (chi-squared test, p > 0.05).
Field study. Before the study was started, a population census was carried out to check the household and individuals to be involved in the study. Clinical examinations were done in the house of the Heads of Neighborhoods, where about 100 people could be assembled. A presumptive diagnosis of leprosy was confirmed by bacteriological and histopathological findings. Lepromin tests were done on patients and their household contacts as well.
For serological testing, 5 ml of venous blood was drawn from every individual. The sera were separated on the same day, stored at - 20ºC and transported to Yogyakarta where the serological tests were performed.
No blood samples were taken from villagers younger than 5 years of age or older than 65. People who did not attend the examination sessions were visited at their homes and examined according to the above procedures.
Serological assays. The serum samples were investigated using two serological assays: an enzyme-linked immunosorbent assay (ELISA) detecting IgM antibodies to the species-specific sugar epitope of phenolic glycolipid-I (PGL-I) antigen of M. leprae (2) and an inhibition-ELISA (ELISA-INH) detecting antibodies to a species-specific epitope on the proline-rich antigen (PRA or 36-kDa protein) of M. leprae (18,19). The semi-synthetic antigen for ELISA was kindly provided by WHO/IMMLEP.
To determine the cut-off value of the positive test, 49 healthy blood donors from urban Yogyakarta were tested. The cut-off value of the positive test was then defined as a given percentile of the optical density (OD) or the percent inhibition, in ELISA or ELISA-INH, respectively, from the healthy blood donors. Sensitivity was determined on the basis of results obtained using sera of 44 paucibacillary (PB) and 41 multiba-cillary (MB) untreated patients.
Data analysis. All data were recorded on special forms and managed using database and statistical software packages in appropriate hardware.
For each assay the sensitivity, specificity and predictive values for detecting cases of MB or PB leprosy were determined. Sensitivity was calculated as: (number of cases positive by test/total number of cases) x 100. Specificity was calculated from the results of tests on sera from healthy blood donors as follows: (number of healthy blood donors negative by the tests/total number of healthy blood donors) x 100.
Based on the individual data, the population could be grouped into the PB and MB patients, household contacts (contacts of PB [CPB] and contacts of MB [CMB]) and healthy individuals (HP).
The differences in antibody levels were analyzed using Student's t test and/or ANOVA (analysis of variance); the differences between frequencies of seropositives were analyzed using the chi-squared test. The standardized prevalence or seropositivity ratios were calculated by indirect methods as the observed/expected ratio after applying age-specific rates in the standard population of each sex to age-specific numbers in each group to calculate the expected value (15,20).
RESULTS
The distribution of the population according to diagnosis is presented in Table 2. In Kaluarang, 21 leprosy cases were detected of which 6 were newly diagnosed and untreated, and 15 were under therapy. In Hulo, 10 cases were diagnosed, 4 untreated and 6 treated; in Jepara, 7 cases were diagnosed, 2 untreated and 5 treated. On the basis of these findings, the prevalence rates were calculated to be 29, 11 and 7 per 1000, in Kaluarang, Hulo and Jepara, respectively. All of the treated cases in these three areas had been on dapsone monotherapy. All of the cases, both those on monotherapy and those not yet treated, were given multidrug therapy from the start of this study. Serum samples were obtained from 91 % of the 2672 individuals examined, providing us with 2430 sera for testing from Kaluarang (696), Hulo (874) and Jepara (860).
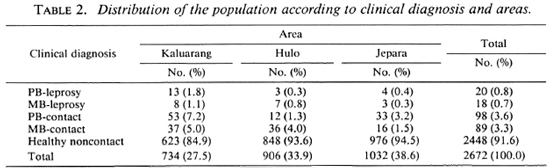
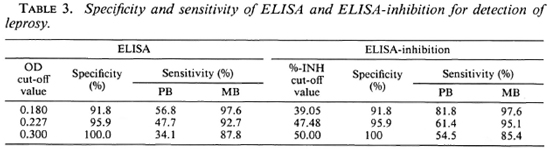
Table 3 shows the specificity and sensitivity of the ELISA and the ELISA-INH for the detection of clinical leprosy using different cut-off values. The specificity of the tests was calculated using the results obtained with sera from 49 healthy blood donors from Yogyakarta. Sensitivity was determined on the basis of the results obtained using sera of 44 PB and 41 MB untreated patients. Both tests were found to be similar as to specificity and sensitivity, irrespective of the cut-off values used. For the purpose of this study, we selected cut-off values of OD 0.180 for the ELISA and of 39.05% for the ELISA-INH. These cut-off values resulted in a specificity of 91.8% and a sensitivity of 97.6% for MB leprosy in both tests, and a sensitivity of 56.8% and 81.8% for PB leprosy in the ELISA and the ELISA-INH, respectively. When the two tests were combined (using OD 0.180 as a cut-off value for the ELISA and 39.05% for the ELISA-INH), the resulting specificity was 83.7% and the sensitivity was 90.9% for PB leprosy and 97.6% for MB leprosy.
Figures 1 and 2 show the seropositivity data according to age and sex in the total population including all three areas. Seropositivity was found to increase rapidly, reaching a peak in the 10-19 year age group and then to decrease steadily at older ages. This age trend was shown to occur in all three areas, independent of sex (data not shown).
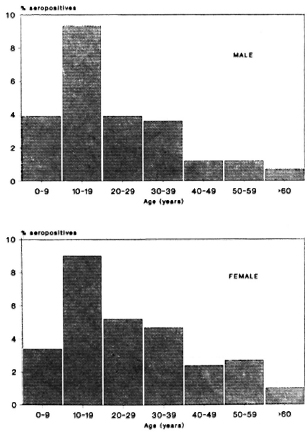
Fig. 1. Frequency of ELISA seropositives according to age and sex in all areas.
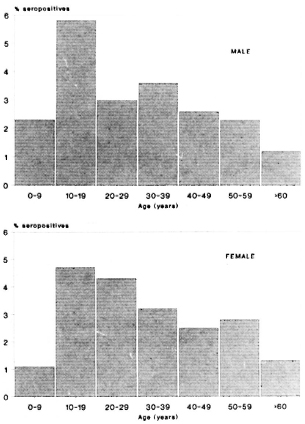
Fig. 2. Frequency of ELISA-INH seropositives according to age and sex in all areas.
Tables 4 and 5 show specific seropositivity rates by age, sex, and area. The age-standardized seropositivity ratio (SPR) and standardized leprosy prevalence ratio (SLPR) in each area according to sex were calculated using the total population of all areas as the standard. It can be seen that the SPR was highest in Jepara, followed by Kaluarang and Hulo, for either the ELISA or the ELISA-INH, irrespective of sex. The SPR did not parallel the SLPR for both males and females; this was highest in Ka-luarang, followed by Hulo and Jepara. The mean antibody levels (data not shown) for both tests were also highest in Jepara, followed by Kaluarang, then Hulo.
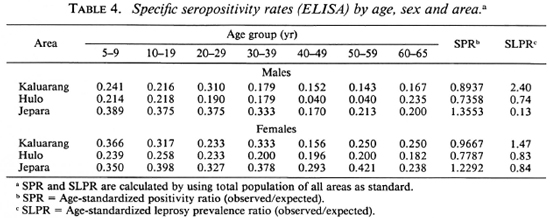
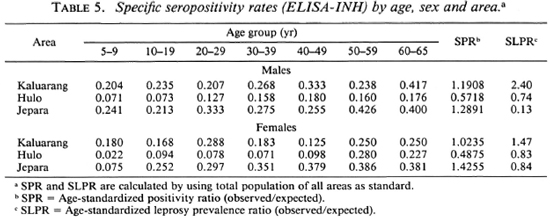
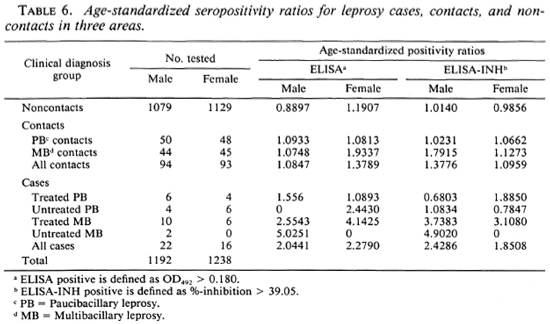
Table 6 shows a comparison of the serological results among the different groups controlled for age and sex. Higher age-standardized seropositivity ratios were found in contacts compared to noncontacts, both for males and females. The age-standardized seropositivity ratio appeared to be higher in MB contacts than PB contacts, for males as well as for females, in both the ELISA and the ELISA-INH (Table 6), although the difference was not statistically significant.(chi-squared test, p > 0.05).
No significant differences were seen in the age-standardized seropositivity ratios between groups with or without a BCG scar (data not shown). The seropositivity ratios did, however, differ among the study areas: higher ratios were found in both BCG-vac-cinated and unvaccinated individuals in Je-para compared to the other areas.
There was also no significant difference in the frequency of seropositives in both tests between the Mitsuda-negativc and Mitsuda-positive healthy individuals (chisquared test, p > 0.05).
DISCUSSION
This study was carried out to evaluate the usefulness of serological tests as indicators of infection at the population level, by collecting and analyzing seroepidemiological data obtained from three areas in Indonesia differing in endemicity for leprosy.
For this purpose, the study included the total population of two isolated villages with high prevalence for leprosy in South Sulawesi (Kaluarang and Hulo). Such locations are ideal for the study of the patterns of leprosy in populations, since they circumvent the well-recognized problems of familial or village clustering of leprosy (7). A third field study was carried out in an area with low leprosy prevalence in Java (Je-para). In this case, the population was too large to be covered completely, so cluster random sampling was used to obtain a representative sample of a size similar to the other two populations. Although this sampling method could, in some cases, be misleading for exploration of the leprosy cases in a population, it still has many advantages over simple random sampling.
For all three areas, the prevalence rates found in this study were higher than the rates reported by the leprosy control services in the same areas. This can probably be attributed to under-reporting by the leprosy control services which only do passive case-finding.
Two serological assays were used in this study based on the detection of antibodies to the species-specific epitopes of the PGL-I and 36 kDa antigens of M. leprae. These two particular assays were chosen because of their high specificity and sensitivity, which have been reported previously (17).
Since the distribution of antibody levels as measured in both assays is unimodal, any criterion for positivity is arbitrary. Since no "gold standard" for M. leprae infection is available, sensitivity can only be determined on the basis of routine diagnosis of clinical cases of leprosy, while specificity has to rely on the assumption that the healthy individuals are not infected. Therefore, the prevalence of seropositivity is always relative and not absolute (9). In this study, the specificity of the assays was determined using results obtained from tests on healthy blood donors from a nonendemic area (Yogyakarta). This served to minimize the possibility of M. leprae infection in this group, while still providing an appropriate background in terms of genetic and environmental factors. The sensitivity of both assays was determined from results obtained on sera from untreated leprosy cases in the clinic. As has already been observed (17), the sensitivity of both assays was found to be appreciably higher in multibacillary than in paucibacillary cases. Using different criteria for positivity (i.e., cut-off values; Table 3), consistent patterns for both tests in all three areas were observed. Considering this, the cut-off values for positivity of OD = 0.180 and percent inhibition = 39.05 were selected for the ELISA and the ELISA-INH, respectively; all results were assessed by this standard.
Since leprosy prevalence is in general extremely low, even in so-called highly endemic areas, the predictive values of even very good tests for disease will consequently be very low as well. Nevertheless, provided that serological assays do in fact provide evidence of past or present infection, they might give information on patterns of M. leprae infection which would form an important addition to that derived from the observed patterns of clinical disease.
An analysis of the serological data revealed a clear age-related pattern, independent of the area (Figs. 1 and 2). The sero-positivity rates were found to increase rapidly up to the age of 10-19 years, followed by a steady decrease through the older ages. This phenomenon was found for both tests, one measuring only IgM and the other predominantly IgG. Overall IgM and IgG levels have been reported to increase during youth and to decrease subsequently with increasing age (3). However, the peak of total immunoglobulin levels appeared in the age group of 20-30 years. Therefore, it is unlikely that our findings reflect this general age trend in immunoglobulin synthesis, but are most likely attributable to the presence of antibodies binding specifically to the respective antigens. Several circumstances may lead to such an age trend (9). Most likely, a peak in the seropositivity rates in the young group reflects a high exposure to infection during this age or the foregoing period. Whatever the reason for this phenomenon, it is clear that the age variable will have to be considered in studies involving comparative serological data analysis.
In contrast to what was reported from a population-based study in Malawi (9), in the present study no difference was detected in seropositivity rates among males and females. It is perhaps relevant to note that in this study population the leprosy prevalence was similar in both sexes.
The age-standardized seropositivity ratios for both tests were, in general, found to be correlated with the prevalence of leprosy in the two areas in South Sulawesi. However, when Jepara was taken into consideration for comparison, this correlation could no longer be demonstrated. Jepara showed the highest seropositivity ratios, although it was the area with the lowest prevalence. There are several possible explanations for these findings. First, it could be that the Jepara population has a different genetic background than the populations in South Sulawesi. This could imply that genetic factors may render individuals more susceptible to M. leprae infection, but not necessarily to disease, since leprosy prevalence is lowest in Jepara. On the other hand, genetic factors might also directly influence serological responses. Secondly, a difference in seropositivity rates independent of the leprosy prevalence could equally be a result of differences in pathogenicity between the strains of M. leprae in the two areas. That M. leprae from different geographical locations might differ in pathogenicity is suggested by the recent work of Kazda, et al. (6), showing that mycobacteria identified as M. leprae by species-specific monoclonal antibodies could be found in the soil in several nonendemic areas. Thirdly, other environmental and population factors may differ in the areas in Java and Sulawesi, such as socioeconomic status, conditions of hygiene and sanitation, and crowding. For instance, the population density is higher in Java compared to Sulawesi. Again, this would imply that transmission in Java is very intense, but that the development of the disease depends on other, as yet unidentified, factors independent of infection. Fourthly, environmental (myco)bacteria could share epitopes with M. leprae, resulting in antibodies which can bind to the "M. leprae-specific" epitopes of the PGL-I and 36-kDa antigens. Or environmental mycobacteria or other infectious agents may possess unrelated antigens which sterically resemble the species-specific epitopes of the PGL-I and 36-kDa antigens. This possibility has been put forward by others to explain high seropositivity rates in endemic populations in French Polynesia (4).
In summary, these findings show that the prevalence of seropositivity does not necessarily parallel the prevalence of disease in populations. Similar findings have been reported from studies in other endemic areas in the world (4,9).
Age-standardized seropositivity ratios were found to be higher in contacts compared to healthy noncontacts. Furthermore, these ratios were higher in contacts of MB patients than in those of PB cases (Table 6). Assuming that seropositivity does reflect present or past infection, transmission of infection would seem to be more intense in contacts, particularly those of MB patients, compared to noncontacts. Consistent with this is the finding that mean antibody levels were higher in contacts compared to non-contacts, and in MB contacts compared to PB contacts (data not shown). Similar findings have been reported using the same and other assays (19), while others did not find any difference in serological status between contacts and noncontacts (9,11). This discrepancy might be caused by the different definitions of household contacts used in these studies.
No association of serological data with a history of BCG vaccination was found, independent of age, sex or area. BCG vaccination does not, therefore, affect antibody responses to M. leprae-specific antigens. Similar results have been reported from a population study (9) and from another study in which leprosy patients were vaccinated with BCG (5).
No difference was found in the seropositivity rates for either test when Mitsuda-positive and -negative individuals were compared. A positive Mitsuda reaction in healthy individuals could reflect infection with crossreactive (myco)bacteria. On the other hand, individuals who have never been infected may also give a negative Mitsuda reaction. This could be an explanation for the absence of a correlation between Mitsuda and serological reactivity. Prospective studies are required to investigate whether Mitsuda-negative but serologically positive individuals are at higher risk of developing leprosy.
This study evaluated the potential use of serological tests in the epidemiology of leprosy infection. Clear patterns could be observed, but results did not fulfill the expectations. In general, it is believed that prevalence of M. leprae infection parallels that of disease, as is the case for many infectious diseases; evidence in support of this assumption in the case of leprosy is limited. The question of whether seropositivity reflects past or present infection with M. leprae is still not settled. If seropositivity does reflect infection, then this study would suggest that the infection rate is not related to the prevalence of disease. However, it is not clear which factors, such as genetic background or crossreactivity with environmental (myco)bacteria, may be influencing the seropositivity in the study population. If such factors are important in determining the nature and the extent of the antibody response, then patterns of seropositivity may not be related to those of disease. In the clarification of such points, the recently developed technology of the polymerase chain reaction, which is able to detect in a sensitive and specific manner the leprosy bacillus itself (13), might be useful as a more direct indicator of (subclinical) infection.
Acknowledgment. We would like to express our gratitude to the Head of the Regional Office of the Ministry of Health, Republic of Indonesia, in both South Sulawesi and Central Java, and to the leprosy officers in the Ministry of Health and in the provinces of Central Java and South Sulawesi for their cooperation. We are indebted to all the people in the villages who voluntarily cooperated in this study.
We thank Dr. P. E. M. Fine and Dr. C. J. Geefhuysen for helpful and stimulating discussions, and Jaka Budi Susila, Tri Yuliati, and Madeleine de Wit for their excellent technical assistance. We thank Dr. Pamela Wright for her critical reading of the manuscript.
The financial support for this project by The Netherlands Leprosy Relief Association (NSL) and by the Science and Technology for Development Programme of the European Community (no. TS2-0111-NL) is greatly appreciated.
REFERENCES
1. Bloom, B. R. and Godal, T. Serial primary health care: strategics for control of disease in the developing world. V. Leprosy. Rev. Infect. Dis. 5(1983)765-780.
2. Brett, S. J., Payne, S. N., Gigg, J., Burgess, P. and Gigg, R. Use of synthetic glycoconjugate containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-453.
3. Buckley, C. E. and Dorsey, F. C. The effect of ageing on human serum immunoglobulin concentrations. J. Immunol. 105(1970)964-972.
4. Cartel, J.-L., Chanteau, S., Boutin, J. P., Plich-art, R., Richez, P., Roux, J. F. and Grosset, J.-H. Assessment of anti-phenolic glycolipid-I IgM levels using an ELISA for detection of M. leprae infection in populations of the South Pacific Islands. Int. J. Lepr. 58(1990)512-517.
5. Douglas, J. T., Hirsch, D. S., Fajardo, T. T., Guido, L. S. and Klatser, P. R. Serological monitoring of previously treated lepromatous patients during a course of multiple immunotherapy treatments with heat-killed Mycobacterium leprae and BCG. Clin. Exp. Immunol. 82(1990)567-573.
6. Douglas, J. T. and Worth, R. M. Field evaluation of an ELISA to detect antibody in leprosy patients and their contacts. Int. J. Lepr. 52(1984)26-33.
7. Fine, P. E. M. Problems in the collection and analysis of data in leprosy studies. Lepr. Rev. 52 Suppl. 1(1981)197-206.
8. Fine, P. E. M. Leprosy: the epidemiology of a slow bacterium. Epidemiol. Rev. 4(1982)161-188.
9. Fine, P. E. M., Ponnighaus, J. M., Burgess, P., Clarkson, J. A. and Draper, C. C. Seroepide-miological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assay using synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
10. Fletcher, R. E., Fletcher S. V and Wagner, E. E. Clinical Epidemiology; the Essentials. Baltimore: Williams and Wilkins, 1988.
11. Godal, T. and Negassi, S. Subclinical infection in leprosy. Br. Med. J. 3(1973)557-559.
12. Gonzalez-abreu, E., Mora, N., Perez, M., Pereira, M., Perez, J. and Gonzalez, A. B. Se-rodiagnosis of leprosy in patients' contacts by enzyme-linked immunosorbent assay. Lepr. Rev. 61(1990)145-150.
13. Hartskeerl, R. A., de Wit, M. Y. L. and Klatser, P. R. Polymerase chain reaction for the detection of Mycobacterium leprae, J. Gen. Microbiol. 135(1989)2357-2365.
14. Ji, B., Tang, Q., Li, Y., Chen, J., Zhang, J., Dong, L., Wang, C, Ma, J. and Ye, D. The sensitivity and specificity of fluorescent leprosy antibody absorption (FLA-ABS) test for detecting subclinical infection with Mycobacterium leprae. Lepr. Rev. 55(1984)327-335.
15. Kahn, H. A. and Sempos, C T. Statistical Methods in Epidemiology. New York: Oxford University Press, 1989.
16. Kazda, J., Irgens, M. and Kolk, A. H. J. Acid-fast bacilli found in sphagnum vegetation of coastal Norway containing Mycobacteria leprae-specific phenolic glycolipid-I. Int. J. Lepr. 58(1990)353-357.
17. Klatser, P. R. and Cardo, P. Esami sierologici. In: Manuale di Leprologia. Nunzi, E. and Leiker, D. L., eds. Bologna: Associazione Italiana Amici di Raoul Follereau-O.C.S.L, 1990, pp. 103-110.
18. Klatser, P. R., de Wit, M. Y. L., Fajardo, T. T., Cellona, R. V., Abalos, R. M., dela Cruz, E. C. Madarnag, M. G., Hirsch, D. S. and Douglas, J. T. Evaluation of M. leprae antigens in the monitoring of a dapsone-based chemotherapy of previously untreated lepromatous patients in Ccbu, Philippines. Lepr. Rev. 60(1989)178-186.
19. Klatser, P. R., de Wit, M. Y. L. and Kolk, A. H. J. An ELISA-inhibition test using monoclonal antibody for the serology of leprosy. Clin. Exp. Immunol. 62(1985)468-473.
20. Kraemer, M. S. Clinical Epidemiology and Bios-tatistics. Berlin: Springer Verlag, 1988.
21. Noordeen, S. K. and Lopez-Bravo, L. The world leprosy situation. Wld. Health Statist. Quart. 39(1986)122-128.
22. Serological tests for leprosy. (Editorial) Lancet 1 (1986) 533-535.
23. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
1. M.D., Department of Dermatology, Faculty of Medicine, Gadjah Mada University, Yogya-karta, Indonesia.
2. M.Sc, N. H. Swcllen-grebel Laboratory of Tropical Hygiene, Royal Tropical Institute, Meibergdreef 39, 1 105 AZ Amsterdam, The Netherlands.
Reprint requests to P. R. Klatser.
Received for publication on 13 February 1991.
Accepted for publication on 26 March 1991.